Abstract
In this paper we provide a focused review of the literature examining neural mechanisms involved in cognitive control over memory processes that can influence, and in turn are influenced, by emotional processes. The review is divided into two parts, the first focusing on working memory and the second on long-term memory. With regard to working memory, we discuss the neural bases of 1) control mechanisms that can select against distracting emotional information, 2) mechanisms that can regulate emotional reactions or responses, 3) how mood state influences cognitive control, and 4) individual differences in control mechanisms. For long-term memory, we briefly review 1) the neural substrates of emotional memory, 2) the cognitive and neural mechanisms that are involved in controlling emotional memories and 3) how these systems are altered in post-traumatic stress disorder. Finally, we consider tentative generalizations that can be drawn from this relatively unexplored conjunction of research endeavors.
Keywords: Emotion, Cognitive Control, Working Memory, Long-term Memory, Prefrontal Cortex, Anterior Cingulate, Amygdala, Hippocampus, Post-traumatic Stress Disorder, Rumination, Depression, Genetics, Human
2. Introduction
The prefrontal cortex has been implicated as playing an important role in cognitive control. Although a variety of models have been proposed to suggest how prefrontal cortex exerts such control, some points of general agreement are relevant for the issues we examine in this paper. First many models emphasize the idea that frontal regions are involved in the selection of processes related to goal-oriented aspects of behavior. For example, Miller and Cohen (2001) have argued that cognitive control acts like a series of switches selecting the processes that will be invoked to reach a goal, much as switches select the route of a train from the departure station to its destination. Although some researchers suggest that the prefrontal cortex does not have a specific organization for such executive processes (e.g., Duncan and Owen, 2000), other researchers view subprocesses as each occurring in distinct regions of prefrontal cortex. This latter viewpoint, for example, has been supported by data from meta-analyses of neuroimaging work (e.g., encoding, response selection, response execution; Nee, Wager & Jonides, 2007) and by the examination of individuals who have sustained brain damage in frontal regions (task setting, performance monitoring, and the initiating and sustaining of responses, Stuss & Alexander, 2007).
In the search for “core” or basic processes underlying cognitive control, work on individual differences suggests at least 3 separable components of executive function: updating, task switching and response selection (Miyake et al. 2000). Other research that spans investigations in human and other primates also supports the idea that inhibition is a core construct, for example, such as the idea that breakdowns in inhibiting responses to previously reinforced information can underlie the perseveration that is a classic sign of frontal damage. The idea that inhibition of responses plays an important role in executive control is a topic that is examined in other articles included in this special issue (e.g., Verbruggen & Logan; Chambers et al.) and to which we make linkages at the end of this review.
Historically, cognitive control mechanisms for the selection (e.g., Chambers et al. 2007) and inhibition (e.g., Aron & Poldrack, 2004) of responses as well as for selection of incoming sensory information (e.g., Kastner & Ungerleider, 2000) have been examined most extensively. In contrast, much less is known about the selection of conceptual information or the selection of information in memory (e.g., Kan & Thompson-Shill, 2004). Although there is a large body of work implicating the dorsolateral prefrontal cortex (DLPFC) in maintaining information in working memory (e.g., Goldman-Rakic 1996), less research has examined how information is selected from working memory. One prominent model suggests that ventral regions of DLPFC are involved in maintaining information in working memory, whereas more dorsal regions are involved in the selection and manipulation of the contents of working memory (Petrides, 2000). Another model argues that a portion of inferior frontal cortex, in particular Brodmann Area (BA) 47, is important in selection among stored conceptual representations whereas BA45 is more involved in post-retrieval selection among active representations (Badre & Wagner, 2007).
An independent line of research has examined the issue of neural systems required for cognitive control over emotional information (for a review see Ochsner & Gross, 2005). Because emotional information receives priority in processing (e.g., Pessoa & Ungerleider, 2004), there are many instances in which there is a need to exert control over the processing of or response to such information. The concept of emotion regulation is considered critical to healthy emotional functioning and is disrupted in a variety of different types of psychopathology. Of note, lateral and medial prefrontal regions have been implicated in cognitive control relevant to emotion, such as suppressing the processing of emotional information or controlling emotional feelings.
These two lines of research on cognitive control – one on cognitive control in working memory and the other on cognitive control of emotional information - have developed somewhat independently, despite evidence that overlapping cognitive and neural mechanisms are involved. Moreover, there is also another independent line of research examining the interface of emotion and working memory without reference to cognitive control. Current issues being addressed include examining whether there are separate systems for the maintenance of emotional vs. non-emotional information (e.g., Mikels et al. 2008) and what effects emotional information has on resolving interference between competing items in working memory (e.g. Levens and Phelps, 2008). There is little connection between these three strands of research, which is unfortunate as the convergence of them has broad implications for mental health disorders.
Hence, here we focus more specifically on the intersection of these three endeavors, namely the neural underpinnings of cognitive control mechanisms in memory that act on or are influenced by emotional information. Despite a dearth of research, cognitive control mechanisms are thought to be very important for keeping emotionally distracting and intrusive thoughts out of memory (e.g., Brewin & Beaton, 2002). Such thoughts are problematic in a variety of psychiatric disorders including depression, anxiety, post-traumatic stress disorder (PTSD), and obsessive-compulsive disorder (OCD). And the neural underpinnings of control mechanisms for emotional information in memory, as we review below, are beginning to be explored. As such, this review is designed to discuss what limited knowledge we have for this important topic.
For this review on the neural structures that underlie control processes involving memory and emotion, we divide the paper into two main sections: one focusing on working memory, and a second on long-term memory. In the section on working memory, we consider the construct rather broadly by focusing on selection processes for what is placed or prioritized in working memory, as well as processes that select among the set of active representations. In the section on long-term memory, we consider both the neural mechanisms that can control how emotional information gets into memory, as well as the processes by which they are retrieved. In the final sections, we speculate on how the cognitive control mechanisms that we have discussed might relate to the response inhibition discussed in some of the other papers in this special issue, and then present some general conclusions that can be drawn from the literature reviewed.
It should be pointed out that the vast majority of literature considered in this review relies almost exclusively on findings from functional magnetic resonance imaging. Interpretations of data from this method are limited in a number of ways. For instance, fMRI cannot isolate brain regions that are critical for a specific function, but only identify those regions that may play a role. This method also cannot distinguish between excitatory and inhibitory neural activity, nor can it determine whether signals reflect activation in a particular brain region or feedback/feedforward connections from other regions (for a recent review of these issues, see Logothetis, 2008). Unfortunately, there is little to no work that examines the neural substrates of control over information in memory related to emotion by examining individuals with brain damage or by using other techniques that temporarily deactivate brain regions, such as transcranial magnetic stimulation (TMS). These methods are important because they can help to isolate those brain regions that are critical for a given function. Some research using these techniques examines two of the three constructs we focus on in this review (i.e., cognitive control, memory, emotion), but we could find none where all three were examined together. For example, TMS has been used to isolate those regions of prefrontal cortex that are involved in control mechanisms that allow for selection of materials in working memory, (e.g. Sandrini et al. 2008) but the linkage with emotional processes has not been investigated. Likewise, lesion studies have isolated those areas of prefrontal cortex important for remembering emotional information, such as the history of risk and reward, (e.g. Clark et al. 2008) but have not examined the specific cognitive control mechanisms that might be involved. As research in this area continues, additional knowledge from studies using these approaches would be invaluable.
3. Working Memory
The research that we review in this section examines neural systems for cognitive control mechanisms related to emotion and working memory. For the purposes of this review, we consider two somewhat distinct “flavors” of the concept of working memory. Traditionally, as studied by cognitive psychologists, working memory is considered the process by which information is maintained or stored on-line for brief periods of time, typically between 3 and 10 seconds, but longer under other circumstances. Often it is investigated through paradigms in which an item is presented, followed by a delay during which the item is not present but must be maintained in mind, after which the item must be identified or recalled, often from among a series of distractors. However, relatively little work examining the neural underpinnings of control mechanisms related to emotion and working memory employ such a paradigm.
Rather, most of the research discussed in this section examines control mechanisms that are important for selecting, usually among a set of representations that are simultaneously active, the one particular representation that should be prioritized. In particular, these control mechanisms are often involved in selecting, which among the active representations, is most relevant for task goals. This flavor of “working memory,” which is conceptualized as the process of maintaining information in an active state for use in goal-directed behavior, is often represented in computational and developmental approaches to working memory (e.g., Morton & Munakata, 2002; Rougier et al. 2005).
In this section, we review research that has relied on one of four major approaches. The first examines neural control mechanisms for selection of task-relevant information in the face of potentially distracting emotional information. The second examines neural control mechanisms over emotional reactions or responses. The third examines changes in neural systems of cognitive control with variations in mood state, and the fourth examines individual differences in neural mechanisms for control of information in working memory related more specifically to ruminative tendencies or genetic variation.
3.1. Control Mechanisms Used to Select Information in the Face of Potentially Distracting Emotional Information
3.1.1. Ignoring emotional information presented simultaneously with task-relevant information
A number of studies have examined the nature of neural systems that are engaged in order to focus on task demands in the face of distracting emotional information. Such mechanisms are important because emotional information captures attention relatively automatically (e.g., Pessoa & Ungerleider, 2004). Hence, researchers have attempted to uncover which brain systems are required to overcome the relatively more automatic bias to prioritize processing of emotional information over more task-relevant information.
One study that investigated this issue used an oddball task in which individuals had to identify targets (circles) that occurred relatively frequently within a series of non-targets (squares). At the same time, distracting scenes were interspersed and were varied parametrically for arousal ranging from neutral to highly arousing with negative valence. The brain regions responding to the targets were dissociable from those responding to the emotional distractors. In particular, the middle frontal gyrus (MFG) responded to targets while deactivating in response to distractors. Conversely, inferior frontal gyrus (IFG) responded to distractors (with the response increasing as a function of the intensity of arousal), but deactivated to targets (Yamasaki, LaBar & McCarthy, 2002). The results were interpreted in the context of a perspective that attentional and emotional functions are segregated into two parallel dorsal and ventral streams in the prefrontal cortex. From this perspective, aspects of emotional processing are in some sense “insulated” from control mechanisms. Given that caudal regions of the anterior cingulate were activated in both tasks, this structure was hypothesized to provide a mechanism whereby information from the two relatively independent streams can be integrated.
However, another body of work using the emotional Stroop task leads to a somewhat different conclusion. In this paradigm, the emotional and non-emotional information is not segregated into two perceptually distinct representations as in the Yamasaki, LaBar & McCarthy (2002) study. Rather, in the emotional Stroop task, which is a variant of the standard color-word Stroop task, one must attend to a word’s ink color while ignoring the meaning of the word. The ability to do so is compared for emotional and non-emotional (i.e., neutral) words, and typically reaction time to emotional words is increased relative to non-emotional words because they automatically attract attention. This attentional bias is not due to the inherent semantic or response conflict as occurs for incongruent trials in the standard color-word Stroop task (e.g. “red” in blue ink). Although the effects are observed most robustly in the emotional Stroop task when the words are related to an individual’s psychopathology (e.g., the word “web” for a spider phobic), or when clinical populations with significant levels of anxiety are tested, modest but significant behavioral effects can be found in individuals without psychopathology (Koven, Heller, Banich, & Miller, 2003).
Initial studies concentrated on determining whether distinct regions of the ACC are engaged by a non-emotional vs. emotional Stroop stimuli. Using a variant of the standard Stroop task in which individuals have to identify the number of words on a screen, participants were more likely to activate rostral regions of the ACC if the words were emotional (Whalen et al., 1998) whereas more caudal and dorsal regions when the words are not emotional (Bush et al., 1998). These studies served as an impetus for making a conceptual distinction between subdivisions of the ACC, with more caudal regions described as the “cognitive division” and more rostral-ventral regions described as the “affective division” (Bush, Luu & Posner, 2000). Consistent with the findings of Yamasaki, LaBar & McCarthy (2002) these results suggest a potential distinction between regions involved in cognitive control for emotional versus non-emotional information.
However, a somewhat different conclusion was reached in a whole-brain exploration of a direct contrast between the standard color-word Stroop task and a color-emotional word Stroop task in the same participants (Compton et al., 2003). In this study, the same DLPFC regions were activated when attentional demands were increased relative to neutral words (i.e.. non-color or non-emotional words), either because the word conflicted with the ink color or because the word was emotional in nature. These findings are consistent with other work suggesting that DLPFC regions are involved in setting a top-down bias or attentional set towards task-relevant information and away from task-irrelevant information (Banich et al. 2000a,b); Milham and Banich, 2005). For both tasks, a top-down bias towards ink color identification and away from word reading is required, regardless of the content of the word. Nonetheless, activity in posterior brain regions differed for the two tasks. Incongruent color words relative to neutral words were associated with increased left parietal activity and decreased activity in the parahippocampal gyrus, while negative emotional words relative to neutral words were associated with bilateral occipito-temporal activity and decreased amygdala activity. These findings suggest that even if DLPFC regions are similarly engaged for top-down biasing, the sites at which they exert their influence may vary depending on whether the information to be ignored is emotional in nature.
A follow-up study, once again directly contrasting activity in the color-word and emotional Stroop tasks, provided additional evidence regarding differential engagement of portions of the ACC for control over emotional vs. non-emotional information (Mohanty et al., 2007). First, consistent with the findings of Bush, Whalen and colleagues, dorsal regions of the ACC were engaged by attentional demand in the standard color-word task (incongruent >neutral) while rostral regions of the ACC were engaged by attentional demand in the emotional Stroop task (emotional>neutral). Second, individual differences in reaction time to incongruent words predicted activation in dorsal ACC while individual differences in reaction time to negative words predicted activation in rostral ACC. Third, the pattern of covariation of activity with other brain regions differed for these two portions of the ACC. Activity in dorsal ACC engendered by attentional demand was more highly associated with DLPFC activity than rostral ACC for both tasks. On the other hand, activity in rostral ACC engendered by attentional demand in the emotional Stroop task was correlated more with amygdala activity than DLPFC activity.
Moreover, activity in this rostral region of the ACC, which has been linked to the regulation of emotional responses (Bush, Luu & Posner, 2000), appears to be sensitive to aspects of cognitive control that are associated with tendencies toward psychopathology. Individuals who are rated high in certain types of anxiety (anxious arousal, anxious apprehension) show decreased activity in this region as compared to non-anxious individuals (Engels et al. 2007),. Such a finding suggests that these individuals may have difficulty exerting cognitive control over emotional information.
Additional work (Herrington et al., 2005) has examined whether control mechanisms vary depending on the valence of the to-be-ignored emotional information by comparing activation observed for positively valenced (e.g., “desire,” “excite”) words to negatively valenced ones (e.g., “hate,” “sad”). Relative to a baseline of neutral words, a similar DLPFC region was engaged regardless of the word’s valence. However, the nature of that engagement differed. Of note, there was an asymmetry such that positive words led to more activity in a portion of left DLFPC (BA 9) than did negative words. This asymmetry of DLPFC activity has been replicated in two additional studies (Engels et al., 2007; Herrington et al. in preparation).
Note that with respect to the findings reported above and in the rest of the review, the reference to emotional valence does not implicate an endorsement of the valence hypothesis of frontal lateralization (pleasant/unpleasant) over the motivational hypothesis (approach/withdrawal), and the issue of which of these hypotheses better accounts for the data is not examined in this article. Rather, valence refers to the property of pleasant or unpleasant emotion in the experimental context. In the studies reviewed, valence and motivation are confounded (as positive valence is typically associated with approach and negative valence with withdrawal). Some research has associated anger (the only negatively valenced emotion associated with approach motivation) with leftward asymmetry, which has been interpreted as challenging the valence view of frontal lateralization. However, Stewart, et al. (in press) found that different types of anger show different lateralization patterns, complicating the picture (e.g., approach-related anger was not associated with leftward asymmetry, but another type of anger associated with anger rumination was). In addition, anger may sometimes have important appetitve qualities rather than being exclusively negative in valence. These and other findings suggest that a definitive model of frontal lateralization for emotion remains to be established.
Nonetheless, the modulation of relative activity across left and right frontal regions by valence is consistent with other work. Studies using EEG have found that more activity over the left hemisphere is associated with processing stimuli of pleasant valence, while more activity over the right hemisphere is associated with processing stimuli unpleasant valence (e.g., Davidson, 1992; for a recent review, see Herrington, Koven, Miller, & Heller, 2006). Moreover, increased activity of left DLPFC has been observed in happy mood states (Habel et al. 2005). This literature, considered along with the fMRI findings discussed above (Herrington et al. 2005, in preparation, Engels et al. 2007), suggests that cognitive control mechanisms are reciprocally influenced by regions involved in emotional processing. Given that regions of orbitofrontal cortex (OFC) are often implicated in the processing of the emotional valence of sensory stimuli and reward value (e.g., O’Dougherty et al. 2001; Lewis, Critchley, Rotshtein & Dolan, 2007), we speculate that there are bottom-up influences from these regions to DLPFC regions involved in cognitive control.
It is noteworthy that a different and more posterior region of DLPFC was found to be sensitive to individual differences in mood state rather than the valence of the to-be-ignored word (Herrington et al., in preparation). Depressed individuals showed greater right than left hemisphere activity, whereas control individuals showed greater left than right hemisphere activity. These findings are consistent with the modulation of EEG asymmetries observed in depressed versus non-depressed individuals (Henriques and Davidson, 1991). Moreover, increased activity of left DLPFC has been observed in happy mood states (Habel et al. 2005).
The fact that a word’s valence influenced more anterior regions of DLPFC whereas mood influenced a more posterior region (Herrington et al. in preparation) is interesting in light of a proposal we have made regarding the role of these two regions in cognitive control. Our model hypothesizes that posterior regions of DLPFC are involved in modulating activity of posterior brain regions to bias toward task-relevant processing, such as ink color identification, and away from task-irrelevant processing, such as word reading in the Stroop task. We have speculated that these regions are involved in more sustained aspects of attentional control. In contrast, we have argued that regions of mid-DLPFC are more involved in selecting the task-relevant representation that must be selected and maintained to guide processing (e.g., the color blue rather than the word “red” in the color-word Stroop task) (e.g., Banich, submitted; Milham, Banich & Barad, 2003), and have speculated that these regions are involved in more transient aspects of attentional control.
In a somewhat parallel manner, activity in posterior regions of DLPFC was found to be sensitive to an individual’s typical mood state, which is a more static phenomenon. We speculate that these regions are involved in biasing processing in posterior brain regions as to produce some of the cognitive characteristics observed in depression (e.g., decreased activity in left posterior DLPFC and/or increased activity in right posterior DLPFC may lead to a deficit in maintaining task relevant processing and hence in executive functioning; see Levin et al., 2007 for review of relevant cognitive deficits in depression). In contrast, activity in more anterior regions of DLPFC was sensitive to the valence of a word, which varied in a more transient manner. This finding is also consistent with the idea that this region of DLPFC is involved in control processes related to selection among the set of potentially task-relevant representations.
In the work reviewed so far, we have discussed the role of the DLPFC and ACC somewhat in isolation from one another. We now turn to studies that examine their roles more in tandem. In one such study, individuals made a decision about centrally presented houses, while ignoring laterally presented faces that could have either neutral or negative emotional expressions (Bishop, Duncan, Brett & Lawrence, 2004). Similar to the emotional Stroop task in which words, either emotional or not must be ignored, in this task faces, either emotional or not are to be ignored. Lateral prefrontal cortex (LPFC) was engaged when the initial set of trials within a block contained a high proportion of negative trials, signaling that the remaining trials in the block would be negative as well. Such findings are consistent with the role of DLPFC in top-down attentional biasing toward task-relevant information that must receive priority in processing despite potent distracting information (e.g., Banich et al. 2000a; Milham, Banich & Barad, 2003). In contrast, rostral regions of the ACC were activated when negative faces appeared in a block that was otherwise composed of a high proportion of neutral faces. The authors argued that rostral ACC is involved in conflict arising from the emotionally salient task-irrelevant information. However, it is not clear exactly what is in “conflict” in this situation.
To address this issue of emotional “conflict,” other researchers (Etkin et al. 2006) have designed an emotional Stroop task in which there is direct conflict between two sources of emotional information. This task is more akin to the traditional color-word Stroop task, in which two sources of color information, one task-relevant and one task-irrelevant, are placed in conflict. In this task, the words “happy” or “fear” were displayed across either a happy face or fearful one. The conflict in this task is between the emotional valence of the word as compared to the emotional valence of the face, and hence probably arises at the semantic level. The researchers focused specifically on activity for incongruent trials in which conflict exists (e.g. the word “fear” across a happy face). They examined activity for incongruent trials preceded by trials that required low attentional control (a congruent trial preceding the incongruent one: CI) as compared to high attentional control (an incongruent trial preceding an incongruent trial: II). As observed in previous studies, there was less behavioral interference for II trials compared to CI trials because the previous incongruent trial had already heightened attentional control.
Of note, they observed greater activity in dorsomedial prefrontal and bilateral DFLPC for incongruent trials when the previous trials required low (CI) rather than high (II) attentional control. The authors interpreted the activation in PFC as being involved in monitoring for the amount of emotional conflict. Within the framework provided earlier, we prefer to think that this finding is consistent with work on the color-word emotional Stroop task that suggests regions of DLPFC are engaged when attentional control must be increased to ignore distracting emotional information. Because the prior trial did not require much attentional control, increased engagement was required on the subsequent incongruent trial. We argue that such engagement occurs whenever distracting information can compete for priority in processing (e.g., Milham, Banich & Barad, 2003). As such, DLPFC activity can be observed even when there is no inherent conflict between task-relevant and task-irrelevant information (e.g., the word “kill” does not conflict with the color green).
In contrast, the pattern of activation for the cingulate was opposite that observed for DLPFC. Greater activity in the rostral anterior cingulate was observed when the prior trial required high attentional control (II) as compared to lower attentional control (CI). Moreover, activity for congruent vs. incongruent trials (regardless of the prior trial) did not differ, suggesting that activity in this region is not merely reflecting how difficult the trials were. Of note, greater activity in the rostral ACC predicted less activity in the amygdala. The authors suggested that the ACC is involved in resolving conflict and in so doing, inhibits amygdala activity that might be involved in activation of the sympathetic nervous system via the hypothalamus. While this is an interesting suggestion, fMRI data is severely hampered in its ability to provide clear and direct evidence for inhibition of one brain region over another (see Aron, 2007 for a thoughtful discussion of this issue in general, and also more specifically with regards to conflict paradigms).
Regardless, these findings are consistent with reduced rostral ACC activity in anxious individuals during performance of an emotion-word Stroop task (Engels et al. 2007). One might speculate that reduced rostral ACC activity may be associated with increased amygdala activity, both of which lead to the increased arousal often observed in anxiety. There have been reports suggesting heightened rostral ACC activity in PTSD, but as we have argued and demonstrated elsewhere (see review in Engels et al., 2007), there are different types of anxiety with different patterns of regional brain activity. The findings on PTSD would benefit from systematic differentiation of apprehensive vs. acute aspects of anxiety.
In a subsequent study, participants performed both this emotional Stroop task as well as a gender Stroop task in which either male or female faces were presented while the word “male” or “female” was positioned across the face. The individual’s task was to identify the face and ignore the word (Egner et al., 2007). The control networks that were activated for the two versions of the task were overlapping but somewhat dissociable. Both tasks activated dorsal regions of the ACC more for incongruent items when the preceding item was incongruent than when it was congruent. Such a finding is consistent with that of Mohanty et al. (2007) who found that both the standard color-word and the emotional-word Stroop task activate this region.
However, Egner et al. (2007) also found that regions of right LPFC become activated for the II>CI contrast in the gender conflict task, but not the emotion conflict task. This lack of engagement for the emotion conflict task is at odds with their earlier findings (Etkin et al. 2006) as well as findings of Compton et al. (2003), Herrington et al. (2005) and Engels et al. (2007), in which DLPFC was activated for both emotional and non-emotional versions of the Stroop task. The reason for the lack of engagement of LPFC in the study of Egner and colleagues is not clear. Conversely, rostral ACC activation was observed for the II>CI contrast in the emotion conflict task but not the gender conflict task. Moreover, activity in the rostral ACC was associated with decreased activity in the amygdala, consistent with the notion that this region in inversely associated with the response of the amygdala (Etkin et al., 2006).
Summary
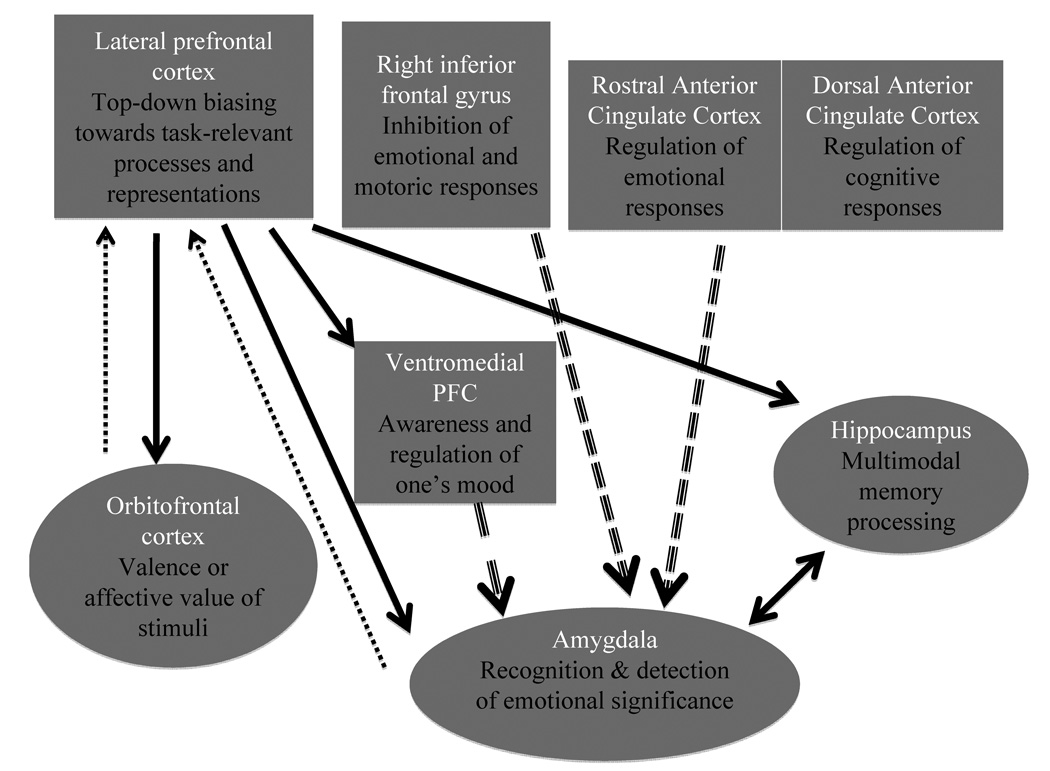
Overall, the findings from the available studies suggest that the DLPFC regions involved in top-down biasing toward task-relevant information and away from task-irrelevant information overlap whether the information to-be-ignored is emotional or non-emotional. There is also some evidence that activity of anterior regions of DLPFC, is influenced by the valence of the information to-be-ignored. Dorsal regions of the ACC have been found engaged when either emotional or non-emotional information must be ignored. Although some argue that this region is involved in resolving conflict (e.g. Egner et al. 2007), we prefer the interpretation that this region is involved in late-stage selection, which is influenced by how well DLFPC is able to implement attentional control (see Banich, submitted; Milham et al. 2002, Milham, et al. 2003). In contrast, rostral and pregenual cingulate regions appear to be recruited specifically when control must be implemented to ignore task-irrelevant emotional information. Some authors have suggested that this region serves to down-regulate activity in the amygdala. Such a relationship will contrast with that discussed later in regards to long-term memory. In that case, modulation of amygdala activity has been proposed to occur more via interaction with lateral PFC and the hippocampus.
3.1.2. Ignoring emotional information when task-relevant information is maintained across a delay
The issue of emotion and working memory when information must be maintained across a delay has received scant attention. In one of the few studies to address this issue, a delayed-response working memory task was given in which 3 faces were shown and the participant’s task was to determine if a probe face presented after a delay was one of the original three. During the delay, two distracting scenes were shown with both scenes being of the same type: either both emotional (highly arousing negative scenes), both neutral, or both scrambled versions of these scenes (Dolcos & McCarthy, 2006).
During the delay period, activity was observed in regions typically involved in working memory, specifically DLPFC, as well as lateral parietal cortices. In contrast, the emotionally distracting information engaged regions involved in emotion including the amygdala and ventrolateral prefrontal cortex (VLPFC). Of note however, the emotionally distracting information was also associated with a decrease in DLPFC activity along with a concomitant drop in performance on the working memory task. The authors interpreted these results as representing competition between emotional and non-emotional systems. They suggest that it is not a mere siphoning of resources that causes the competition, since DLPFC activity was increased (rather than decreased) when novel distracting faces rather than emotional scenes were used during the delay (Dolcos et al., 2008). Such findings are consistent with the theorizing of a competition in which subgenual regions of the ACC dominate during emotional processing and LPFC during cognitive processing (Drevets & Raichle, 1998).
However, there is at least some evidence that this dichotomy may not be quite so strict. Although the amygdala is thought typically to be a structure highly involved in the processing of emotional information, it has also been found to be active during processing in a non-emotional working memory task. This effect was observed in a study in which individuals were asked to do an N-back working memory task with non-emotional words (Schaefer, et al., 2006). In the N-back task, individuals see a series of items and must respond every time the current item matches the stimulus N items ago, where N can be 1, 2 or 3 depending on the condition. This task not only requires individuals to maintain information in working memory, but also to update and manipulate those contents in order to compare the current item to the changing set of items held in working memory. The faster an individual was on a 3-back version of the task, the greater was the activity in the left amygdala. As discussed earlier, regions of the PFC such as the rostral ACC appear to be related to amygdala function, and as we discuss later in the section on long-term memory, there are hints of LPFC modification of amygdala function as well (e.g., Depue et al. 2007). Thus, the findings of this study suggest the possibility of a reciprocal influence of the amygdala on PFC functioning. In particular, Schaefer and colleagues suggest that what links the two regions is the role of both working memory and emotion areas in goal-related behavior. To the degree that the amygdala is involved in processing information that is attentionally relevant, it may up-regulate regions of the PFC involved in maintaining that information for goal-oriented processes.
Summary
Currently very little work has specifically examined the influence of emotional processing on tasks that tap working memory from the perspective of maintaining information across a delay. This area appears to be one that is ripe for further investigation since there is contradictory evidence on whether working memory capacity is diminished or enhanced if portions of the emotional neural circuitry are engaged. Answering such a question would seem important for a number of psychiatric disorders. In anxiety disorders and depression for instance, it is thought that verbal working memory may be co-opted either because of verbal thoughts regarding worry or depressive ruminations, respectively. In disorders such as OCD and PTSD, visual working memory may also be co-opted by images of harm to oneself or others, or flashbacks of traumatic experiences, respectively.
3.2. Cognitive control over emotional information, reactions, and responses
One paradigm designed to examine cognitive control over information in working memory is the refreshing paradigm pioneered by Johnson and colleagues (2005). In this paradigm, individuals see words on the screen that they must say out loud. In the refresh condition, an asterisk appears afterwards and the individual must say the previously presented word. In the repeat condition, which acts as a control, the word is simply shown on the screen and the word must be repeated. These researchers have proposed that refreshing is an executive process that allows thoughts or representations to be put into the foreground and maintained or augmented. A meta-analysis of experiments utilizing this technique has revealed extensive activation across middle and superior frontal regions, especially in the left hemisphere and the anterior cingulate when comparing activation for a refresh condition versus a repeat condition (Johnson et al., 2005). Subsequent work has suggested a critical role for the middle frontal gyrus, as rTMS to this area results in a specific elongation of responses to refresh, but not repeat trials (Miller et al. 2008).
The effect of emotional context in this paradigm was investigated by showing three words simultaneously, two of which were neutral and one of which was emotional. The neural systems engaged by refreshing a neutral word as compared to an emotional one were identical, except for additional activation in anterior orbitofrontal cortex (BA 10) when a neutral word had to be refreshed. The authors interpreted this finding as suggesting that orbitofrontal regions might play a role in controlling emotional responses that might interfere with on-going processing of the neutral word (Johnson et al., 2005, Exp. 6).
Other work has more directly examined the ability to control emotional reactions or responses. Often in these studies individuals are asked to suppress the emotional response or to attempt to “re-appraise” it in a more cognitive and less emotional manner. Some of the earliest work examined the ability to suppress responses to sexually arousing pictures as compared to just viewing them (Beauregard et al., 2001). This study identified regions of the right superior frontal gyrus and a pregenual region (part of the “affective” division) of the ACC. A subsequent study by the same group compared brain activation when individuals must inhibit a sad response to pictures as compared to just viewing them (Levesque et al. 2003). This process also engaged portions of the right PFC, specifically regions of the right OFC (BA11) and right DLPFC (BA 9). Furthermore, activity in these regions was related to how much the individuals self-reported a change in sadness, with more activation associated with greater ratings of sadness. Unlike the prior study, there was no significant change in activity in the ACC associated with this type of attempt to control emotion.
In other studies, individuals were not asked to suppress emotion, but rather to re-appraise information in non-emotional terms. For example, when shown a picture of women crying outside a church, individuals were told to think of an alternative interpretation from the one most obvious – that someone had died. This re-appraisal might instead involve imagining that the women were at a wedding and were overjoyed at the new marriage. When reappraising highly negative scenes as compared to just viewing them, increased activation has been observed in lateral and medial PFC (BA 6, 8, 32) along with a concomitant decrease in activation of the amygdala and medial OFC. Of note, the degree of activity in the dorsal (“cognitive”) region of the ACC predicted the degree of the drop in self-reported negative affect across individuals during reappraisal (Ochsner et al. 2002), a finding that has been replicated (Phan et al. 2005). These findings suggest that cognitive control regions play an important role in re-appraisal and controlling emotional responses. When individuals are not told to regulate their response to sad emotion (Phan et al. 2005) or to maintain such a response (Schaefer et al., 2002), activation in the amygdala increases, suggesting that the amygdala is a region to which such cognitive control is directed.
Further research indicates that the regions involved in regulation may vary depending on whether the negative emotion is to be down-regulated, as previously discussed, or up-regulated (e.g., if viewing a picture of a ferocious dog, imagine it is about to bite you). Both processes activate the previously noted regions of LPFC and the dorsal regions of the ACC implicated in cognitive control, suggesting a common set of control regions regardless of valence. However, another set of regions showed different activation during the down-regulation as compared to the up-regulation of negative emotion. In particular, during down regulation, there was greater activity of right lateral inferior cortex (LIFC), a region that has been previously implicated in inhibition (e.g., Aron, Robbins & Polrack, 2004). In contrast, up-regulation led to increased activity in left rostromedial PFC, which has been implicated in the retrieval of emotions. In both cases, amygdala activation was modified depending on task demands (i.e., increased during retrieval of negative emotions and decreased during suppression) (Ochsner et al. 2004). To preview, these findings are consistent with those examining the suppression of negative information in long-term memory that 1) suggests that activity in right IFG associated with such suppression and 2) changes in amygdala activity when negative memories are suppressed (Depue et al. 2007).
Other researchers have examined the degree to which such regulatory mechanisms over negative affect may be disrupted in depression (Johnstone et al., 2007). As in prior studies, individuals were shown negative pictures and were told either to 1) attend to emotion invoked by a picture (no regulation) 2) increase the emotion or 3) decrease the emotion. When comparing the decrease condition to the attend condition, several findings were noted. For control individuals, increased activation was observed in left lateral and left ventrolateral areas of PFC (BA 8, 45/47) as well as the insula. Depressed individuals, however, showed bilateral PFC activity. Furthermore, the relationship between ventromedial prefrontal cortex (VMPFC) activity and amygdala activity differed between the groups consistent with prior work suggesting that the VMPFC may act as a mediator by which lateral areas of prefrontal cortex exert control over the amygdala (Urry et al. 2006). In particular, the greater the activity in VMPFC for controls, the less the amygdala activity. In contrast, the greater the activity in VMPFC activity in depressed individuals, the greater the amygdala activity. This finding suggests that attempts at reappraisal are counterproductive in the depressed group and may lead to greater rather than less engagement of neural areas involved in emotion, such as the amygdala.
Finally, researchers have attempted to disentangle the effect of controlling a negative mood state from the content of the representation associated with that mood state. In this study (Cooney et al. 2007), individuals were asked to remember a happy autobiographical memory and as expected, this condition yielded activation in OFC, temporal cortex and the parahippocampal gyrus. Afterwards, they were induced into a sad mood state by watching a film clip of a girl dying of cancer. Next, they were asked to recall the positive autobiographical memory, which lessened their sad mood. Notice that in this study the individual does not attempt to actively modulate or exert control over the mood induced by either the film clip or the autobiographical memory. The investigators examined which brain areas were more active for the second recall of the positive autobiographical memory (after sad mood induction) as compared to the first recall (prior to sad mood induction). This contrast yielded increased activity in VMPFC and subgenual regions of the ACC, but not in the dorsolateral and caudal regions of the ACC that have been implicated in cognitive control. As such, this study helps to implicate more dorsal regions of PFC as playing a role in effortful control over emotional thoughts rather than being engaged whenever emotion is being processed.
Summary
The research using reappraisal has implicated LPFC and sometimes regions of the dorsal ACC as being important for regulating emotional responses or reactions. Additional evidence suggests that this regulation acts on the activity of the amygdala, possibly via the VMPFC and/or sub-genual ACC.
3.3 Mood state influences on cognitive control
There has been relatively little research designed to examine how mood states affect cognitive control mechanisms involved in working memory. At least one study (Gray, Braver & Raichle, 2002) suggests that emotion exerts its influence by modulating activity in regions involved in cognitive control. In this study, individuals watched short videos to induce one of three emotional states (pleasant/approach, unpleasant/withdrawal, or neutral). Individuals were scanned while performing two versions of a 3-back working memory task: one using words and one using faces. N-back tasks, as discussed earlier, do not just tap the ability to maintain information in working memory, but also the ability to manipulate the contents of working memory (e.g., instructions involve maintaining the last two items that occurred and selecting the item from two trials ago to compare to the current trial). Emotion appeared to modulate DFLPC activity induced by the N-back task. Furthermore, activity in bilateral regions of BA 9 appeared to be driven equally by contributions from the task-specific nature of the information (e.g., words or faces) and the emotional mood that had been induced. In particular, activity in this region was greatest for the word task during unpleasant mood and the face task during pleasant mood. In contrast, activity in this region was least for the word task during a pleasant mood and the face task during an unpleasant mood. The authors suggest that these results argue that systems for emotion and cognitive control are interrelated. These results are consistent with the findings of Herrington et al. (2005), who found that DLPFC activity could be modulated by the valence of the information to-be-ignored. However the effects occurred bilaterally in the study of Gray and colleagues (2002) rather than varying by hemisphere depending on emotional valence, as was the case in Herrington et al. (2005).
In another study, individuals were induced into either a neutral emotional state via ambient air or a negative emotional state via the smell of rotten yeast while performing a 2-back verbal working memory task. Like the Gray et al. (2002) study, males showed activity in DLPFC regions that reflected an interaction between working memory processing and emotional state. In contrast, no such interaction was observed for females (Koch et al. 2007).
One can also examine the effect of mood on control mechanisms in working memory by comparing performance of individuals who are depressed versus those who are not. Non-medicated individuals with depression showed greater DLPFC activity in comparison of a 2-back vs. 1-back condition relative to controls even though task performance was not significantly different. Moreover, they showed greater dorsal ACC activity even though the group difference did not reach statistical significance (Matsuo et al. 2007). This finding is consistent with other work indicating that individuals who have only recently started anti-depressant medication (within the past 2 weeks) show greater PFC activity in left inferior (BA6/44), left middle (BA46) and ACC (BA 32) regions than controls, even though performance between the groups does not differ (Harvey et al. 2005). Such data are also consistent with a larger body of work suggesting that executive functions are compromised in depressed individuals, and that people who are depressed have to exert more cognitive control and effort to maintain levels of performance comparable to those of non-depressed controls (see Levin et al. 2007, for a review). The increased engagement of regions of PFC involved in cognitive control mechanisms in depressed individuals seems to reflect such compromise.
Summary
The relatively few studies in existence suggest that negative mood can modulate activity in cognitive control regions associated with memory maintenance and updating.
3.4. Individual Differences
Another way to examine the neural underpinnings of the relationship between cognitive control mechanisms involved in working memory and emotion is to investigate individual differences in the relationship between these functions. To date, there is very little research on this topic (although, we will return to this topic a bit more in the section on long-term memory, especially as it relates to PTSD). One characteristic that appears, on the face of it, to lie at the confluence of these processes is rumination, which is the tendency to actively maintain a specific set of thoughts, usually negative in valence.
Depressive rumination is defined as repetitively and passively focusing attention on one’s experience of negative mood states and the possible causes, meanings and implications of that mood (Nolen-Hoeksema, 1991). The prevailing viewpoint, mainly taken from a clinical perspective, is that individuals who tend to ruminate as measured by self-report questionnaires like the Ruminative Response Styles (RRS) questionnaire (Nolen-Hoeksema & Morrow, 1991) are attentionally inflexible. For example, ruminators have difficulty abandoning rules being maintained in working memory in the Wisconsin Card Sorting Task even though they are given feedback indicating that the rule is no longer correct (Davis & Nolen-Hoeksema, 2000).
Research in our laboratory and others has suggested that ruminators’ attentional inflexibility may be linked specifically to an impairment in cognitive control mechanisms that have been proposed to keep previously relevant, but now irrelevant, information out of working memory (Joormann, 2006; Joormann & Gotlib, 2008; Whitmer & Banich, submitted). For example, one study used a backward inhibition paradigm (Mayr & Keele, 2000). In paradigm, one switches between different tasks across trials. Responses are slower if one has to return immediately back to a previous task (e.g, pick the oddly shaped item, pick the largest item, pick the oddly shaped item) as compared to a new task e.g. pick the oddly shaped item, pick the largest item, pick the moving item). This finding has been interpreted as demonstrating that access to representations in working memory of very recent task sets (e.g. pick the oddly shaped item) is more difficult than access to less recent task sets. This phenomenon is often interpreted as automatic inhibition of previous but no longer relevant task sets (Mayr & Keele, 2000). We have found that individuals with a high tendency to ruminate do not have trouble switching their attentional focus from one task to another, but they do have trouble inhibiting mental representations of previous task demands when they switch their attention to new task demands (Whitmer & Banich, 2007). Hence, ruminators’ attention may remain fixated on certain thoughts because inhibitory mechanisms do not effectively remove information that is no longer needed from working memory. Such findings are consistent with work linking working memory processes with cognitive control. For example, the ability to manipulate the contexts of working memory predicts the ability to maintain on-task thoughts (Kane et al., 2007) and the ability to suppress unwanted thoughts (Brewin & Smart, 2005).
When confronted with emotionally negative information, the negative bias associated with depressive rumination coupled with a faulty inhibitory mechanism may make it difficult to be distracted away from such thoughts (Whitmer, submitted). Consistent with this idea, functional imaging studies have found that when ruminators are presented with emotionally negative material, the amygdala shows sustained activity as compared to non-ruminators (Ray, et al. 2005).
Interestingly, a review of the literature on people who ruminate but are not clinically depressed suggests that this deficit is not specific to the valence of information being controlled; instead rumination appears to be associated with a tendency to fixate attention on any information perceived to be important whether it is emotionally neutral, negative or positive (e.g. Whitmer & Banich, 2007; Joormann & Gotlib, 2008; Davis & Nolen-Hoeksema, 2000; (see Whitmer, submitted for a longer discussion of this issue). This inflexibility in attention will primarily become maladaptive when an individual’s attentional focus is also biased to selectively process negative information over neutral or positive information, as may occur in depressed individuals or in those individuals with negative cognitive tendencies like pessimism, low self-esteem or cognitive distortions (Ciesla & Roberts, 2007; Whitmer, submitted).
At present, very little research has been done to discern the neural mechanisms underlying an increased tendency to engage in rumination. Some research has attempted to determine if an overactive amygdala may be linked to excessive rumination. In support of such a hypothesis, two studies have found that in clinically depressed individuals, increased tendencies to ruminate were moderately associated with sustained amygdala activity in response to evaluation of emotionally negative words as compared to emotionally positive words (Siegle et al., 2002; Siegle, Carter & Thase, 2006). However, these findings are insufficient to conclude that depressive rumination is caused by an overreactive amygdala for two reasons. First, the correlation between rumination and sustained amygdala activity may actually be due to underlying variables. For example, these studies did not control for the influence of depressed mood and therefore sustained amygdala activity may be due to rumination’s association with depressed mood. Sustained amygdala activity may also simply reflect heightened negativity in clinically depressed people who ruminate and may not be due to some more inherent mechanism involved in prolonging rumination. Future research clearly needs to control for these other explanatory factors. Second, an overactive amygdala may not be causing overmaintenance of negative information (i.e., rumination) but instead may be merely reflecting the overmaintenance of negative information. For example, faulty mechanisms in working memory (e.g., inhibitory deficits) may cause the negative information to be overly maintained and sustained amygdala activity may just be the result of such deficits in control of working memory.
Another fMRI study examined a tendency to ruminate in individuals who were not clinically depressed while they looked at emotionally negative pictures and instructed to either regulate their emotional reaction or to just look at the picture (Ray et al., 2005). When participants just looked at the pictures, an increased tendency to ruminate was associated with increased amygdala activity, in line with the studies discussed above. However, if participants were told to diminish their emotional response then rumination was actually associated with decreased amygdala activity. This study may have two implications. First, it suggests that ruminators are quite capable of exerting cognitive control over their emotions but that their problem may lie in their failure to use such cognitive control to override their emotional thoughts when not explicitly told to do so. In line with such an idea, other studies have shown a similar effect for non-emotional information. For example, ruminators can switch their attention between non-emotional stimuli if explicitly told to do so (e.g., in a task switching paradigm Whitmer & Banich, 2007) but not if they have to learn on their own from negative feedback that a switch is needed (e.g. WCST; Davis & Nolen-Hoeksema, 2000). Second, these findings may argue against a hypothesis that proposes that an overactive amygdala is causing ruminators to overmaintain negative information. If that were the case, then ruminators should have had difficulty in diminishing amygdala activity when downregulating negative emotions. Therefore, these results instead suggest that other deficits (e.g., in cognitive control mechanisms for working memory) are causing overmaintenance of negative information.
Unfortunately, there is a dearth of research done on the role of other brain regions, such as the PFC, in driving an increased tendency toward rumination. Therefore, there is a clear need for future research to pinpoint the neural underpinnings of ruminative tendencies and their relationship to neural structures involved in cognitive control.
Summary
In sum, depressive rumination is associated with an impaired ability to keep previously relevant, but now irrelevant information, out of working memory. A consequence of this inability may be an inflexible cycle of thoughts about such information. If personality traits or negative mood bias attention towards negative information in particular, such inflexible thinking may increase negativity and thereby increase negative mood. The neural underpinnings of such control mechanisms as they relate to negative emotional thought remain to be determined.
4. Long Term Memory
The question of how emotional memories are controlled and regulated is one that has fascinated scientists and clinicians since the time of Freud, who suggested that memories may be repressed to keep anxiety at bay. In more recent times, researchers have been examining the neural mechanisms involved in the suppression of emotional thoughts, as well as the neural underpinnings of control mechanisms in individuals who may experience seemingly little control over retrieval of memories, such as seen in those who have been traumatized or have PTSD, and experience unwanted flashbacks of disturbing memories. In this section of the paper we examine 1) the neural substrates of emotional memory, 2) the cognitive and neural mechanisms that allow for control over emotional memories, emphasizing the suppression of negative memories and 3) cognitive control mechanisms in PTSD.
4.1. The Neural Substrates of Emotional Memory
Memories for emotional events are more persistent and vivid than other memories (Christianson, 1992; Phelps, 2004, 2006). A key conceptual issue that warrants attention is the fact that recent studies examining emotional memory have focused on the highly arousing nature of emotional stimuli or experimental contexts as the key component contributing to enhanced memory (Cahill, 2000; Canli et al., 1999; Dolcos, LaBar, & Cabeza, 2005). Several studies have demonstrated that the highly arousing nature of emotional stimuli and not their unpleasant valence, as self-reported by subjects, promotes enhanced emotional memory (Cahill & McGaugh, 1990; Hamann, 2001; Dolcos, LaBar, & Cabeza, 2004).
Previous research has shown that the amygdala and hippocampus are necessary for the enhanced memory observed for emotional material and contexts (Cahill et al., 1995; LaBar & Phelps, 1998; Phelps & LeDoux, 2005). Recent neuroimaging studies have further illustrated that amygdala and hippocampus activation during encoding of emotional stimuli is related to better recollection of those stimuli (Cahill et al., 2001 Hamann et al., 1999; Canli et al., 2000; Dolcos, LaBar, & Cabeza, 2004, 2005).
Insights into how these structures may be involved in emotional memory have been derived from studies that examine the anticipation of aversive or negative stimuli. Increased activity in right DLPFC and dorsal ACC has been observed during anticipation of viewing aversive items, but not when they are actually being viewed. This finding suggests that these regions are involved in modulatory control processes. Consistent with this interpretation, the activation of right DLFPC was associated with self-reports of increased negative affect (Nitschke et al. 2006).
In contrast, the amygdala and hippocampus exhibit activity both during anticipation and actual viewing of aversive stimuli, and may be the sites at which DLPFC regions exert their control. Activity in these two regions has been related to subsequent memory for aversive stimuli. In particular, dorsal amygdala and anterior hippocampus activations in anticipation of aversive emotional stimuli are positively associated with immediate recognition of the stimuli, whereas ventral amygdala activation in response to aversive emotional stimuli is positively associated with delayed recognition of the stimuli. Increased attention to potential threat/aversion likely drives the dorsal amygdala activation and the hippocampus is brought on-line because it has been proven adaptive in the past to remember emotional, in this case aversive, stimuli. Ventral amygdala activation is likely present because of its role in modulating consolidation of emotional memory via the hippocampus (Mackiewicz et al., 2006).
Summary
Based on the body of neuroimaging studies on emotional memory, it is evident that the amygdala and hippocampus work in tandem. It has been argued that the amygdala can modulate the encoding and storage of hippocampal-dependent memories. In contrast the hippocampus, by forming episodic representations of emotional information, can in turn influence the amygdala when emotional events are encountered (Phelps, 2004).
4.2. Control over Emotional Memories – Non-clinical samples
4.2.1. Behavioral evidence from the Think/No-Think Paradigm
The Think/No-Think paradigm (TNT) has been used to provide unique insight into the cognitive and neural mechanisms involved in the suppression and amplification of emotional memories (Depue et al., 2006, 2007). The TNT paradigm, derived from the Go/No-go task, requires individuals to exert control over memory representations as opposed to motor responses. The task is divided into three phases. In the training phase, participants learn cue-target pairing until they reach a very high degree of accuracy. In the experimental phase, only the cue is shown. For some cues, the individual is asked to think about or re-remember the target (Think condition) while for other cues, the individual is told to try and not let the target enter consciousness (No-Think condition). During this phase of the experiment participants are also given repeated attempts, usually between 6 and 12, to invoke either re-remembering or suppression of the target, depending on condition. For a subset of items, no cue is shown during the experimental phase and they serve as a baseline for the final test phase. In the test phase the individual is shown the cue and asked to recall the target that was paired with it. Typically compared to baseline, recall of items in the Think condition is enhanced and recall in the No-Think condition is reduced. These effects are amplified with increasing chances to exert cognitive control (e.g. 12 vs. 6 repetitions).
Initial studies used non-emotional word-word pairings to examine this aspect of cognitive control (Anderson & Green, 2001; Anderson et al., 2004). More recently our laboratory has used the paradigm to examine control over emotional and pictorial stimuli (Depue et al., 2006). Specifically, we compared control for neutral and negative pictorial material information that was equated for arousal. We found that emotional and pictorial information were susceptible to similar cognitive control mechanisms as previously observed for non-emotional words. More importantly, however, we found that the effects of cognitive control were enhanced for emotional as compared to non-emotional material. Hence, recall of information was greater for emotional information in the Think condition while recall of information was poorer for emotional than non-emotional information in the No-Think condition. Moreover, this effect increased with attempts at control (i.e. 10 vs. 5 attempts).
4.2.2. Neural substrates of the suppression of emotional memories
Although behavioral work with the TNT task illustrates that cognitive mechanisms can successfully exert their effect on emotional memories, they are not able to highlight the specific neural mechanisms that allow for such control. A recent study in our laboratory provides some insight into this question. Our study was built on prior findings (Anderson et al., 2004) in which regions of DLPFC and ACC were found to show increased activation for No-Think trials than Think trials when non-emotional words were used for the cue-target pairings. In addition, this study revealed that greater activity in DLPFC correlated with reduced activity in the hippocampus during No-Think trials to reduce the likelihood of retrieval.
Using our negative emotional stimuli from our prior study in the same TNT paradigm, we focused on the neural mechanisms involved in control of such information (Depue et al., 2007) since this issue is highly relevant for psychiatric disorders such as PTSD and OCD. Our results suggest that the ability to suppress or not think about emotional memory invokes control mechanisms that are separable those involved in the elaboration of memory. These control mechanisms are composed of at least two distinct pathways that appear to exert their influence at different times during the multiple attempts at cognitive control. In the first half of attempts at control (attempts 1–6), increased activity in inferior prefrontal regions are associated with reduced activity in the thalamus and visual cortex, which may reflect a modulatory influence of prefrontal over sensory components of memory representation. In the second half of attempts at cognitive control (attempts 7–12), increased activity in lateral prefrontal regions is associated with decreased activity in the hippocampus and amygdala, which may reflect modulation of memory processes and emotional components of memory representation, respectively. Finally, the overall timing of these effects may be orchestrated by a modulatory influence from frontopolar cortex that first predicts inferior and, then afterwards, lateral prefrontal regions. This work directly supports the previous findings of Anderson et al. (2004), which demonstrated a relationship between activity in DLPFC and the hippocampus, which was interpreted as a potential mechanism for reducing encoding and/or retrieval processes during No-Think trials.
One possible interpretation of these sets of findings is that these prefrontal areas are involved in directly modulating activity in posterior, hippocampal and amygdalar regions, and that the negative association reflects inhibition of one region over activity in the other. However, as discussed earlier, it is impossible to demonstrate this definitively with MRI data as they do not allow for the demonstration of causal relationships, such as those that can be observed with TMS (Aron, 2007). A potential alternative interpretation is that variations in activity in medial frontal regions are explained by how engaged a subject is in a given task. According to this argument, it is difficult to determine an “absolute” baseline for medial temporal regions in fMRI studies as in some cases more activity can be observed during a fixation baseline than during performance, for example, of an odd/even task (Stark and Squire, 2001). The proposed explanation is that when people aren’t engaged in task demands, they are thinking to themselves (e.g., ruminating) which leads to activity in medial temporal regions. By this account, if individuals are more engaged in task demands on No-Think than Think and fixation trials, there will be less time for self-generated thoughts, less engagement of hippocampal cortex and memory mechanisms (i.e. more rumination), and therefore less activity in these regions.
Although a logical possibility, we do not find it a compelling argument Regardless of whether the decreased hippocampal activity on No-Think trials is called “inhibition”, “suppression” or “decreased engagement in the task”, the point remains that memory structures involved in retrieval are significantly less active on No-Think trials than Think trials or during whatever thoughts may be occurring during the fixation baseline. Were this decreased activity just the result of being more engaged in task demands for No-Think than Think trials, one would expect that the engagement in the No-Think task would remain steady or decrease across trials, as one gets more practice at exerting cognitive control. Hence, this hypothesis makes the opposite prediction of what we observed. Steady or decreasing engagement in task demands on No-Think trials would lead to steady or increasing activity in medial temporal regions across trials. Rather, what we observed was that activation in hippocampal regions decreased over No-Think trials, which is more suggestive of an active control process that works to suppress or inhibit retrieval processes. Moreover, the degree of activity in prefrontal regions in cognitive control regions across individuals predicted the degree of (de)activation in the hippocampus and amygdala. Hence, we believe a more parsimonious explanation is that these prefrontal regions exert modulatory influence over hippocampal regions that allow for reduced activation of memories that are to-be-forgotten or suppressed.
Other recent work from ERP research using the TNT paradigm lends further support for the idea that cognitive control mechanisms can be used to exert control over memory (Bergstrom et al., 2007). The results showed that ERP components were significantly different in Think as compared to No-Think trials. Early portions of the ERP (200–300 ms) showed increased positive amplitude over frontal leads but increased negativity over parietal-occipital leads for Think compared to No-Think trials. This pattern is thought to index strategic processes that are involved in voluntarily controlling recollection. In addition, a late (500–800 ms) parietal positivity was observed that was specific to Think items that were remembered. This late positivity has been suggested to index conscious recollection of memories. Hence this set of findings provides additional evidence that the strength of memories is indeed reduced on No-Think as compared to think trials.
4.2.3. Differences between the suppression and retrieval of emotional memories
Our research has also suggested that the cognitive control mechanisms involved in the suppression, inhibition or reduction of the strength of memories may be somewhat different than those involved in the retrieval and enhancement of memories. Behaviorally, we have found that during the retrieval of memory in the Think conditions as measured by effective recall of both emotional and non-emotional information increased enhanced linearly as a function of the number of attempts at cognitive control. In contrast, the cognitive control mechanisms involved in memory suppression do not appear to simply be the converse - a decrease in memory as a function of repetition (Depue et al., 2006). One of the most intriguing findings from our behavioral studies was the apparent paradoxical effect in which a) a small number of attempts to forget or not retrieve emotional information actually led to marginally significant better recall for that information, whereas b) only when the number of attempts to forgot or not retrieve emotional information was further increased, was there a significant drop in recall. Thus, the relationship between attempts at suppressive cognitive control and subsequent recall was non-monotonic.
There are some hints in our data that the non-monotonic nature of the relationship between suppression of repetitions and subsequent recall, appears to be related to the vividness or elaborateness of stimuli, and is not necessarily driven only by whether or not the content being controlled is emotional. This idea would be consistent with the suggestion by Anderson and colleagues that only intrusive memories are subject to suppression (Anderson, et al., 2004; Levy and Anderson, 2002), as well as findings discussed earlier that the arousal induced by emotional stimuli rather than their emotionality per se drives subsequent recall.
Evidence for this assertion comes from the following data. In our behavioral work (Depue et al., 2006), we found that the non-monotonic function for suppressive control of emotional vs. non-emotional information is greater for pictures than words; clearly the pictures are more elaborate, rich and vivid representations of information than word stimuli. In addition, in our neuroimaging study (Depue et al. 2007) we observed that the initial hippocampal activity (suppression attempts 1–3) was greater for No-Think items that were successfully suppressed than for those that were not. Hence we speculate that cognitive control may be easier to exert over items that have a more well-elaborated memory representation. This idea is supported by recent work in computational modeling (Norman, et al., 2004) suggesting that more elaborated representations may be easier to control.
Such an explanation provides a tentative means of interpreting the clinical phenomenon that painful and disturbing memories require “working through” or “revisitation” before they can be processed and suppressed (Freud, 1904). It may be that memories must be elaborated before they can be effectively controlled. This idea is also consistent with clinical descriptions of therapeutic approaches to PTSD, where enhancement of traumatic memories often precedes their suppression (Bower and Sivers, 1998). In contrast, ruminative aspects of OCD and depression may represent conditions where these control mechanisms are compromised (Van Der Kolk et al., 1996). Hence, individual differences in the effectiveness of control mechanisms of suppression may contribute to the susceptibility of disorders such as OCD and PTSD (de Silva and Marks, 2001) and merit further investigation.
Summary
Cognitive control mechanisms appear to be able to modulate memory for emotional memories more effectively than for non-emotional memories. Increased effectiveness in forgetting specific memories appears to be associated with two somewhat dissociable pathways. The first involves inferior frontal cortex, which is associated with reductions in activity in sensory processing regions, suggesting that it may aid in the suppression of sensory aspects of the memory . The second involves the middle frontal gyrus, which is associated with reduction in activation in the aymgdala and hippocampus, suggesting that it may play a role in suppressing multimodal and emotional aspects of the memory via control over the. Furthermore, cognitive control processes that lead to the diminution vs. retrieval and enhancement of emotional information are not converse processes. Rather suppression of negative emotional information appears to be more successful when that information is initially better elaborated.
4.3 Cognitive control and Post-traumatic Stress Disorder (PTSD)
Cognitive processing in individuals with PTSD has received much attention, due in large part to the nature of several PTSD symptoms, such as intrusive thoughts and hypervigilance. These symptoms as well as behavioral data suggest that two main processes affected in PTSD are memory (Amir et al., 1996; Vrana et al., 1995) and attention/cognitive control (MacLeod et al., 2002; Matthews & MacLeod, 2002). Illustrating their importance, cognitive processing variables and memory disorganization have also been found to predict PTSD severity both at 3 and 6 months post onset. This association holds even when controlling for depression, suggesting this may be distinct to PTSD (see review by Brewin & Holmes, 2003). For the most part, these two processes - cognitive control and memory - have been conceptualized as independent. However, it is likely there is a relationship between the two processes and we therefore explore the potential interrelationship here.
4.3.1. Control mechanisms in PTSD
4.3.1.1. Behavioral evidence
It is frequently observed that people with PTSD are hypervigilant (MacLeod et al., 2002; Matthews & MacLeod, 2002), which is the result of a strong attentional bias for threat. Experiments using the emotional Stroop task have demonstrated an attentional bias for threat in people with PTSD above and beyond that observed in the normal population. Specifically, individuals with PTSD are slower at naming the color of words that are relevant to their trauma rather than trauma words in general, regardless of the trauma experienced. Results are robust across different media and modalities (Constans, 2006) and across individuals with PTSD resulting from different types of trauma, including rape (Cassidy et al., 1992; Foa et al., 1991), sexual abuse (McNally et al., 2000), combat (Constans et al., 2004; McNally, English, & Lipke,1993), and accidents (Bryant & Harvey, 1995; Buckley, Blanchard, & Hickling, 2002). Deficits are positively correlated with symptom severity (McNally et al., 2000).
However, these effects may be moderated by the degree to which an individual dissociates. According to DePrince & Freyd (1999), dissociation results when thoughts, emotions, and experiences are not normally integrated. Freyd has also characterized dissociative experiences similarly, stating that “dissociative experiences are characterized by a disruption in integration of consciousness, attention, and/or memory” (Freyd et al., 1998, pg. S91). High levels of dissociative experience have been related to a history of trauma (Freyd et al., 1998) and studies have suggested that dissociation is an important construct in PTSD (Bremner et al., 1992; Koopman et al., 1994).
Of interest, dissociation appears to influence aspects of attention and cognitive control. One study found that high levels of dissociation were associated with more interference on a Stroop task than low levels (Freyd et al., 1998). Following up on this finding, DePrince and Freyd (1999) found that although high dissociation was indeed associated with high levels of interference on a classic Stroop task, this interference was reduced when simultaneously attempting to recall the words. Low levels of dissociation were associated with the opposite pattern. This finding suggests that attentional context may moderate the effect of dissociation on alterations in cognitive control. Of note, high dissociators could better exert cognitive control when also engaged in another task, consistent with their ability to exert control by dissociating when placed in a traumatic context. The same study also found a relationship between dissociation and memory, such that high dissociators were better able to recall the neutral words than the trauma words, with low dissociators being able to recall the trauma words better than the neutral words (DePrince & Freyd, 1999). This finding suggests that these alterations in cognitive control mechanisms can influence the nature of memories that are retrieved.
4.3.1.2. Neuroimaging evidence
Neuroimaging techniques have been used to examine the neural bases of control mechanisms in PTSD. Before discussing this research, however, it is instructive to consider how individuals with PTSD respond in general to traumatic or threatening information. Neuroimaging indicates that individuals with PTSD have increased amygdala activation to fear-related stimuli, including fearful faces and trauma related words (Rauch et al., 2000; Shin et al., 2004, 2005; Nemeroff et al., 2006). It is thought that this increased amygdala activation is part of the larger neural system that leads to hypervigilance to threat seen in people with PTSD. The degree of amgydala hyperactivation in response to trauma cues in individuals with PTSD is linked to symptom severity (Pissota et al., 2002; Fredrikson & Fermark, 2003; Shin et al., 2004). Furthermore, Shin and colleagues (2005) have found that increased amygdala activation in people with PTSD is functionally associated with decreased VMPFC activity, which has been suggested by Johnstone and colleagues (2007) to mediate the relationship between lateral prefrontal area and control over the amygdala. Supporting this idea is a recent meta-analysis by Etkin & Wager (2007) which notes evidence across neuroimaging studies for decreased VMPFC activity as well as decreased anterior cingulate activation in individuals with PTSD.
A small body of work has more directly examined the neural bases of cognitive control in individuals with PTSD. These have generally used variants of the Stroop task. Shin and colleagues (2001) found that combat veterans with PTSD showed decreased ACC activation as compared to combat veterans with no PTSD when performing a counting emotional Stroop task. Interestingly, they did not show the same ACC deficit when performing a non-emotional counting Stroop task, suggesting a specific deficit only in response to emotional activation. Bremner and colleagues (2004) found that when performing an emotional Stroop task, women with PTSD demonstrated decreased blood flow in the ACC as compared to women with similar trauma histories but no PTSD. Conversely, when performing a classic Stroop task, the women with no PTSD demonstrated increased blood flow in visual association cortex and right inferior parietal cortex. Taken together, these studies suggest that people with PTSD fail to recruit the ACC to the degree it is needed when they must maintain and direct attention in the face of distracting information that is threatening.
4.3.2. The nature of memory in PTSD
People with PTSD demonstrate enhanced recall of trauma-related materials (Brewin & Holmes, 2003). Concordant with studies of emotional memory in general, people with PTSD are not just more likely to recall trauma-related materials, but the memories of these materials or actual events are often vivid and long-lasting (Brewin & Holmes, 2003). Experimental tasks have demonstrated that people with PTSD have greater explicit (e.g., Vrana et al., 1995) and implicit (e.g. Amir et al., 1996) memory for trauma related material as compared to non-trauma related material. Not only do people with PTSD demonstrate increased recall of trauma related material, but they also exhibit difficulty forgetting trauma material. In a directed forgetting study by McNally and colleagues (1998), women with a history of childhood sexual abuse with PTSD exhibited deficits in recalling positive and neutral words, but not trauma words.
Despite the fact that autobiographical memories of trauma in people with PTSD are lacking in detail and coherence (Brewin & Holmes, 2003), flashbacks are often dominated by sensory detail. Most flashbacks are disjointed and fragmentary, with the person vividly re-experiencing specific aspects of the trauma in great detail. Flashbacks, as compared to autobiographical memories, appear to be happening in the present. In one study (Reynolds & Brewin, 1998), flashbacks were reported as the most frequent intrusion by 43% of patients with PTSD as compared to 9% of patients with depression and 0% of non-patients. Based on results of studies like this, it has been hypothesized that flashbacks may be distinctive to PTSD (Brewin & Holmes, 2003).
Paradoxically, it has been found that increased recall of trauma related material is coupled with difficulty in retrieving autobiographical memories of the trauma (Buckley et al., 2000). Clinicians note observations of clients with PTSD reporting confusion, disorganization, and forgetfulness associated with the trauma memory, although they simultaneously report that such memories are vivid and persistent (Herman, 1992). Multiple studies have demonstrated an association between trauma history and overgeneral memory, such that more severe trauma history is predictive of more overgeneral memory of the trauma (Kuyken & Brewin, 1995; McNally et al., 1994, 1995). Furthermore, people with PTSD are more physiologically responsive to autobiographical trauma scripts than generic trauma scripts (McNally et al., 1998).
4.3.3. Control over traumatic memory in individuals with PTSD
In order to study the neural mechanisms involved in the recall of traumatic memories as well as re-experiencing (including flashbacks) of the trauma, script-driven paradigms have been frequently employed in fMRI and PET research (e.g. Shin et al., 2004; Osuch et al., 2001; Lanius et al., 2001, 2004). In a typical script-driven paradigm, each participant constructs an autobiographical narrative of a traumatic experience and some other neutral emotional experience, which acts as the control. During the fMRI or PET scan, the participant is read their script aloud and instructed to recall the specific memory in the script and to remember sensory details of the experience. It has been verified by participants that this paradigm does induce PTSD symptoms, including re-experiencing of the trauma (Rauch et al., 1996).
In general, script-driven studies have found decreased activation or blood flow in ACC, medial and inferior frontal cortices, and the thalamus while there is increased activation or blood flow in the amygdala and other limbic and paralimbic structures in people with PTSD (for review, see Lanius et al., 2006). A functional connectivity study found that in participants with PTSD who reported a reliving or flashback response to the trauma script, show increased interrelationships between activity in the right ACC and a set of regions including occipital cortex, right parietal cortex, and posterior cingulate cortex. In contrast, control participants activity in right ACC was associated with a different set of regions: left PFC and the left anterior cingulate cortex (Lanius et al., 2004). Lanius and colleagues (2004) suggest these patterns reflect the phenomenon that PTSD participants experience the memories primarily visually, whereas control participants experience the memories more linguistically, in the form of a narrative. This interpretation is in line with the paradoxical behavioral findings of increased recall of trauma-related material being associated with decreased autobiographical recall of the trauma. The predominant activity in the right hemisphere for individuals with PTSD is also consistent with evidence suggesting that various right hemisphere regions are critically involved in response to threat (for review, see Nitschke & Heller, 2002; Nitschke, Heller, & Miller, 2000).
Additionally, these data suggest that some mechanisms of cognitive control have gone awry. Decreased ACC activation as well as decreased medial and inferior frontal activation suggests a shift from a task-oriented focus in which attention is directed toward performance and monitoring behavior in light of a goal to a threat-oriented state, in which attention is directed broadly toward the assessment of danger. This shift is associated with a deficit or at least a marked decrease in cognitive control functions reflected, for example, in an inability to inhibit a response to emotional distractors. In addition, the decreased thalamus activity and increased limbic activity suggests emotion-driven sensory overload. Decreased activation of medial and inferior frontal regions would further contribute to reduced regulation of this responding that is emotionally-driven and sensory in nature.
However, individuals with PTSD may dissociate from, rather than re-experience, traumatic memories. Although the majority of PTSD participants report a reliving or flashback experience to the trauma script, approximately 30% of participants dissociate when they are listening to the trauma script (Lanius et al., 2006). Therefore, Lanius and colleagues (2002) examined brain activation in a subset of participants in a script-driven fMRI study who reported dissociating while listening to the script. These participants demonstrated increased activation in the anterior cingulate cortex, inferior and medial frontal cortices, and temporal, parietal, and occipital cortices. Lanius and colleagues (2002) report that their pattern of findings is consistent with other imaging studies of dissociation. These findings are quite opposite of those discussed previously in people experiencing reliving/flashbacks during the trauma script. In fact, this pattern of increased activation in these cognitive control regions suggests people who dissociate are better able to maintain attention in the face of distraction and suppress responding that is emotionally-driven and sensory in nature. Dissociation provides some cognitive advantage or survival value, in that it allows people to exhibit cognitive persistence in the face of high emotional distraction.
Summary
Individuals with PTSD show increased responsiveness to threat stimuli, which appears to be strongly associated with amygdala activation. Initial studies suggest that control over the amydala may occur via cingulate and other prefrontal mechanisms. Memory processing is also altered in PTSD, such as trauma-related memories are often vivid in nature, but non-trauma related information is overgeneral. Poor control over trauma-related memories appears to be associated with decreased cingulate as well as lateral and medial prefrontal activation. In contrast, individuals who exhibit dissociation show increased activation in these areas, suggestive of increased cognitive control.
IV. LINKAGE TO REPONSE INHIBITION
As this paper appears in a special issue related to inhibition, it is worthwhile to consider how the processes discussed in this paper relate to those discussed in other papers in this issue. In many of the instances described above, cognitive control has been conceptualized as a potential mechanism for selecting certain types of information or processes over others. For example, in Stroop and conflict paradigms, tasks require task-relevant information or processes to be selected over task-irrelevant information or processes. In task-switching paradigms, the current task set must be selected over a prior onel in memory tasks, a target must be distinguished from among distractors and in emotion regulation paradigms, a mood, emotional response, feeling or thought must be selected over others. In describing many of these paradigms, researchers use the word “inhibitory processing” to describe the effects. For example, papers often refer to “inhibiting a memory” or “inhibiting word reading”.
To understand what these phrases mean requires considering two major theoretical conceptualizations of inhibition. One viewpoint is that inhibition is an active process – it is a cognitive control mechanism whereby a process or access to a representation is interrupted or stopped (see the discussion of neural circuitry of the “kill switch” in the Stop-Signal paradigm; Chambers, Garvan & Bellgrove, this issue). Another viewpoint is that is really an epiphenomenon of competition between two or more alternatives (see Verbruggen and Logan, this issue, in which they use horse-race model between two alternatives to explain performance in the Stop-Signal paradigm). Which viewpoint characterizes the effects of cognitive control related to memory and emotion described in the current manuscript, and what, if any of the neural machinery that we have described is related to that involved in response inhibition?
Models of response inhibition have focused on the role of the basal ganglia and its connection with frontal regions, especially right inferior frontal cortex (for a more detailed description see Aron et al. 2007). Corroborative evidence for the importance of these areas comes from studies examining clinical populations, such as individuals with ADHD or individuals who are substance dependent, who may exhibit deficits in response inhibition, alterations in brain activation on response inhibition tasks compared to controls, and differential effects of drugs that alter neurotransmission (for a longer discussion see Chambers, Garavan & Bellgrove, this issue; Jentsch, Groman & James, this issue). For the most part, these brain regions are not those that we have highlighted, which in contrast, have included dorsolateral prefrontal cortex, ventromedial prefrontal cortex and the anterior cingulate cortex.
However, there may be more overlap between these sets of regions than meets the eye. In particular, recent computational models have suggested that basal ganglia mechanisms gate information in prefrontal cortex, sending a signal either to retain the current information in working memory or to replace it (O’Reilly & Frank, 2006). Because these effects are thought to depend on relative activation of the Go vs. No-go pathways within the basal ganglia, their effect will be obscured in the neuroimaging studies described above because fMRI does not the spatial resolution to discrimination between them. Moreover this model suggests that such a mechanism is shaped through reinforcement learning that including not only the basal ganglia but also the amgydala. Another common region is pre-SMA, which in our studies and many others, co-occurs with activity in dorsal ACC when cognitive control is required. As noted by others in this issue (Chambers, Garavan & Bellgrove) pre-SMA has been implicated in response inhibition as well. This raises the possibility that inhibitory circuits involving the basal ganglia and portions of the pre-SMA are invoked whether inhibitory processes act on motor responses or other types of representations, such as those in working memory. Although these dorsal regions of the cingulate are sometimes also engaged when emotional information must be ignored or controlled, a more anterior and ventral region of cingulate cortex is usually involved. Whether the functions of these portions of the cingulate are identical but just act on different representations (cognitive vs. emotion) or whether the nature of the process performed by these two cingulate regions is fundamentally different remains to be seen.
Right inferior frontal cortex (RIFC) is implicated both in response inhibition and in memory suppression. Exactly what distinguishes the role played by RIFC from that played by dorsal cingulate/preSMA remains to be determined. It may be that RIFC is involved in the override of motor-plans, but cingulate regions in aspects of the selection of stimulus-response mappings or the channel of information (e.g. word/color, auditory/visual) that will be used to guide responding (see Milham &Banich, 2005, for a discussion of this potential role of dorsal ACC). Another outstanding issue is the role that DLPFC may play with regards to inhibition. Generally it is thought of as a region whose activity is involved in top-down selection of task-relevant information (see for example Banich et al. 2000a, 2000b) under conditions of attentional demand. However, to the degree that “inhibiting” one response or memory for another requires a change in the attention set or context that is used to select responding, DLPFC may be involved. As can be seen, although there are potential points of contact between the processes involved in response inhibition and inhibition or control over memories (both short-term and long-term, emotional or non-emotional), but the exact nature of their potential overlap remains to be determined.
V. CONCLUSIONS
Research examining the interface of cognitive control, memory and emotion is still in its infancy, despite the large implications for mental health disorders such as Post-traumatic Stress Disorder. In Figure 1 we provide an overview of the main regions that appear to be involved and their interrelationships. One major issue of contention is whether there are general cognitive control mechanisms that are invoked over both emotional and non-emotional memory information, or whether these control mechanisms are somewhat separable. At present, some tentative generalizations may be possible. The lateral prefrontal regions and the anterior cingulate are recognized as playing a large role in cognitive control, and they appear to so do over emotional information in both working memory and long-term memory. However, there appears to be at least some specialization of these control mechanisms in the cingulate, with more anterior and pregenual regions playing a more predominant role when the information is emotional in nature.
Figure 1. The major regions involved in cognitive control over memory representations that are associated with emotional information or emotional processes.
Areas involved in cognitive control are depicted by rectangles, whereas the regions on which such control is exerted are depicted by ovals. Arrows from one structure to another indicate the direction of influence. Dotted lines represent potential feedback mechanisms and dashed lines indicate potential inhibitory influences.
The sites at which these brain regions exert their control may differ for emotional and non-emotional information. For emotional information, the sites at which control is implemented include ventral and orbitofrontal regions, the amygdala, regions processing sensory aspects of the memory, and the hippocampus. The exact circuitry by which this control is exerted however remains an issue of debate, with some individuals suggesting direct control of the amygdala and hippocampus and others suggesting mediation via intermediate relay stations.
Evidence exists at present that such processes and pathways are altered in individuals with tendencies related to psychopathology, such as depressive ruminations, or individuals who are experiencing PTSD. A challenge for future research will be to determine what aspects of cognitive control and neural systems are compromised similarly across these disorders, what are specific to each disorder, and how they may be remedied by therapeutic interventions.
ACKNOWLEDGEMENTS
Writing of this paper was supported by NIMH P50 079485 to the first, fifth and sixth authors. The first author would also like to thank her students, the second, third and fourth authors, and her colleagues, the fifth and sixth authors, for the interesting and stimulating collaborative research that they have performed with her related to the topic of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, McNally RJ, Wiegartz PS. Implicit memory bias for threat in posttraumatic stress disorder. Cognitive Therapy and Research. 1996;20:625–635. [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JDE. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Aron A. The neural basis of inhibition in cognitive control. The neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron A, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn W. Converging evidence for a fronto-basal-ganglia-network for inhibitory control of action and cognition. Journal of Neuroscience. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropscyhologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for a unified account. Current Directions in Psychological Science. (submitted). [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional “set”: Evidence from fMRI. Cognitive Brain Research. 2000a;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z-P, Wright A, Shenker J, Magin R, Barad V, Gullett D, Shah C, Brown C. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000b;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Lèvesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21 doi: 10.1523/JNEUROSCI.21-18-j0001.2001. RC165(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström ZM, Velmansa M, de Fockerta J, Richardson-Klavehna A. ERP evidence for successful voluntary avoidance of conscious recollection. Brain Research. 2007;1151:119–133. doi: 10.1016/j.brainres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bower GH, Sivers H. Cognitive impact of traumatic events. Development and Psychopathology. 1998;10:625–653. doi: 10.1017/s0954579498001795. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick S, Bret,t E, Fontanna A, Rosenheck R, Charney D. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. American Journal of Psychiatric Rehabilitation. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Advances in Biological Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Beaton A. Thought suppression, intelligence, and working memory capacity. Advances in Behavior Research and Therapy. 2002;40:923–930. doi: 10.1016/s0005-7967(01)00127-9. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Holmes EA. Psychological theories of posttraumatic stress disorder. Clinical Psychology Review. 2003;23:339–376. doi: 10.1016/s0272-7358(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Smart L. Working memory capacity and suppression of intrusive thoughts. Journal of Behavior Therapy and Experimental Psychiatry. 2005;36:61–68. doi: 10.1016/j.jbtep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing of threatening information in posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Hickling EJ. Automatic and strategic processing of threat stimuli: A comparison between PTSD, panic disorder, and nonanxiety controls. Cognitive Therapy and Research. 2002;26:97–115. [Google Scholar]
- Buckley TC, Blanchard EB, Neil WT. Information processing and PTSD: A review of the empirical literature. Clinical Psychology Review. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging – Validaiton study with functional MRI. Human Brain Mapping. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Modulation of long-term memory in humans by emotional arousal: Adrenergic activation and the amygdala. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. New York: Oxford University Press; 2000. pp. 425–446. [Google Scholar]
- Cahill L, McGaugh JL. Amygdaloid complex lesions differentially affect retention of tasks using appetitive and aversive reinforcement. Cognitive, Affective & Behavioral Neuroscience. 1990;104:532–543. doi: 10.1037//0735-7044.104.4.532. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MG. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of Learning and Memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. fMRI indentifies a network of structures correlated with retention of positive and negative emotional memory. Psychobiology. 1999;27:441–452. [Google Scholar]
- Cassidy KL, McNally RJ, Zeitlin SB. Cognitive processing of trauma cues in rape victims with post-traumatic stress disorder. Cognitive Therapy and Research. 1992;16:283–295. [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. Journal of Neurophysiology. 2007;98:3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Emotion and memory: Research and theory. Hillsdale, NJ: Lawrence Earlbaum Associates; 1992. [Google Scholar]
- Ciesla JA, Roberts JE. Rumination, negative cognition, and their interactive effects on depressed mood. Emotion. 2007;7:555–565. doi: 10.1037/1528-3542.7.3.555. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Banich MT, Mohanty A, Milham MP, Herrington J, Miller GA, Scalf PE, Webb A, Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cognitive, Affective, and & Behavioral Neuroscience. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Constans JI. Information-processing biases in PTSD. In: Vasterling JJ, Brewin CR, editors. Neuropsychology of PTSD. New York: The Guilford Press; 2006. pp. 3–26. [Google Scholar]
- Cooney RE, Joormann J, Atlas LY, Eugène F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–1774. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research. 2000;24:699–711. [Google Scholar]
- DePrince AJ, Freyd JJ. Dissociation, attention and memory. Psychological Science. 1999;10:449–452. [Google Scholar]
- Depue BE, Banich MT, Curran T. Suppression of emotional and nonemotional content in memory: effects of repetition on cognitive control. Psychological Science. 2006;17:441–447. doi: 10.1111/j.1467-9280.2006.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- de Silva P, Marks M. Traumatic experiences, post-traumatic stress disorder and obsessive-compulsive disorder. International Review of Psychiatry. 2001;13:172–180. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. NeuroImage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences U S A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, Diaz-Granados P, Wang L, McCarthy G. Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortx activityduring a delayed-response working memory task. Neuropsychologia. 2008;46:326–335. doi: 10.1016/j.neuropsychologia.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm179. (epub ahead of publication). [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Mohanty A, Herrington JD, Banich MT, Webb AG, Miller GA. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology. 2007;44:352–363. doi: 10.1111/j.1469-8986.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder and specific phobia. The American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulated in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. Processing of threat-related information in rape victims. Journal of Abnormal Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Furmark T. Amygdaloid regional cerebral blood flow and subjective fear during symptom provocation in anxiety disorders. Annals of the. New York Academy of. Sciences. 2003;985:341–347. doi: 10.1111/j.1749-6632.2003.tb07092.x. [DOI] [PubMed] [Google Scholar]
- Freud S. Freud’s psycho-analytic procedure. Standard Edition. 1904;7:249–254. [Google Scholar]
- Freyd JJ, Martorello SR, Alvarado JS, Hayes AE, Christman JC. Cognitive environments and dissociative tendencies: Performance on the standard Stroop task for high versus low dissociators. Applied Cognitive Psychology. 1998;12:S91–S103. [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London B Biologial Sciences. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences U S A. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. NeuroImage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton SD, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Harvey P-O, Fossati P, Pochon J-B, Levy R, Lebastard G, Lehéricy S, Allilaire J-F, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Herman JJ. Trauma and recovery. London: Pandora Books; 1992. [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, Fisher JE, Stewart JL, Banich MT, Webb AG, Miller GA, Heller W. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–207. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Koven NS, Miller GA, Heller W. Mapping the neural correlates of dimensions of personality, emotion, and motivation. In: Canli T, editor. Biology of personality and individual differences. New York: Guilford; 2006. pp. 133–156. [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels A, Banich MT, Webb AG, Miller GA. Localization of asymmetric brain function in emotion and depression. doi: 10.1111/j.1469-8986.2009.00958.x. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cognitive, affective, and behavioral neuroscience. 2005;5:339–361. doi: 10.3758/cabn.5.3.339. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37:269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson-Schill SL. Selection from perceptual and conceptual representations. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:466–482. doi: 10.3758/cabn.4.4.466. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18:614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider L. Mechanisms of visual attention in the human cortex. Annual Reviews of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Stöcker T, Shah NJ, Amunts K, Kircher T, Schneider F, Habel U. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Koopman C, Classen C, Spiegel D. Predictors of posttraumatic stress symptoms among survivors of Oakland/Berkeley, Calif., firestorm. The American Journal of Psychiatry. 1994;151:888–894. doi: 10.1176/ajp.151.6.888. [DOI] [PubMed] [Google Scholar]
- Koven N, Heller W, Banich MT, Miller GA. Relationships of distinct affective dimensions to performance on an emotional Stroop task. Cognitive Therapy and Research. 2003;27:671–680. [Google Scholar]
- Kuyken W, Brewin CR. Autobiographical memory functioning in depression and reports of early abuse. Journal of Abnormal Psychology. 1995;104:585–591. doi: 10.1037//0021-843x.104.4.585. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. American Journal of Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. Journal of sychiatric Research. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Eugxne F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux J-M, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. Emotion processing effects on interference resolution in working memory. Emotion. 2008;8:267–280. doi: 10.1037/1528-3542.8.2.267. [DOI] [PubMed] [Google Scholar]
- Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognitive Therapy and Research. 2007;31:211–233. [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17:742–748. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the effect of sex differences for amygdala and hippocampus function in emotional memory. Proceedings of the National Academy of Sciences. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the cause of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Matthews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition Emotion. 2002;16:331–354. [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, Sanches M, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao J-H, Soares JC. Prefrontal hyperactivaiton during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry. 2007;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- Mayr U, Keele SW. Changing internal constraints on action: the role of backward inhibition. Journal of Experimental Psychology: General. 2000;129:4–26. doi: 10.1037//0096-3445.129.1.4. [DOI] [PubMed] [Google Scholar]
- McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: Use of the modified Stroop paradigm. Journal of Traumatic Stress. 1993;6:33–41. [Google Scholar]
- McNally RJ, Clancy SA, Schacter DL, Pitman RK. Cognitive processing of trauma cues in adults reporting repressed, recovered, or continuous memories of childhood sexual abuse. Journal of Abnormal Psychology. 2000;109:355–359. [PubMed] [Google Scholar]
- McNally RJ, Lasko NB, Macklin MB, Pitman RK. Autobiographical memory disturbance in combat-related posttraumatic stress disorder. Behavior Research and Therapy. 1995;33:619–630. doi: 10.1016/0005-7967(95)00007-k. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Litz BT, Prassas A, Shin LM, Weathers FW. Emotional priming of autobiographical memory in post-traumatic stress disorder. Cognition Emotion. 1994;8:351–367. [Google Scholar]
- McNally RJ, Metzger LJ, Lasko NB, Clancy SA, Pitman RK. Directed forgetting of trauma cues in adult survivors of childhood sexual abuse with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 1998;107:596–601. doi: 10.1037//0021-843x.107.4.596. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Reuter-Lorenz PA, Beyer JA, Fredrickson BL. Emotion and working memory: evidence for domain-specific processes for affective maintenance. Emotion. 2008;2:256–266. doi: 10.1037/1528-3542.8.2.256. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity & functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the Stroop task. Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus E, Cohen N. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the Stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller B, Verstynen T, Johnson M, D’Esposito M. Prefrontal and parietal contributions to refreshing: An rTMS study. Neuroimage. 2008;39:436–440. doi: 10.1016/j.neuroimage.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal function. Annual review of neurosicnece. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contribution to complex “Frontal Lobe” taks: a latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho M-HR, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate corte subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active versus latent representations: a neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40:255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: A state of the science review. Journal of Psychiatric Research. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W. The neuropsychology of anxiety disorders: Affect, cognition, and neural circuitry. In: D’haenen H, den Boer JA, Westenberg H, Willner P, editors. Textbook of Biological Psychiatry. 2002. [Google Scholar]
- Nitschke JB, Heller W, Miller GA. Anxiety, stress, and cortical brain function. In: Borod JC, editor. The Neuropsychology of Emotion. New York: Oxford University Press; 2000. [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Detre GJ, Polyn SM. Center for the Study of Brain, Mind, and Behavior. Princeton, NJ: Princeton University; 2004. How inhibitory oscillations can train neural networks and punish competitors. (Tech. Rep. No. 04-3) [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. The Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- O’Dougherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal .cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18:283–238. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biological Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in Brain Research. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Experimantal Brain Research. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Clinical Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pissota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrickson M. Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. European Archivesof Psychiatry and Clinical Neuroscience. 2002;252:68–75. doi: 10.1007/s004060200014. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychology. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A function MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Reynolds M, Brewin CR. Intrusive cognitions, coping strategies and emotional responses in depression, post-traumatic stress disorder and a non-clinical population. Behavior Research and Therapy. 1998;36:135–147. doi: 10.1016/s0005-7967(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Rougier NP, Noelle DC, Braver TS, Cohen JD, O’Reilly RC. Prefrontal cortex and flexible cognitive control rules without symbols. Proceedings of the National Academy of Sciences U S A. 2005;102:7338–7343. doi: 10.1073/pnas.0502455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Rossini P, Miniussi C. Lateralized contribution of prefrontal cortex in controlling task-irrelevant information during verbal and spatial working memory tasks: rTMS evidence. Neuropsychologia. 2008;46:2056–2063. doi: 10.1016/j.neuropsychologia.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Braver TS, Reynolds JR, Burgess GC, Yarkoni T, Gray JR. Individual differences in amygdala activity predict response speed during working memory. Journal of Neuroscience. 2006;26:10120–10128. doi: 10.1523/JNEUROSCI.2567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Marzol Peters P, Metzge L, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Levin RL, Sass SM, Heller W, Miller GA. Anger style, psychopathology, and regional brain activity. Emotion. doi: 10.1037/a0013447. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical transactions of the Royal Society of London Series B, Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Gresichar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. American Journal of Psychiatry. 1996;153:83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Roodman A, Beckham JC. Selective processing of trauma-relevant words in post-traumatic stress disorder. Journal of Anxiety Disorders. 1995;9:515–530. [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL. The emotional counting stroop apardigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ. The cognitive mechanisms of depressive rumination: A review. Psychological Bulletin. (submitted). [Google Scholar]
- Whitmer AJ, Banich MT. The generalizability of inhibitory deficits in individuals who rumination. Cognition & Emotion. (submitted). [Google Scholar]
- Whitmer AJ, Banich MT. Inhibition versus switching deficits in different forms of rumination. Psychological Science. 2007;18:546–553. doi: 10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proceedings of the National Academy of Sciences U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]