Abstract
A novel nanoparticle-based dual-modality positron emission tomograph/magnetic resonance imaging (PET/MRI) contrast agent was developed. The probe consisted of a superparamagnetic iron oxide (SPIO) core coated with PEGylated phospholipids. The chelator 1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic acid (DOTA) was conjugated to PEG termini to allow labeling with positron-emitting 64Cu. Radiolabeling with 64Cu at high yield and high purity was readily achieved. The 64Cu-SPIO probes produced strong MR and PET signals, and were stable in mouse serum for 24 hr at 37 °C. Biodistribution and in vivo PET/CT imaging studies of the probes showed a circulation half-life of 143 min, and high initial blood retention with moderate liver uptake, making them an attractive contrast agent for disease studies.
INTRODUCTION
Multimodality imaging combines two or more distinct imaging modalities in a way that leverages the respective strengths and weaknesses of each imaging technology. Examples include combined PET-CT (1), PET-MR (2, 3) and MR-fluorescence imaging (4, 5), which often generates results superior to both modalities operating separately. The combination of positron emission tomography (PET) with x-ray computed tomography (CT) has become the gold standard in oncologic imaging (1). Positron emission tomography is the most sensitive human molecular imaging modality and produces whole body images of functional and molecular information. CT rapidly acquires whole-body images at high resolution but exposes patients and experimental animals to substantial doses of ionizing radiation. Magnetic resonance imaging (MRI), in comparison, uses no ionizing radiation and provides soft tissue contrast superior to CT. Combining PET with the high-resolution, spectroscopic, or contrast enhanced abilities of MRI would produce a breakthrough in the detection and monitoring of disease (2).
Nanoparticles possess unique characteristics that make them well suited as probes for molecular imaging (6). Particles can be synthesized in a systematic fashion with tight control over nanoparticle diameter to produce particles with narrow size distributions. As particles become smaller, their surface area to volume ratio increases significantly. Engineering of nanoparticle surface chemistry allows the surface area to be decorated with therapeutic molecules, imaging agents, targeting ligands, or nucleic acids. Distinct ligands and reporters can be attached to a single particle to allow multiplexing and multi-functionality. A single nanoparticle can be conjugated with a large number of targeting ligands, increasing the affinity of the nanoparticle to its biological target through a phenomenon known as multivalency. Additionally, a nanoparticle can be conjugated with a large number of reporter molecules (e.g. fluorophores, radionuclides), increasing signal-to-noise in imaging applications.
The unique qualities of nanoparticles described above make them superior in many ways to traditional low molecular weight MRI contrast agents and PET probes (7). Compared to gadolinium-based MRI contrast agents, nanoparticle MRI contrast agents circulate in the blood for longer periods of time, offer greater sensitivity, and may produce fewer side-effects (8). Radiolabeled nanoparticles can circulate for longer periods of time than small molecule PET tracers and can carry greater numbers of radionuclides (9). Nanoparticles are therefore a promising material for the construction and evaluation of combined PET/MRI probes.
Herein we report the development of a novel dual-modality nanoparticle for PET/MR imaging. The probe in this work has a superparamagnetic iron oxide nanoparticle (SPIO) core with a micelle coating composed of functionalized PEGylated lipids. The nanoparticles were modified with DOTA to allow chelation of copper-64 for PET imaging. The nanoparticles were labeled at high specific activity and produced strong PET and MRI contrast. The pharmacokinetics of this novel probe was measured using microPET/CT and organ biodistribution.
EXPERIMENTAL PROCEDURES
Materials
Superparamagnetic iron oxide (SPIO) crystalline cores (generously provided by Dr. Charles O’Connor, University of New Orleans) were synthesized using a reverse micelle process (10, 11). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (DSPE-PEG2000-amine) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000] (DSPE-mPEG5000) were purchased from Avanti Polar Lipids Inc. (Alabaster, AL). 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid mono(N-hydroxysuccinimide ester) (DOTA-NHS) was purchased from Macrocyclics Inc. (Dallas, TX). 64Cu was produced on a Washington University School of Medicine Cyclotron (Model CS-15, Cyclotron Corp.) by the 64Ni(p,n)64Cu reaction with the nuclide having a specific activity of 220 ± 55 mCi/μg at the end of bombardment, as previously described (12). All buffers used for 64Cu labeling were passed through a Chelex 100 column before use. Water was deionized to 18 MΩ•cm using an E-Pure water filtration system (Barnstead International, Dubuque, IA). Wiretrol II capillary micropipets were purchased from the Drummond Scientific Company (Broomall, PA). Chelex 100 resin (50–100 mesh), hydrochloric acid, hydroxylamine hydrochloride, ferrous ammonium sulfate hexahydrate, 1,10-phenanthroline, ethylenediaminetetraacetic acid (EDTA), 10 mM phosphate buffered saline (PBS), chloroform, and mouse serum were purchased from Sigma-Aldrich Inc. (St. Louis, MO).
Preparation of micelle-coated SPIOs
Micelle-coated SPIOs (mSPIOs) were prepared as previously described (13) with slight modifications. DSPE-PEG2000-amine and DSPE-mPEG5000 were individually dispersed in chloroform at 25 mg/mL and stored at −20 °C prior to the coating procedure. To prepare amino-functionalized mSPIOs, DSPE-PEG2000-amine (9 μmol) and DSPE-mPEG5000 (34 μmol) were added to 24 mL chloroform in a round-bottomed flask. Uncoated SPIOs suspended in toluene (20 mg Fe) were then added to the flask and the mixture was placed in a rotary evaporator. Pressure was slowly reduced to 10 mTorr to remove all solvents, resulting in a dry film of nanoparticles and PEG-phospholipids. The physical adsorption of PEGylated lipids onto particles was due to a hydrophobic interaction between cetyltrimethylammonium bromide capping ligands on the nanoparticle surface and acyl chains of phospholipids. The film was heated at 60 °C for 30 minutes under vacuum. Next, the flask was removed from vacuum and the film hydrated using deionized water with gentle agitation followed by sonication, producing a clear, brown solution of mSPIOs. Empty PEG-phospholipid micelles were separated from mSPIOs by ultracentrifugation (Optima XL-100K; Beckman Coulter Inc., Fullerton, CA) for 10 hrs at 280,000 g (4 °C). The supernatant containing empty micelles was removed and the nanoparticle pellet was resuspended in PBS to 10 mg Fe/mL and passed through a 0.1 μm Anotop syringe filter (Whatman International Ltd., UK).
Conjugation of mSPIOs with DOTA-NHS-Ester
DOTA-NHS-ester was reacted with 1 mL of 10 mg Fe/mL amino-functionalized mSPIOs at 1 mM in PBS (pH 7.5) overnight at 4°C. The product, DOTA-mSPIO, was separated from excess DOTA-NHS-ester by dialysis using a 100 kD Float-A-Lyzer (Spectrum Laboratories, Inc., Rancho Dominguez, CA) against 2L of phosphate buffer for 48 hrs at 4°C with a total of 8 buffer changes. The number of DOTA groups per mSPIO, estimated at 5–10, is proportional to the percentage of initial DSPE-PEG2000-amine present during the micelle coating procedure (14, 15).
Iron Concentration Determination
Iron concentration of DOTA-mSPIO solutions were determined using a 1,10-phenanthroline assay after conjugation of DOTA to mSPIOs (16, 17). Solutions of hydroxylamine hydrochloride (10 mg/mL), sodium acetate (125 mg/mL), 1,10-phenanthroline (1 mg/mL) and hydrochloric acid (6 M) were freshly prepared using deionized water. In a 200 μL PCR tube, 17 μL of hydrochloric acid was added to 18 μL of dilute nanoparticles and incubated in a thermocycler at 100°C for 10 minutes. The resulting solution of dissolved mSPIOs was combined with 10 μL of hydroxylamine hydrochloride and 855 μL of sodium acetate, followed by addition of 100 μL 1,10-phenanthroline. After 10 minutes the absorbance of the sample at 510 nm was measured using a Safire microplate reader (Tecan Group Ltd., Männedorf, Switzerland) and compared to the absorbance of a standard curve created using a ferrous ammonium sulfate hexahydrate solution.
Relaxation Measurement
Relaxation measurements were carried out using a 0.47 T Minispec mq20 (Bruker Optics, Billerica, MA) according to previous studies (13). Briefly, transverse magnetization decay curves were acquired using a Carr-Purcell-Meiboom-Gill sequence, while longitudinal magnetization recovery curves were acquired using an inversion-recovery sequence. Curves were fit using Origin 6 analysis software (OriginLab Corp., Northampton, MA) to obtain T2 time constants. Relaxivity values (r2) were calculated using T2 time constants and the iron concentration of measured samples.
Radiolabeling of DOTA-mSPIO with 64Cu
In general, DOTA-mSPIOs were labeled with 64Cu and analyzed according to Rossin et al (18). DOTA-mSPIOs (5mg in 610 μL) were incubated with 64CuCl2 (3 mCi in 2.2 μL, 0.1 M HCl) in 0.1 M ammonium acetate buffer (pH 5.5) at 37 °C for 1 hr in a thermomixer. The solution was then challenged with 5 μL of 10 mM EDTA or DTPA at 37°C for 5 min with mixing to remove nonspecifically bound 64Cu. Labeling yield was determined by radiochemical thin layer chromatography (radio-TLC). A small amount of the of the 64Cu-labeled mSPIO (64Cu-mSPIO) solution was applied to an ITLC-SG plate (Pall Corporation, East Hills, NY) and developed using a 1:1 mixture (v/v) of 10% (w/v) ammonium acetate and methanol. The TLC plate was then measured using a Bioscan 200 imaging scanner (Bioscan Inc., Washington DC) and the labeling yield was found to be 94%. The 64Cu-labeled mSPIOs were then passed through a centrifugal desalting column (Pierce Biotechnology, Inc., Rockford, IL) to remove the small amount of unbound 64Cu contained in the sample.
Radiochemical purity (RCP, the percentage of the total radioactivity that was bound to DOTA-mSPIOs) of 64Cu-mSPIO was evaluated by radio-fast protein liquid chromatography (radio-FPLC) using an Amersham Biosciences ÄKTA FLPC system (GE Healthcare, Piscataway, NJ) equipped with a UV detector (280nm) and fitted with an in-line Model 170 radioisotope detector (Beckman Coulter Inc., Fullerton, CA). A 100 μL sample of dilute 64Cu-mSPIO was applied to a Superose 12 gel filtration column (GE Healthcare, Piscataway, NJ) and eluted with 20 mM HEPES and 150 mM NaCl at a flow rate of 0.8 mL/min. The RCP of 64Cu-labeled mSPIO was found to be > 95%, indicating that less than 5% of the total radioactivity present in the sample was free 64Cu. The nanoparticle solution was then diluted with PBS to obtain doses suitable for biodistribution and imaging studies. The serum stability of 64Cu-mSPIO was determined by incubating labeled nanoparticles in mouse serum at 37°C with constant shaking for up to 24 hr. The samples were subjected to radioTLC analysis after 0, 1, 3, 6, and 24 hr incubation to measure the amount of activity dissociated from 64Cu-mSPIOs.
Size Determination
Dynamic light scattering (DLS) was used to measure the hydrodynamic diameter of uncoated SPIOs and DOTA-mSPIOs using a NICOMP 380 ZLS Submicron Particle Sizer (Particle Sizing Systems, Inc., Santa Barbara, CA) equipped with a 632.8 nm He-Ne laser and an Avalanche Photodiode detector at a fixed 90° scattering angle. DLS measurements were performed on DOTA-mSPIO samples chelated with “cold” (i.e. stable) isotopes of copper (Cu-mSPIO ) according to the same procedure used for radiolabeling with copper-64. DLS of uncoated, hydrophobic SPIOs was performed in toluene. Dilute nanoparticle samples (0.4 mL) were placed in prewashed cylindrical optical cells and incident laser light was adjusted using a neutral density filter to obtain a scattering intensity of 300 KHz. Samples were scanned at 23 °C for 30 min in a temperature-controlled sample chamber and data are reported as the volume-weighted hydrodynamic diameter.
Uncoated SPIOs were stored in toluene prior to surface modification and were analyzed using transmission electron microscopy (TEM) to ensure monodispersity. TEM micrographs were produced by applying dilute SPIO samples to copper TEM grids allowing the organic solvent to evaporate. Grids were then imaged using a Hitachi H-7500 TEM. Micrographs were analyzed for particle size and distribution using the NIH ImageJ.
Biodistribution
All animal studies were performed in compliance with guidelines set by the Washington University Animal Studies Committee. Female BALB/c mice (Charles River Laboratories, Inc., Wilmington, MA) weighing approximately 20 g were anesthetized using 1–2 % isoflurane and given tail vein injections of 100 μCi of purified 64Cu-mSPIO at a dose of 10 mg Fe/kg body weight. Mice (n = 4 per time point ) were sacrificed by cervical dislocation at 10 min, 1 hr, 4 hr, and 24 hr post-injection and organs of interest were collected, blotted dry, weighed, and assessed for radioactivity by counting for one minute in a Gamma 8000 scintillation counter (Beckman Coulter Inc., Fullerton, CA). A standard dose was prepared and measured along with the samples to allow calculation of the percentage injected dose (%ID) for the various tissues and organs. Data were corrected for 64Cu decay and the percentage injected dose per gram of tissue (%ID/g) and the percentage injected dose per organ (%ID/organ) were calculated according to the following equations:
| Equation 1 |
| Equation 2 |
Small-Animal PET/CT Imaging Studies of 64Cu-mSPIOs
MicroPET imaging scans were performed using a microPET Focus 120 and (Siemens Medical Solutions USA, Inc., Malvern, PA). Female BALB/c mice (20–25 g) were anesthetized using 1–2 % isoflurane and given tail vein injections with 100 μCi of purified 64Cu-mSPIO (10 mg Fe/kg body weight) in 100 μL PBS. The anesthetized animals were monitored for physiological signs. Mice were reanesthetized and imaged in the supine position at 1 hr, 4 hr, and 24 hr post-injection. PET scan acquisition time for the 10 min, 1 hr, and 4 hr images was 10 min, while the PET scan acquisition time for the 24 hr image was 20 min. Mice were scanned on a microCAT II (Siemens Medical Solutions USA, Inc., Malvern, PA) immediately after microPET. Analysis of microPET images was accomplished using vendor software (ASIPro, Siemens Medical Solutions USA, Inc.) on decay-corrected images. Coregistration of microPET and microCT images was performed using fiducial markers in the animal transfer bed and the Amira software package (Mercury Computer Systems, Inc., Chelmsford, MA). The small animal studies were performed following an IACUC-approved protocol.
Phantom Study
To demonstrate the ability of mSPIOs to function as a dual imaging agent, we prepared a microcentrifuge tube of 64Cu-mSPIOs for PET and MR imaging. Radiolabeled nanoparticles were added to a tubes containing PBS to a final concentrations of 10 and 25 μg Fe/mL. The tubes were placed upright in the bed of a Focus 120 microPET and scanned for 10 minutes. After a sufficient amount of time to allow 64Cu to decay, the samples were then imaged in a 7.0-T Pharmascan magnet (Bruker BioSpin, Billerica, MA). A T2-weighted proton density scan was obtained using a multi-slice multi-echo sequence (MSME-PD-T2, Bruker Paravision 4) using the following parameters: 1000 ms repetition time (TR); 12 echo times (TE) ranging from 12–144 ms; 180° flip angle, 3cm FOV; 1mm slice thickness, 2 averages.
RESULTS
Nanoparticle Formation and Characterization
Dual PET/MRI nanoparticles (Figure 1A) were prepared by first encapsulating monocrystalline superparamagnetic iron oxide nanoparticles (SPIOs) in PEG-phospholipid micelles (13, 19, 20). This technique exploited the hydrophobic interaction between capping ligands on the nanoparticle surface and hydrocarbon chains of PEGylated phospholipids, leaving the PEG moieties exposed to confer water solubility and reduce protein absorption. High magnetic field gradients surround the surface of the iron oxide core, allowing sensitive detection by MRI. The DOTA-functionalized PEG-phospholipid coating permits labeling with 64Cu for sensitive detection by PET imaging. SPIOs used in this experiment were highly monodisperse (Figure 1B), having an average diameter of 6.2 nm with size variation of 9%, as determined by TEM. Functionalized micelle-coated SPIOs (mSPIOs) were prepared by coating nanoparticles with a 1:5 molar ratio of amino-PEG2000 phospholipids to methoxy-terminated mPEG5000 phospholipids. Empty micelles were separated from mSPIOs using ultracentrifugation and the yield, calculated from Fe concentration measurements, and was found to be 44%. The r2 relaxivity of mSPIOs, measured using a 0.47 T Bruker Minispec, was 209 ± 26 mM−1 · sec−1.
Figure 1.
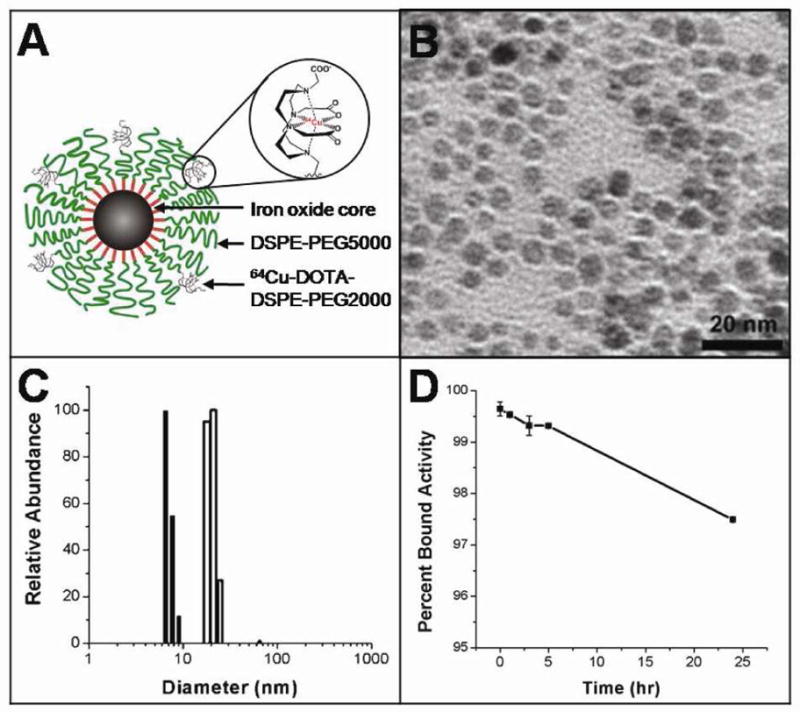
(A) Schematic representation dual-modality 64Cu-mSPIO. (B) Transmission electron micrograph of uniform monocrystalline superparamagnetic iron oxide nanoparticles. (C) Dynamic light scattering showing highly monodisperse populations of mSPIO before (closed bars) and after (open bars) encapsulation in PEGylated lipid micelle. (D) In vitro stability of 64Cu-mSPIO over the course of 24 hr incubation in 37 °C mouse serum.
Amine-modified mSPIO were conjugated with DOTA-NHS-ester to allow incorporation of PET imaging isotopes. Dialysis was used to separate DOTA-mSPIOs from excess DOTA-NHS-ester, and dynamic light scattering was employed to measure the hydrodynamic diameter of the DOTA-mSPIO (Figure 1C). We found that DOTA-mSPIO had a hydrodynamic diameter of 20.3 ± 1.9 nm as determined by dynamic light scattering, while prior to lipid-PEG encapsulation the SPIO cores had an average diameter of 7.1 ± 0.7 nm, consistent with the TEM measurement.
64Cu-Radiolabeling of DOTA-mSPIO
To construct the dual-modality PET/MR contrast agent, the positron-emitting radionuclide 64Cu (t1/2 =12.7 hr; β+ = 0.653 MeV, 17.4%; β− = 0.578 MeV, 39%) was used to radiolabel mSPIOs to allow small animal PET imaging and biodistribution studies. Copper-64 is commonly used in PET imaging and radiopharmaceuticals (21) because it can be produced at high specific activity in biomedical cyclotrons (22, 23), and chelation of 64Cu to various macrocyclic ligands can provide an efficient route for radiolabeling a wide variety of macromolecules and nanoparticles.
Labeling of DOTA-mSPIOs was achieved by incubation with 64Cu at 37 °C for 1 hr in 0.1 M ammonium acetate buffer, pH 5.5 (radiolabeling in acetate buffer was found to be superior to labeling in 0.1 mM ammonium citrate buffer, pH 5.5). It was found that DOTA-mSPIOs could be labeled to a specific activity of at least 2–4 GBq/mmol Fe (10–20 μCi/μg Fe) with a yield of 94%. Nonspecifically bound 64Cu was removed from 64Cu-mSPIOs by addition of excess EDTA following completion of the labeling reaction, and 64Cu-EDTA was separated from 64Cu-mSPIOs using a desalting column. The radiochemical purity of the labeled nanoparticles, defined as the percentage of the total radioactivity that was bound to DOTA-mSPIOs, was determined by radio-FPLC to be > 95%. Incubation of 64Cu-mSPIOs at 37 °C for 24 hr in mouse serum indicated high in vitro stability of 64Cu radiolabeling (Figure 1D).
Biodistribution Studies
The pharmacokinetics and biodistribution of 64Cu-mSPIO were studied to evaluate the nanoparticle’s potential as dual-PET/MRI in vivo as an imaging probe. Radiolabeled nanoparticles were administered via tail vein injection, and circulation half-life and accumulation in major organs were investigated; the results of organ biodistribution, presented as percent injected dose per gram tissue are shown in Figure 2 and Table 1. To allow dual PET/MR imaging with 64Cu-mSPIOs, the dose of particles administered needed to be within the detection limits of both modalities. Because PET is more sensitive than MRI (24), the amount of administered iron oxide was the limiting factor when formulating doses for injection. Mice (n = 4) weighing approximately 20 g were injected with a 10 mg Fe/kg dose of 64Cu-mSPIOs (100 μCi, radiochemical purity > 95%), an amount of iron oxide used for typical in vivo magnetic resonance imaging experiments. Each dose of particles contained 100 μCi of 64Cu (t1/2 = 12.7 hr) to allow imaging up to 24 hr post-injection. 64Cu-labeled mSPIOs exhibited high blood retention at 10 minutes and 1 hr post-injection of 47.5 ± 4.6 and 37.3 ± 12.9 %ID/g, respectively. Blood retention of 64Cu-labeled mSPIOs decreased to 7.9 ± 0.8 %ID/g at 4 hr post-injection and to 1.0 ± 0.05 %ID/g by 24 hr post-injection. Accumulation in the heavily vascularized tissues of the heart and lungs generally followed the pattern of blood retention. The circulation half-life of labeled nanoparticles was 143 ± 21 minutes, substantially longer than 64Cu-DOTA alone, which undergoes rapid renal excretion (circulation half-life ≈ 6 minutes) (25, 26).
Figure 2.
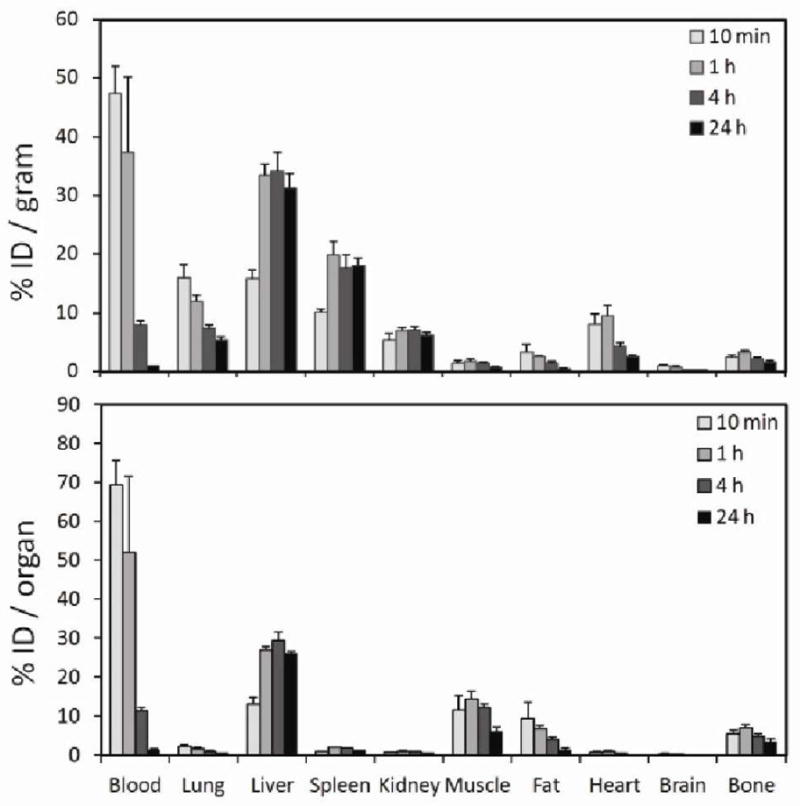
Biodistribution data of dual-PET/MRI 64Cu-mSPIO (10 mg Fe/kg body weight) in BALB/c mice. Groups of mice (n = 4) were sacrificed at 10 min, 1 hr, 4 hr, and 24 hr and select organs were measured for activity. Data are expressed as percent injected dose per gram tissue (%ID/g, top) and percent injected dose per organ (%ID/organ, bottom) ± one standard deviation.
Table 1.
Organ biodistribution as percent injected dose per gram tissue
| Organ | 10min | 1hr | 4hr | 24hr |
|---|---|---|---|---|
| blood | 47.47 +/− 4.64 | 37.31 +/− 12.87 | 7.89 +/− 0.76 | 1.01 +/− 0.05 |
| lung | 16.06 +/− 2.03 | 11.98 +/− 1.01 | 7.29 +/− 0.64 | 5.37 +/− 0.52 |
| liver | 15.85 +/− 1.41 | 33.42 +/− 1.85 | 34.20 +/− 3.08 | 31.33 +/− 2.33 |
| spleen | 10.07 +/− 0.56 | 19.96 +/− 2.27 | 17.52 +/− 2.32 | 18.05 +/− 1.27 |
| kidney | 5.26 +/− 1.16 | 6.87 +/− 0.63 | 7.03 +/− 0.65 | 6.20 +/− 0.38 |
| muscle | 1.37 +/− 0.49 | 1.78 +/− 0.30 | 1.45 +/− 0.14 | 0.72 +/− 0.13 |
| fat | 3.23 +/− 1.40 | 2.51 +/− 0.16 | 1.41 +/− 0.28 | 0.49 +/− 0.12 |
| heart | 8.00 +/− 1.72 | 9.46 +/− 1.76 | 4.29 +/− 0.66 | 2.52 +/− 0.35 |
| brain | 0.95 +/− 0.14 | 0.77 +/− 0.20 | 0.31 +/− 0.01 | 0.30 +/− 0.02 |
| bone | 2.47 +/− 0.39 | 3.28 +/− 0.29 | 2.17 +/− 0.28 | 1.62 +/− 0.27 |
Organs of the reticuloendothelial system (RES) experienced moderate nanoparticle uptake. The liver and spleen showed a low initial accumulation of 15.8 ± 1.4 and 10.1 ± 0.6 %ID/g, respectively. At 1 hr post-injection the liver and spleen uptake reached 33.4 ± 1.8 and 20.0 ± 2.3 %ID/g, respectively, and generally maintained those values for the remainder of the study. It has been shown previously that 64Cu dissociated from the 64Cu-DOTA complex will undergo transchelation by proteins such as superoxide dismutase, and metallothionein, and ceruloplasmin (27–29) in the liver. Therefore, while liver uptake at later time points was prominent, some contribution may have been due to an accumulation of disassociated 64Cu.
Kidney uptake remained relatively constant, averaging 5–7 %ID/g for all time points. Renal excretion of 64Cu-labeled mSPIOs was assessed by measuring urine activity using a metabolism cage. The activity of urine at 4 hr post-injection was 1.1 %ID/g at 4 hr post-injection and 5.0 %ID/g at 24 hr post-injection. This implies that although 64Cu-labeled mSPIOs were larger than the cut-off for glomerular filtration (30), their individual 64Cu-DOTA-PEGylated lipids were able to traverse the fenestrae of glomerular capillaries when disassociated from the nanoparticle surface. All other organs evaluated showed low (muscle, fat, bone) or negligible (brain) uptake. Animals were not perfused prior to counting, so some contribution of activity in the harvested organs may have been due to remaining blood.
Small Animal PET Imaging Studies
The PET imaging capabilities of dual-modality 64Cu-labled mSPIOs were analyzed by small animal microPET imaging performed using a microPET Focus 120. In this study, BALB/c mice were administered 100 μCi of 64Cu-mSPIO (10 mg Fe/kg body weight) in 100 μL PBS via tail vein injection and imaged at 1 hr, 4 hr, and 24 hr post-injection. Accurate comparison of microPET images with ex vivo biodistribution data required additional anatomical information that was provided by microCT scans. Mice were scanned on a microCAT II immediately after acquisition of microPET images, and coregistration was accomplished using fiducial landmarks on the animal bed. Examples of coregistered images obtained after administration of 64Cu-mSPIO are shown in Figure 3.
Figure 3.
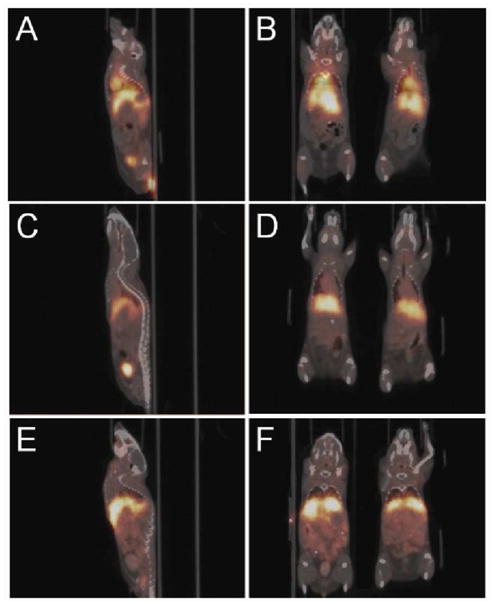
Coregistered microPET/microCT of BALB/c mice administered 100 μCi of 64Cu-mSPIOs (10 mg Fe/kg body weight, 100 μL injection volume). Whole body sagittal (A–E) and coronal (B–F) PET images are decay corrected and scaled by min/max frame: (A, B) 1 hr; (C, D) 4 hr; and (E, F) 24 hr post-injection.
MicroPET images showed a general agreement with the results of the nanoparticle biodistribution studies. High levels of activity were observed in the heart and carotid arteries at 1 hr post-injection (Figure 3A, 3B), indicating a large amount of nanoparticles in circulation. The strong presence of circulating nanoparticles was also observed in the descending abdominal aorta, aortic bifurcation, and common iliac arteries. Less activity was observed in the heart at 4 hr post-injection (Figure 3C, 3D). This absence of activity in the heart indicated a marked decrease in circulating nanoparticles relative to levels at 1 hr post-injection, corresponding well to the decrease in blood activity observed from the 1 hr to 4 hr ex vivo biodistribution. Uptake of 64Cu-mSPIOs by the liver was also clearly apparent, suggesting significant amounts of nanoparticles were sequestered by the RES at 4 hr post-injection.
By 24 hr post-injection microPET images showed little activity in the heart (Figure 3E, 3F), corresponding to negligible amounts of circulating nanoparticles. While the liver and spleen showed high uptake at 24 hr, widespread activity could also be observed in gastrointestinal (GI) tract. This activity indicated that 64Cu-mSPIOs (and their metabolic products) present in the liver underwent biliary excretion into the GI tract to be eliminated in the feces.
Phantom Studies
To verify the dual imaging capability of 64Cu-mSPIOs, nanoparticle samples underwent PET and MR scans using standard techniques. Figure 4 shows T2-weighted and microPET images of 10 and 25 μg Fe/mL samples of labeled magnetic nanoparticles. Iron oxide cores in 64Cu-mSPIO produced a characteristic darkening (signal hypointensity) at increasing iron concentrations, seen in Figure 4A. A strong PET signal was produced from 64Cu chelated to PEG-phospholipid micelle coating (Figure 4B).
Figure 4.
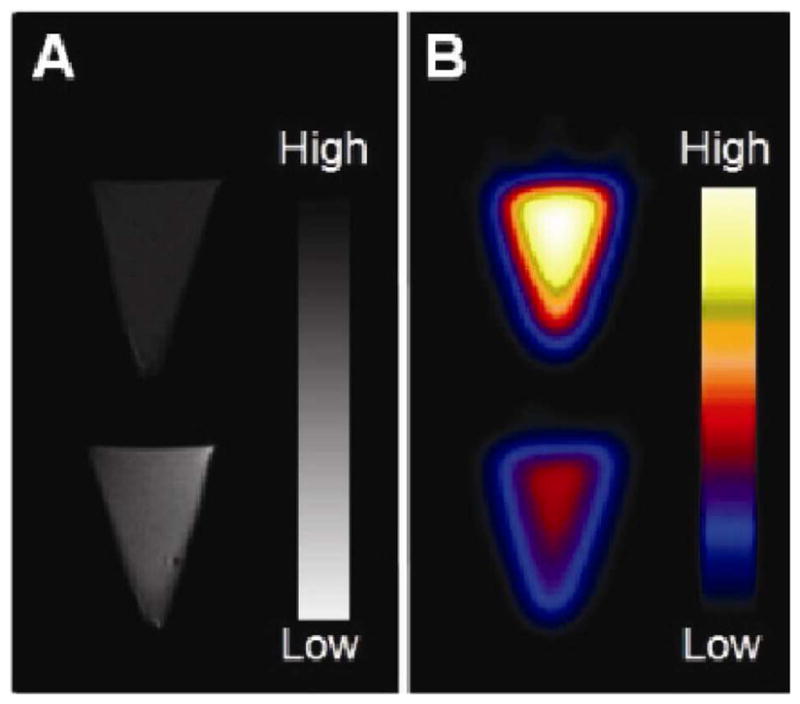
PET and MR imaging of 64Cu-mSPIO phantoms. (A) T2-weighted image of 25 μg Fe/mL (top) and 10 μg Fe/mL (bottom) of 64Cu-labeled magnetic nanoparticles (scale bar corresponds to high and low iron concentration). (B) Decay-corrected microPET image of 25 μg Fe/mL (top) and 10 μg Fe/mL (bottom) of 64Cu-labeled magnetic nanoparticles (scale bar corresponds to high and low copper-64 concentration).
DISCUSSION
The combination of positron emission tomography (PET) with magnetic resonance imaging (MRI) offers many opportunities (31–34). MRI produces high resolution soft issue images as well as functional and spectroscopic information. PET provides quantitative, whole-body imaging with high sensitivity. Interest in combining PET with MRI has surged (2, 35–37), and structured nanomaterials have emerged as a possible platform for the development of dual-PET/MR imaging agents. Although dual modality PET/MRI imaging does not necessarily require a combined PET/MRI contrast agent, the use of 64Cu-mSPIO in dual modality PET/MRI imaging offers certain potential advantages, including enhanced PET contrast, better circulation half-life, and better correlation between functional (PET) and morphological (MRI) information. While magnetic nanoparticles have been used extensively with MRI to study a wide range of biological phenomena such stem cell tracking (13, 38), cancer detection (39–41), and atherosclerotic monitoring (42–44), the synthesis and use of nanoparticles as PET molecular imaging agents have only recently been explored in detail (45–48). Nanoparticles are well suited to the design of PET probes, because their relatively large surface area (as compared with small-molecule PET agents) can be labeled to a high specific activity, allowing for increased sensitivity of detection and increased payload of targeting ligands and therapeutic isotopes.
Encapsulation of magnetic nanoparticles in micelles of PEGylated lipids has many advantages over traditional polymer- and carbohydrate-based approaches. Unlike polymeric encapsulation, the modular nature of this coating scheme allows the functionalization of nanoparticle surfaces with multiple lipid species and alternative chemical groups on PEG termini to be achieved in situ by simple adjustment of the stoichiometry of the initial lipid solution. Additionally, polymer coatings commonly applied to nanoparticles are relatively bulky and possess surface chemistry that result in nanoparticles that experience high RES uptake (26). Carbohydrate coatings traditionally used in precipitation-based nanoparticle synthesis limit the core size that can be encapsulated (49) and generally require post-synthesis surface functionalization. Several functionalized PEG-phospholipids are commercially available and inexpensive, and PEGylated lipid coatings have been applied to nanoparticles ranging from 3 nm (50) to 35 nm (51) using multiple chemical functionalities (20, 52).
64Cu-mSPIOs produced strong MR and PET signal (Figure 4) and were found to be stable in serum for 24 hr (Figure 1D). The potential of this nanoparticle as novel imaging agent was investigated by ex vivo biodistribution and in vivo PET imaging. Biodistribution results showed high initial blood retention with moderate liver uptake (Figure 2). 64Cu-mSPIO had a circulation life time of 143 minutes, a value that compares well with previous reports of quantum dots and silica nanoparticles with PEGylated lipid coatings (53, 54). This result suggests that the circulation profile is dominated by the coating and that lipid-PEG coatings can be generalized to other classes of nanoparticle cores with differing chemistries.
PET/CT imaging showed high levels of 64Cu in several regions. In general, microPET imaging correlated well with biodistribution findings. The heart and carotid arteries were clearly visible at early time points, indicating a high level of 64Cu-mSPIO blood retention (Figure 3A, 3B). While the liver and spleen showed high uptake at 24 hr post-injection, widespread activity could also be observed in gastrointestinal (GI) tract (Figure 3E, 3F). This activity was an indication that 64Cu-mSPIOs (and their metabolic products) sequestered in the liver at earlier time points underwent biliary excretion into the GI tract to be eliminated in the feces. Since iron oxide nanoparticles have been used in humans with few observed side effects (55, 56), and 64Cu has been used in patients (57–60) and showed no toxicity, 64Cu-mSPIO probes have a high potential for clinical applications.
In summary, we report the development and in vivo evaluation of a novel dual-PET/MR nanoparticle composed of a monocrystalline superparamagnetic iron oxide (SPIO) core and a physically adsorbed layer of PEGylated lipids. This is in contrast to the few published reports of dual-modality PET/MRI nanoparticles have used either dextran-based (61, 62) or protein-based (7, 63) coatings. We have characterized the physical and magnetic properties of 64Cu-mSPIO probes, and evaluated their potential as a dual-modality imaging agent using quantitative ex vivo biodistribution and in vivo microPET/CT imaging. We are currently investigating the properties of these and other dual-PET/MRI nanomaterials and their application to disease detection and treatment in atherosclerosis and cancer models.
Acknowledgments
This work was supported by the National Heart Lung and Blood Institute of the NIH as Programs of Excellence in Nanotechnology (HL80711 to GB and HL080729 to MJW), and as a Center of Cancer Nanotechnology Excellence (CA119338 to GB). The production of 64Cu was supported by the National Cancer Institute (CA86307 to MJW).
References
- 1.Czernin J, Allen-Auerbach M, Schelbert H. Improvements in Cancer Staging with PET/CT: Literature-Based Evidence as of September 2006. Journal of Nuclear Medicine. 2007;48:78S. [PubMed] [Google Scholar]
- 2.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14:459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, Welling A, Hofmann F, Oostendorp RA, Gansbacher B, Nekolla SG, Bengel FM, Botnar RM, Schwaiger M. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med. 2009;50:1088–1094. doi: 10.2967/jnumed.108.060665. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Regino CA, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Preparation and preliminary evaluation of a biotin-targeted, lectin-targeted dendrimer-based probe for dual-modality magnetic resonance and fluorescence imaging. Bioconjug Chem. 2007;18:1474–1482. doi: 10.1021/bc0701085. [DOI] [PubMed] [Google Scholar]
- 5.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–871. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 6.Lee SK, Chen X. Dual-Modality Probes for In Vivo Molecular Imaging. Mol Imaging. 2009;8:87–100. [PubMed] [Google Scholar]
- 7.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 8.Xie J, Huang J, Li X, Sun S, Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem. 2009;16:1278–1294. doi: 10.2174/092986709787846604. [DOI] [PubMed] [Google Scholar]
- 9.Shokeen M, Fettig NM, Rossin R. Synthesis, in vitro and in vivo evaluation of radiolabeled nanoparticles. Q J Nucl Med Mol Imaging. 2008;52:267–277. [PubMed] [Google Scholar]
- 10.Feltin N, Pileni MP. New technique for synthesizing iron ferrite magnetic nanosized particles. Langmuir. 1997;13:3927–3933. [Google Scholar]
- 11.Seip CT, O’Connor CJ. The fabrication and organization of self-assembled metallic nanoparticles formed in reverse micelles. Nanostructured Materials. 1999;12:183–186. [Google Scholar]
- 12.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, Anderson CJ, Welch MJ. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 13.Laconte LE, Nitin N, Zurkiya O, Caruntu D, O’Connor CJ, Hu X, Bao G. Coating thickness of magnetic iron oxide nanoparticles affects R(2) relaxivity. J Magn Reson Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 14.Ducongé F, Pons T, Pestourie C, Hérin L, Thézé B, Gombert K, Mahler B, Hinnen F, Kühnast B, Dollé F, Dubertret B, Tavitian B. Fluorine-18-Labeled Phospholipid Quantum Dot Micelles for in Vivo Multimodal Imaging from Whole Body to Cellular Scales. Bioconjug Chem. 2008;19:1921–1926. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 15.Johnsson M, Hansson P, Edwards K. Spherical Micelles and Other Self-Assembled Structures in Dilute Aqueous Mixtures of Poly(Ethylene Glycol) Lipids. The Journal of Physical Chemistry B. 2001;105:8420–8430. [Google Scholar]
- 16.Yu Z. Klinik für Kardiologie. Charité-Universitätsmedizin Berlin; Berlin: 2006. p. 60. [Google Scholar]
- 17.Yu Z, Grafe M, Meyborg H, Fleck E, Li Y. In Vitro Characterization of Magnetic Resonance Imaging Contrast Agents for Molecular Imaging. ASH Annual Meeting Abstracts. 2006;108:3944. [Google Scholar]
- 18.Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, Welch MJ. 64Cu-labeled folate-conjugated shell cross-linked nanoparticles for tumor imaging and radiotherapy: synthesis, radiolabeling, and biologic evaluation. J Nucl Med. 2005;46:1210–1218. [PubMed] [Google Scholar]
- 19.van Tilborg G, Mulder W, Deckers N, Storm G, Reutelingsperger C, Strijkers G, Nicolay K. Annexin A5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjug Chem. 2006;17:741–749. doi: 10.1021/bc0600259. [DOI] [PubMed] [Google Scholar]
- 20.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J Biol Inorg Chem. 2004;9:706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, Anderson C. Production and applications of copper-64 radiopharmaceuticals. Methods Enzymol. 2004;386:237–61. doi: 10.1016/S0076-6879(04)86011-7. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy D, Shefer R, Klinkowstein R, Bass L, Margeneau W, Cutler C, Anderson C, Welch M. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nuclear Medicine and Biology. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy D, Bass L, Cutler P, Shefer R, Klinkowstein R, Herrero P, Lewis J, Cutler C, Anderson C, Welch M. High purity production and potential applications of copper-60 and copper-61. Nuclear Medicine and Biology. 1999;26:351–358. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 24.Frangioni J. New Technologies for Human Cancer Imaging. Journal of Clinical Oncology. 2008;26:4012. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones-Wilson T, Deal K, Anderson C, McCarthy D, Kovacs Z, Motekaitis R, Sherry A, Martell A, Welch M. The in vivo behavior of copper-64-labeled azamacrocyclic complexes. Nuclear Medicine and Biology. 1998;25:523–530. doi: 10.1016/s0969-8051(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 26.Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS. microPET-based biodistribution of quantum dots in living mice. J Nucl Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bass L, Wang M, Welch M, Anderson C. In Vivo Transchelation of Copper-64 from TETA-Octreotide to Superoxide Dismutase in Rat Liver. BIOCONJUGATE CHEMISTRY. 2000;11:527–532. doi: 10.1021/bc990167l. [DOI] [PubMed] [Google Scholar]
- 28.Woodin K, Heroux K, Boswell C, Wong E, Weisman G, Niu W, Tomellini S, Anderson C, Zakharov L, Rheingold A. Kinetic Inertness and Electrochemical Behavior of Copper (II) Tetraazamacrocyclic Complexes: Possible Implications for in Vivo Stability. European Journal Of Inorganic Chemistry. 2005;23:4829–4833. [Google Scholar]
- 29.Mirick G, O’Donnell R, DeNardo S, Shen S, Meares C, DeNardo G. Transfer of copper from a chelated 67Cu-antibody conjugate to ceruloplasmin in lymphoma patients. Nuclear Medicine and Biology. 1999;26:841–845. doi: 10.1016/s0969-8051(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 30.Choi H, Liu W, Misra P, Tanaka E, Zimmer J, Ipe B, Bawendi M, Frangioni J. Renal clearance of quantum dots. Nature Biotechnology. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann MD. Whole-body PET/MRI: the future in oncological imaging. Technol Cancer Res Treat. 2005;4:577–582. doi: 10.1177/153303460500400512. [DOI] [PubMed] [Google Scholar]
- 32.Cherry SR. The 2006 Henry N. Wagner Lecture: Of mice and men (and positrons)--advances in PET imaging technology. J Nucl Med. 2006;47:1735–1745. [PubMed] [Google Scholar]
- 33.Ruf J, Lopez Hanninen E, Bohmig M, Koch I, Denecke T, Plotkin M, Langrehr J, Wiedenmann B, Felix R, Amthauer H. Impact of FDG-PET/MRI image fusion on the detection of pancreatic cancer. Pancreatology. 2006;6:512–519. doi: 10.1159/000096993. [DOI] [PubMed] [Google Scholar]
- 34.Seemann MD, Meisetschlaeger G, Gaa J, Rummeny EJ. Assessment of the extent of metastases of gastrointestinal carcinoid tumors using whole-body PET, CT, MRI, PET/CT and PET/MRI. Eur J Med Res. 2006;11:58–65. [PubMed] [Google Scholar]
- 35.Catana C, Procissi D, Wu Y, Judenhofer MS, Qi J, Pichler BJ, Jacobs RE, Cherry SR. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc Natl Acad Sci U S A. 2008;105:3705–3710. doi: 10.1073/pnas.0711622105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichler BJ, Judenhofer MS, Wehrl HF. PET/MRI hybrid imaging: devices and initial results. Eur Radiol. 2008;18:1077–1086. doi: 10.1007/s00330-008-0857-5. [DOI] [PubMed] [Google Scholar]
- 37.Pichler BJ, Wehrl HF, Kolb A, Judenhofer MS. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med. 2008;38:199–208. doi: 10.1053/j.semnuclmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walczak P, Ruiz-Cabello J, Kedziorek DA, Gilad AA, Lin S, Barnett B, Qin L, Levitsky H, Bulte JW. Magnetoelectroporation: improved labeling of neural stem cells and leukocytes for cellular magnetic resonance imaging using a single FDA-approved agent. Nanomedicine. 2006;2:89–94. doi: 10.1016/j.nano.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58:261–269. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- 40.Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia. 2006;8:214–222. doi: 10.1593/neo.05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13:95–99. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 42.Wickline SA, Lanza GM. Nanotechnology for molecular imaging and targeted therapy. Circulation. 2003;107:1092–1095. doi: 10.1161/01.cir.0000059651.17045.77. [DOI] [PubMed] [Google Scholar]
- 43.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 44.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 45.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley KL, Welch MJ, Hawker CJ. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 46.Rossin R, Muro S, Welch MJ, Muzykantov VR, Schuster DP. In vivo imaging of 64Cu-labeled polymer nanoparticles targeted to the lung endothelium. J Nucl Med. 2008;49:103–111. doi: 10.2967/jnumed.107.045302. [DOI] [PubMed] [Google Scholar]
- 47.Sun G, Hagooly A, Xu J, Nystrom AM, Li Z, Rossin R, Moore DA, Wooley KL, Welch MJ. Facile, efficient approach to accomplish tunable chemistries and variable biodistributions for shell cross-linked nanoparticles. Biomacromolecules. 2008;9:1997–2006. doi: 10.1021/bm800246x. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Rossin R, Turner JL, Becker ML, Joralemon MJ, Welch MJ, Wooley KL. An assessment of the effects of shell cross-linked nanoparticle size, core composition, and surface PEGylation on in vivo biodistribution. Biomacromolecules. 2005;6:2541–2554. doi: 10.1021/bm050260e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarrett B, Frendo M, Vogan J, Louie A. Size-controlled synthesis of dextran sulfate coated iron oxide nanoparticles for magnetic resonance imaging. Nanotechnology. 2007;18:35603. doi: 10.1088/0957-4484/18/3/035603. [DOI] [PubMed] [Google Scholar]
- 50.Dubertret B, Skourides P, Norris D, Noireaux V, Brivanlou A, Libchaber A. In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 51.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, Tang CY, Gordon RE, Nicolay K, Meijerink A, Fayad ZA, Mulder WJM. Improved Biocompatibility and Pharmacokinetics of Silica Nanoparticles by Means of a Lipid Coating: A Multimodality Investigation. Nano Letters. 2008;8:2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 52.Mulder WJM, Koole R, Brandwijk RJ, Storm G, Chin PTK, Strijkers GJ, de Mello Donega C, Nicolay K, Griffioen AW. Quantum Dots with a Paramagnetic Coating as a Bimodal Molecular Imaging Probe. Nano Letters. 2006;6:1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 53.Ducongé F, Pons T, Pestourie C, Hérin L, Thézé B, Gombert K, Mahler B, Hinnen F, Kühnast B, Dollé F. Fluorine-18-Labeled Phospholipid Quantum Dot Micelles for in Vivo Multimodal Imaging from Whole Body to Cellular Scales. Bioconjug Chem. 2008;19:1921–1926. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 54.van Schooneveld M, Vucic E, Koole R, Zhou Y, Stocks J, Cormode D, Tang C, Gordon R, Nicolay K, Meijerink A. Improved Biocompatibility and Pharmacokinetics of Silica Nanoparticles by Means of a Lipid Coating: A Multimodality Investigation. Nano Lett. 2008;8:2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 55.Anzai Y, Blackwell KE, Hirschowitz SL, Rogers JW, Sato Y, Yuh WT, Runge VM, Morris MR, McLachlan SJ, Lufkin RB. Initial clinical experience with dextran-coated superparamagnetic iron oxide for detection of lymph node metastases in patients with head and neck cancer. Radiology. 1994;192:709–715. doi: 10.1148/radiology.192.3.7520182. [DOI] [PubMed] [Google Scholar]
- 56.Heesakkers R, Hövels A, Jager G, van den Bosch H, Witjes J, Raat H, Severens J, Adang E, van der Kaa C, Fütterer J, JB MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncology. 2008;9:850–856. doi: 10.1016/S1470-2045(08)70203-1. [DOI] [PubMed] [Google Scholar]
- 57.Anderson CJ, Dehdashti F, Cutler PD, Schwarz SW, Laforest R, Bass LA, Lewis JS, McCarthy DW. 64Cu-TETA-octreotide as a PET imaging agent for patients with neuroendocrine tumors. J Nucl Med. 2001;42:213–221. [PubMed] [Google Scholar]
- 58.Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ, Siegel BA. An Imaging Comparison of 64Cu-ATSM and 60Cu-ATSM in Cancer of the Uterine Cervix. J Nucl Med. 2008;49:1177–1182. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osborn SB, Szaz KF, Walshe JM. Studies with radioactive copper (64Cu and 67Cu): abdominal scintiscans in patients with Wilson’s disease. Q J Med. 1969;38:467–474. [PubMed] [Google Scholar]
- 60.Walshe JM, Potter G. The Pattern of the Whole Body Distribution of Radioactive Copper (67Cu, 64Cu) in Wilson’s Disease and Various Control Groups. QJM. 1977;46:445–462. [PubMed] [Google Scholar]
- 61.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jarrett BR, Gustafsson B, Kukis DL, Louie AY. Synthesis of 64Cu-labeled magnetic nanoparticles for multimodal imaging. Bioconjug Chem. 2008;19:1496–1504. doi: 10.1021/bc800108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, Cheon GJ, Chang Y, Yoo J, Cheon J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl. 2008;47:6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]


