Abstract
Continuous decline in cognitive performance accompanies the natural aging process in humans, and multiple studies in both humans and animal models have indicated that this decrease in cognitive function is associated with an age-related increase in oxidative stress. Treating aging mammals with exogenous free radical scavengers has generally been shown to attenuate age-related cognitive decline and oxidative stress. The present study assessed the effectiveness of the superoxide dismutase/catalase mimetics EUK-189 and EUK-207 on age-related decline in cognitive function and increase in oxidative stress. C57/BL6 mice received continuous treatment via osmotic minipumps with either EUK-189 or EUK-207 for 6 months starting at 17 months of age. At the end of treatment, markers for oxidative stress were evaluated by analyzing levels of free radicals, lipid peroxidation and oxidized nucleic acids in brain tissue. In addition, cognitive performance was assessed after three and six months of treatment with fear conditioning. Both EUK-189 and EUK-207 treatments resulted in significantly decreased lipid peroxidation, nucleic acid oxidation, and reactive oxygen species (ROS) levels. In addition, the treatments also significantly improved age-related decline in performance in the fear conditioning task. Our results thus confirm a critical role for oxidative stress in age-related decline in learning and memory and strongly suggest a potential usefulness for salen-manganese complexes in reversing age-related declines in cognitive function and oxidative load.
Keywords: Aging, Oxidative stress, Superoxide dismutase/catalase mimetics, Free radicals, Antioxidants, Fear conditioning, Learning
1. Introduction
An age-associated decline in learning and memory has been well documented in humans (Davis et al., 2003). While in its mild form, age-related decline in memory function is not life-threatening, it becomes much more dramatic in the pathological form exhibited by patients with Alzheimer’s disease. In addition, individuals exhibiting mild cognitive impairment have a greater chance of developing Alzheimer’s disease compared to the general population (Petersen et al., 2001). Such age-related loss of memory is not unique to humans, and is present in a variety of mammals, including rats and mice (Barnes et al., 1990; Foster et al., 1996). While there is still no consensus regarding the nature of the biological process(es) that underlies age-related decline in cognitive function, it has been frequently proposed that the aging process itself is linked to the accumulation of oxidative damage in neurons. Reactive oxygen species (ROS) are formed as a by-product of cellular metabolism. The formation of free radicals by normal cellular respiration usually results in a low steady state level of ROS (Boveris and Chance, 1973; Finkel and Holbrook, 2000), which can be handled by cellular antioxidant defense mechanisms. However, during oxidative stress, ROS production increases and surpasses the capacity of endogenous free radical scavengers such as superoxide dismutase and catalase. In addition, the brain is especially sensitive to oxidative stress because it utilizes high levels of oxygen, contains large amounts of lipids that free radicals can readily react with, and exhibits a lower level of antioxidants compared to other tissues (Halliwell, 1992). Indeed, aging is associated with increased free radical levels and damage associated with oxidative stress in mammalian brain, including lipid peroxidation, protein oxidation, and oxidized nucleic acids (Calabrese et al., 2004; Cini and Moretti, 1995; Hamilton et al., 2001; O’Donnell and Lynch, 1998; Siqueira et al., 2005; Sohal et al., 1994). The reason for this age-related increase in oxidative stress in the brains of aged mammals is still unclear, although mitochondria preparations from the brains of aged rodents exhibit a significant age-dependent increase in superoxide and hydrogen peroxide production (Sawada and Carlson, 1987; Sohal et al., 1994), and several studies have reported age-associated decreases in superoxide dismutase activity and catalase activity in rat brain (Rao et al., 1990; O’Donnell et al., 2000). Whether age-related increase in oxidative stress is directly responsible for age-dependent decline in memory and cognitive function remains unclear. Nevertheless, age-associated cognitive deficits have been correlated with increased oxidative stress in various regions of the mammalian brain (Butterfield et al., 2006; Forster et al., 1996; Fukui et al., 2001; Nicolle et al., 2001), and supplementing diets of aging mammals with antioxidants or free radical scavengers has generally been shown to attenuate age-related cognitive decline and oxidative stress (Carney et al., 1991; Raghavendra and Kulkarni, 2001; Stoll et al., 1994). Such findings support the idea that free radical accumulation might indeed impair memory function. It would therefore seem that antioxidant molecules should be beneficial for treating age-associated declines in learning and memory in humans. However, to apply such treatments to humans would require enormous amounts of free radical scavengers as they react on a stoichiometric basis with reactive oxygen and nitrogen species. An alternative approach would be to use low molecular weight synthetic molecules that function like the enzymes superoxide dismutase and catalase, that is, act catalytically against ROS. Two such molecules, EUK-189 and EUK-207, have previously been shown to improve cognitive performance and decrease oxidative stress in middle-aged wild type mice (Liu et al., 2003). In the current study, the effects of these compounds on age-related learning and memory impairment and on markers of oxidative stress in the brain were tested in older mice, at a lower dose, and for longer periods of time. The results provide strong evidence that ROS accumulation and oxidative stress are directly related to age-associated decline in learning and memory. In addition, our results also support potential therapeutic applications for synthetic superoxide dismutase and catalase mimetics for treating age-related decline in cognitive function.
2. Materials and Methods
2.1 Materials
EUK-189 and EUK-207 were synthesized as described previously (Doctrow et al., 2002; Malfroy-Camine and Doctrow, 2003). The oxo8dG/oxo8G antibody was purchased from QED Bioscience (San Diego, CA). All other chemicals were purchased from Sigma, unless indicated otherwise.
2.2 Mice and treatments
Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals; all protocols were approved by the Institutional Animal Care and Use Committee of the University of Southern California. We used 85 C57BL/6N Sim male mice obtained from the National Institute on Aging. Before experiments, mice were housed 4-5 per cage and placed in the same room with a 12-h light/dark cycle and behavioral testing was performed during the last 6 h of the light cycle. Mice were allowed free access to food and water, and their weights ranged from 27 – 36 g. Before surgery, mice were randomly assigned to five of the following groups (17 mice per group): vehicle control and EUK-treated groups (1.5 mM or .15 mM EUK-189 or EUK-207). An additional group of 10 male mice were used for the untreated 16-month control group. Before implantation, Alzet 2004 miniosmotic pumps were loaded with either EUK-189 or EUK-207 at 1.5 mM or .15 mM in 5% mannitol, or 5% mannitol alone (as vehicle control group) and then primed for at least 40 h in 5% mannitol at 37°C. The minipumps were then implanted s.c. in the 17-month-old mice according to the manufacturer’s recommendations. Briefly, mice were anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg) by i.p. injection. A small 1-cm incision was then made to the hip area of the mice and a small pocket was formed by spreading the s.c. connective tissues apart. The pump was placed into the prepared pocket, and the wound was then closed with sutures. Pumps delivered the drugs at 0.25 μl/h for a 28-day period, and the calculated drug infusion rates were ≈ 9 nmol/day for the 1.5 mM doses of EUK-compounds and ≈ 0.9 nmol/day for the .15 mM doses of EUK compounds. The lower concentration is equivalent to a dose of 15 and 16 μg/kg/day for EUK-189 and EUK-207, respectively (assuming a 30 g mouse). This is a much lower dose than has ever been tested for efficacy in previous studies. Control mice were implanted with mini-pumps filled with vehicle alone (5% mannitol). During the 6-month treatment, pumps were replaced 5 more times with new ones at the original sites at the end of each 28-day period of implantation. In some of the mice, the new pumps had to be placed on the opposite hip area during the 6 month treatment due to skin damage at the original site of implantation. In addition, body weights were recorded at the end of the 6 month treatment in order to assess the effects of EUK-189 and EUK-207 on overall health.
2.3 Behavioral analysis
Fear Conditioning
Experiments were run in a conditioning chamber consisting of a Plexiglas cage (29 × 29 × 29 cm) with a grid floor composed of 26 stainless steel rods (0.48 cm in diameter; Coulbourn Instruments, Allentown, PA). The apparatus was located in a sound-attenuating box located in a room that is separated from the main laboratory. A personal computer controlled the experimental events and a video camera monitoring system was used to continuously record behavior for off-line scoring of freezing. The chamber was wiped with 70 % ethanol before and after each training and testing session and each mouse was placed in the chamber individually. On day 1 of training, mice were put in the chamber and after 3 min they received three separate tones that were terminated with a foot-shock (tone: 20 sec, 80 dB, 2 kHz; foot shock: 1 sec, 0.8 mA; intertrial interval, 1 min apart). 30 sec after the final foot shock, mice were returned to their home cages. Twenty-four hours after training, mice were tested for conditioning to the context by placing them into the conditioning chamber for 8 min, but neither foot shock nor tone was given. When fear conditioning was performed at 23-months of age, the visual properties of the conditioning chamber were changed and the chamber was cleaned with 4 % acetic acid instead of 70 % ethanol in order to minimize any effects fear conditioning at 20-months of age might have on fear conditioning performance at 23-months of age.
Behavioral analysis
A time-sampling procedure performed by a trained observer blind to the experimental conditions was used. Briefly, every 10 seconds, each mouse is judged as either freezing or active. Freezing is defined as the absence of all visible movement of the body and vibrissae, aside from movement necessitated by respiration. The percentage of freezing is then calculated for the 8 minute trial by dividing the number of freezing episodes by the total number of observations (48) and multiplying by 100. In addition, freezing was also assessed on the training day prior to the delivery of the first shock in order to quantify baseline freezing levels. Baseline freezing levels were subtracted from the freezing responses measured during the context test in order to get % cumulative freezing time. For comparison, and to confirm age-associated decline in cognitive function, fear conditioning was also measured in 16-month-old mice.
In order to control for potential sensory deficits, mice were also tested for auditory and visual functions, and for nociception. Hearing was assessed by administering an auditory startle threshold test. Briefly, mice were placed in a sound-attenuating chamber individually, and after a 3-minute acclimation period, they were presented with a series of 1 second 2 kHz or 4 kHz tones. Each series started out at 80 dB and increased by 10 dB until 100 or 110 dB was reached. Each tone was separated by 15 seconds and responses to every tone were recorded. As previously reported, we observed that aged mice had hearing impairment, and this impairment precluded testing of the animals in the cue test as is often performed in fear conditioning. Vision was evaluated using a forepaw-reaching test. Mice were held by their tail and placed up side down in mid air next to a platform, and their ability to correctly reach towards the platform was assessed. Special care was taken in order to keep the whiskers away from the platform, and each mouse was tested with two different types of platforms. Nociception was evaluated with a tail-flick latency test. Every mouse was placed in a beaker in order to restrain them, and after the mouse calmed down their tale was placed on a 51°C hot plate. Tail-flick latency was defined as the length of time that elapsed between placing the tail on the hot plate and tail flicking. Overall health of the animals was assessed by daily inspection and by monitoring body weights.
2.4 Assays for lipid peroxidation, nucleic acid oxidation, and ROS content
For biochemical studies, mice were anesthetized with isoflurane and killed by decapitation. Brains were rapidly extracted, placed on a chilled platform, and the cerebellum and pons were removed and discarded. The brains were then cut in half sagitally, and each half was immediately frozen on dry ice, and stored at – 70 °C until assayed. Lipid peroxidation, nucleic acid oxidation, and free radical content were measured in brain homogenates from 23-month-old vehicle controls, EUK-189- or EUK-207-treated C57 mice, and 16-month-old untreated control mice.
The levels of lipid peroxidation were quantified by the thiobarbituric acid-reactive substances (TBARS) assay as previously described (Bruce and Baudry, 1995) with minor modifications. One sagital half from each brain was homogenized in 2.5% SDS containing 6.25 μM deferoxamine and 12.5 μM probucol (to prevent further oxidation). Four hundred microliters of homogenates were added to an aqueous solution consisting of 375 μl of 20% acetic acid solution (pH 3.5) and 225 μl of 1.33% thiobarbituric acid, and the mixture was heated at 95 °C for 1 h. One ml of a 15:1 1-butanol/pyridine solution was added, and TBARS were extracted into the organic layer by centrifugation at 4,000 x g for 10 min. The amounts of TBARS were determined by spectrophotometry at 532 nm and were calculated as nmol malondialdehyde equivalent per mg of protein according to a standard curve prepared from malonaldehyde bis(dimethyl acetal).
Oxidized nucleic acid content was determined using a DNA dot/blot procedure. Briefly, genomic DNA was extracted from 205 μl of the homogenized brain tissue mentioned above using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). Ten μg of extracted DNA was then digested with Hind III overnight and then precipitated with 3 M sodium acetate and 100 % ethanol. The resulting DNA pellet was then resuspended in 6 X SSC by heating to 95 °C for 10 min and then chilled on ice. One μg of extracted digested DNA was then blotted onto a NitroPure nitrocellulose membrane (GE Healthcare Life Sciences, Piscataway, NJ) that had been previously soaked in 6 X SSC. After allowing the membrane to dry at room temperature, the DNA was fixed onto the membrane using UV irradiation. The blot was then developed using the avidin-biotin-horseradish peroxidase complex (ABC) method with reagents and instructions of the VECTASTAIN Elite ABC kit from Vector Laboratories (Burlingame, CA). The membrane was washed with PBS and then incubated in 10% normal horse serum diluted in PBS for 1 h at room temperature. The membrane was then incubated in the presence of a monoclonal antibody to oxo8dG/oxo8G (1:1,000) in 5% horse serum diluted in PBS overnight at 4 °C. The blot was then washed in PBS, incubated in biotinylated anti-mouse IgG (1:200 in PBS with 5% normal horse serum) for 2–3 h, washed in PBS, and incubated in the avidin–biotin complex solution for 45 min. After the diaminobenzidine reaction, the membrane was washed with water and then air dried. Blots were scanned and then quantified using Image J Software, and the results were expressed as % of the corresponding values assayed in the 16-month-old control mice.
Reactive oxygen species (ROS) levels were quantified via the 2′-7′-dichlorofluorescein-diacetate (DCFH-DA) assay previously described (Siqueira et al., 2005) with some minor modifications. Briefly, the remaining sagittal half of each brain was homogenized in 50 mM phosphate buffer at pH 7.4 (10% wt/vol) and centrifuged at 11,000 x g for 15 min to sediment insoluble materials. DCFH-DA was then added to a portion of the homogenate to a final concentration of 100 μM and the reaction mixture was incubated for 30 min at 37 °C. The reaction was then stopped by placing it on ice and the formation of oxidized fluorescent 2′-7′-dichlorofluorescein (DCF) was measured with a fluorimeter (HORIBA Jobin Yvon Flouromax-3) using excitation and emission wavelengths at 488 nm and 525 nm respectively. The final results were corrected for protein concentration and then expressed as % of the corresponding values in 16 month-old mice. All steps were performed in the dark and DCF formation was also monitored immediately after DCFH-DA was added to the homogenate (t = 0 min) in order to subtract background autofluorescence.
2.5 Statistical Analysis
All statistics were performed using GraphPad Prism 4.03 software. One-way ANOVA was used to test if the means of each experimental group were significantly different, and if the overall p value was < 0.05, then multiple comparisons between the experimental groups were tested using Newman-Keuls post-hoc analysis. Pearson’s correlation coefficients were determined for the linear relationships between markers of oxidative stress and fear conditioning performance and two-tailed p values were calculated for each Pearson correlation coefficient in order to assess the significance of the relationship.
3. Results
3.1 Effects of SOD/catalase mimetics on fear-conditioning learning
C57 BL/6N mice were treated continuously with EUK-189 or EUK-207 administered through subcutaneously implanted osmotic mini-pumps. After 3 months and 6 months of continuous treatment, cognitive performance was assessed using a contextual fear conditioning paradigm.
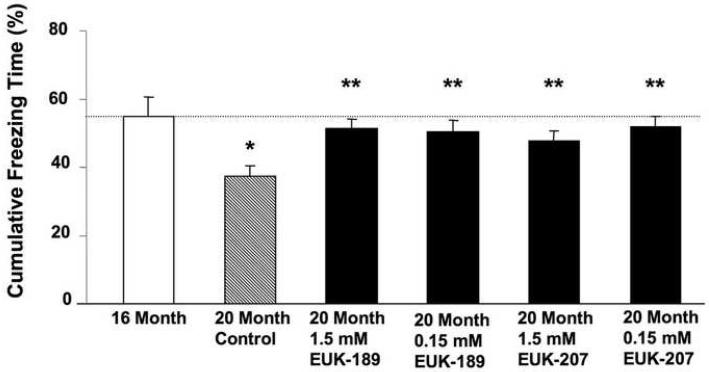
Vehicle control mice exhibited a significant decline in freezing response during the context test at both 20 months and 23 months of age when compared to 16-month-old mice (Figs. 1 & 2), thus suggesting an age-associated decline in learning and memory in this strain of mice. Chronic treatment with either EUK-189 or EUK-207 at both concentrations significantly increased freezing responses exhibited by 20 and 23-month-old mice (Figs. 1 & 2). The 20 month-old treated groups performed just as well as the 16 month-old mice; therefore it appears that EUK-189 and EUK-207 were able to block the cognitive deficits that arose between 16 and 20 months of age. The effects of chronic treatment with either EUK-189 or EUK-207 on freezing response in 23-month-old mice were not as striking as those seen in 20-month-old mice, but the treatments still significantly increased learning and memory performance in the fear conditioning task (Fig. 2). There were no significant differences between the two compounds in their ability to protect against age-associated cognitive decline, and both 1.5 mM and .15 mM concentrations provided similar results (Figs. 1 & 2). In addition, no significant defects in vision or nociception were present in all the groups tested (Table 1); in addition, the prolonged treatments with either compounds did not appear to produce any ill effects or weight changes (Table 1), suggesting that age-related differences observed in contextual fear conditioning were due to learning and memory deficits and not to differences in visual or pain perception or overall health conditions.
Figure 1. Effects of 3-month chronic treatment with EUK-189 or EUK-207 on context fear conditioning.
17 month-old mice were treated for 3 months with a low (0.15 mM) or a high (1.5 mM) concentration of EUK-189 or EUK-207 and then trained in a contextual fear conditioning paradigm and tested 24 h after training. Results were calculated as percent time the mouse expressed freezing behavior during the 8-min observation period for context minus the percent time the mouse expressed freezing behavior prior to training. Shown are means ± SEM of 9-17 mice. One-way ANOVA indicated that the effect of age was significant (*p <0.05 vs. 16 month-old mice), as was the effect of EUK-189 and EUK-207 (**p <0.05 vs. vehicle control).
Figure 2. Effects of 6-month chronic treatment with EUK-189 or EUK-207 on context fear conditioning.
After 6 months of treatment, 23 month-old mice were retrained in a contextual fear conditioning paradigm, but in a new context, and tested 24 h after training. Results were calculated as percent time the mouse expressed freezing behavior during the 8-min observation period for context minus the percent time the mouse expressed freezing behavior prior to training. Shown are means ± SEM of 9-14 mice. One-way ANOVA indicated that the effect of age was significant (*p <0.001 vs. 16 month-old mice), as was the effect of EUK-189 and EUK-207 (**p <0.05 vs. vehicle control).
Table 1. Body weight, nociception, and vision test results for SOD-catalase mimetic treated mice.
| Treatment | Body weight Mean ± SD (grams) |
Tail-flick latency Mean ± SD (sec) |
Forepaw reaching test (% success) |
|---|---|---|---|
| Vehicle | 30.8 ± 2.7 | 1.50 ± 0.52 | 100 |
| 1.5 mM EUK-189 | 30.1 ± 2.3 | 1.45 ± 0.44 | 100 |
| 0.15 mM EUK-189 | 30.9 ± 2.0 | 1.55 ± 0.44 | 100 |
| 1.5 mM EUK-207 | 31.3 ± 2.7 | 1.30 ± 0.45 | 100 |
| 0.15 mM EUK-207 | 30.3 ± 2.9 | 1.32 ± 0.39 | 100 |
Summary of the data for body weights after 6 months of treatment, tail-flick latency (nociception) and forepaw reaching test (vision). There were no statistically significant differences for the three parameters between the different treatment groups.
3.2 Effects of SOD/catalase mimetics on brain oxidative stress
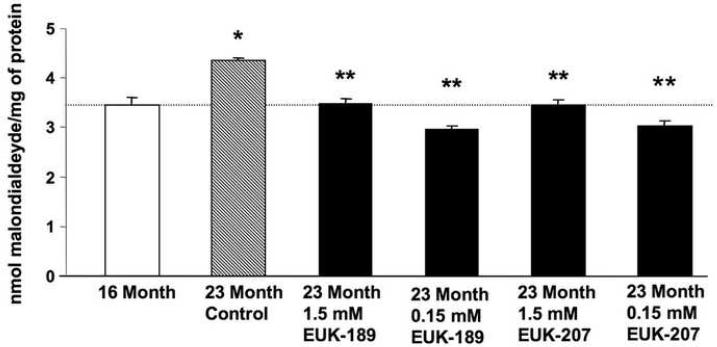
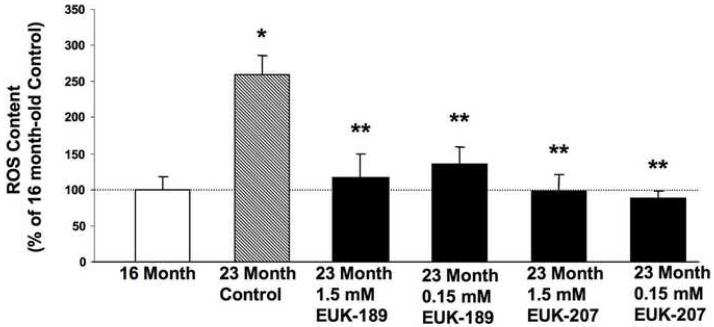
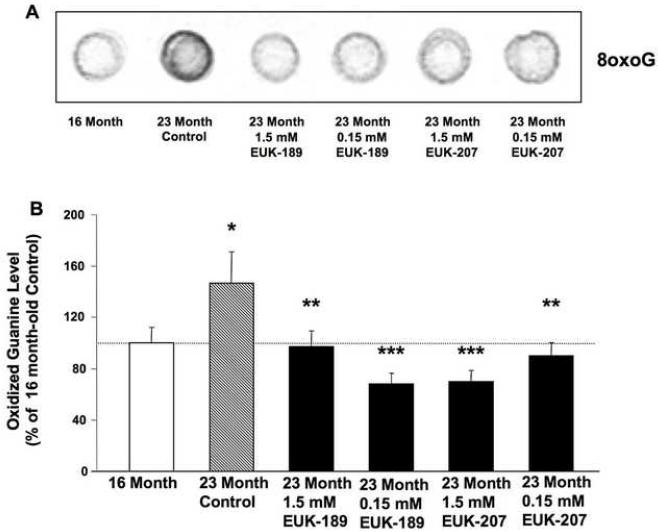
In order to assess the effects of EUK-189 or EUK-207 on age-related oxidative stress, mice treated as described above were sacrificed at the end of the 6 month long treatment and their brains, minus cerebellum and pons, were harvested and homogenized. Brain homogenates were then used to measure markers for oxidative stress, which included lipid peroxidation (levels of equivalent malondialdehyde), ROS levels and oxidized nucleic acids. For comparison, and to confirm age-associated increase in oxidative stress, markers for oxidative stress were also measured in 16-month old mice. Lipid peroxidation significantly increased between 16 and 23 months of age (Fig. 3). A 6-month chronic treatment with either EUK-189 or EUK-207 at both concentrations significantly decreased age-dependent increase in lipid peroxidation (Fig. 3). A similar age-associated increase in free radical levels (Fig. 4), and oxidized guanine (Fig. 5) was observed between 16 and 23 months of age. Treatment with either EUK-189 or EUK-207 almost completely reversed age-associated increases in both ROS levels and oxidized guanine and at both concentrations. Like for the behavioral study, there were no significant differences between the two compounds in their ability to protect against age-associated oxidative stress. Treatment with both compounds at 1.5 mM and .15 mM concentrations resulted in similar reduction in lipid peroxidation and ROS content in the brains of these mice, although EUK-189 at a concentration of 0.15 mM and EUK-207 at a concentration of 1.5 mM produced a more significant reduction in oxidized guanine (Fig. 5).
Figure 3. Effects of chronic treatment with EUK-189 or EUK-207 on lipid peroxidation in brain homogenates.
At the end of the 6 month treatment, mice were decapitated and their brains (minus cerebellum and pons) were removed and homogenized. Lipid peroxidation was then quantified by the thiobarbituric acid-reactive substances (TBARS) assay. Lipid peroxidation was also determined in brain homogenates from 16 month-old control mice. Levels of lipid peroxidation were expressed as nmol malondialdehyde equivalent per mg of protein. Shown are means ± SEM of 8-10 mice. One-way ANOVA indicated that the effect of age was highly significant (*p <0.001 vs. 16 month-old mice), as was the effect of EUK-189 and EUK-207 (**p <0.001 vs. vehicle control).
Figure 4. Effects of chronic treatment with EUK-189 or EUK-207 on reactive oxygen species (ROS) levels in brain homogenates.
At the end of the 6-month treatment, brain ROS content was quantified by incubating brain homogenates with 2′-7-dichlorofluorescein diacetate (DCFH-DA). ROS content was also determined in brain homogenates from 16 month-old control mice. Results were expressed as percentage of 16 month value. Shown are means ± SEM of 8-10 mice. One-way ANOVA indicated that the effect of age was highly significant (*p <0.001 vs. 16 month-old mice), as was the effect of EUK-189 and EUK-207 (**p <0.001 vs. vehicle control).
Figure 5. Effects of chronic treatment with EUK-189 or EUK-207 on oxidized nucleic acids in brain homogenates.
At the end of the 6-month treatment, oxidized guanine was quantified by extracting DNA from brain homogenates and then blotting onto a membrane, which was then probed with an anti-oxo8dG/oxoG antibody. Oxidized nucleic acid content was also determined in brain homogenates from 16 month-old control mice. (A) Representative 8oxoG dot blot. (B) Results were expressed as percentage of 16 month value. Shown are means ± SEM of 8-10 mice. One-way ANOVA indicated that the effect of age was significant (*p <0.05 vs. 16 month-old mice), as was the effect of EUK-189 and EUK-207 (**p <0.05 vs. vehicle control, ***p <0.01 vs. vehicle control).
3.3 Correlation between cognitive function and brain levels of markers for oxidative stress
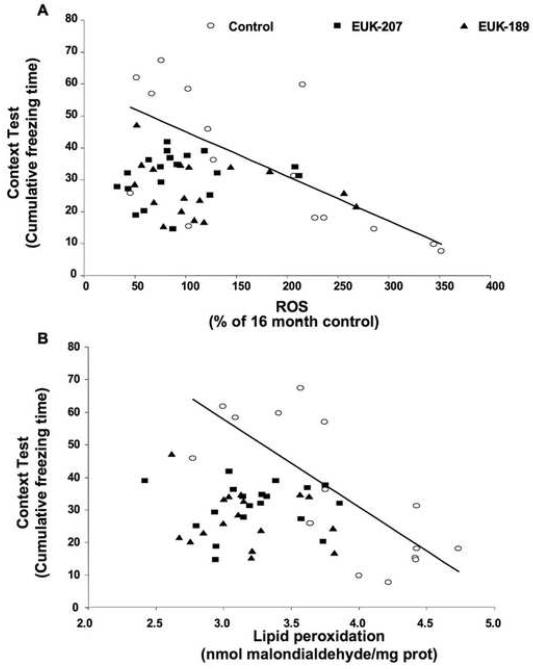
In order to further define the relationship between learning and memory performance and brain oxidative stress, we assessed the correlation between performance in the contextual fear conditioning paradigm and brain levels of ROS and lipid peroxidation. Individual data for contextual fear conditioning and brain ROS content (Fig. 7A) or lipid peroxidation (Fig. 7B) were plotted for 16-month-old control mice, 23-month-old vehicle control mice, and 23-month-old EUK-189 and EUK-207 treated mice (as there were no significant differences between the two doses of both compounds, results for both doses of EUK-189 or EUK-207 were combined). The linear relationship between contextual fear conditioning performance and oxidative stress was then assessed by determining the significance of the Pearson’s correlation coefficient. We observed a significant negative correlation between contextual fear conditioning performance and brain ROS content (Fig. 7A, ρ= −0.68) and lipid peroxidation levels (Fig. 7B, ρ= −0.76) in control mice. This indicates that oxidative load accounts for about 50-60% of the variability in learning and memory in aged mice. However, this correlation was lost in 23-month-old mice treated with either EUK-189 or EUK-207 (Figs. 7A & 7B).
4. Discussion
A large body of evidence supports a role for oxidative stress in age-dependent decline in cognitive function. The decline in learning and memory aged mice exhibit when tested with the spatial swim maze has been correlated with an increase in protein carbonyl content in cerebral cortex (Forster et al., 1996). Lipid peroxidation is also significantly much higher in hippocampus and inferior parietal lobule of elderly individuals who exhibit mild cognitive impairment (Butterfield et al., 2006). Aged rats that perform just as well as young rats in spatial learning tasks do not show the increased levels of nucleic acid and protein oxidation in hippocampus that aged cognitively impaired rats do (Nicolle et al., 2001). Furthermore, age-dependent cognitive decline in rodents can be attenuated by treating them with antioxidants and free radical scavengers, such as alpha-lipoic acid (Stoll et al., 1994), melatonin (Raghavendra and Kulkarni, 2001), and N-tert-butyl-alpha-phenylnitrone (Carney et al., 1991). Our results further strengthen the relationship between oxidative stress and age-associated deficits in learning and memory by showing that the age-dependent increased oxidative stress and cognitive decline that arise between 16 months and 23 months of age in mice are significantly reduced by chronic treatment with the SOD/catalase mimetics EUK-189 or EUK-207. Numerous studies have shown that aging is associated with decrease in fear conditioning in rats and mice and our data are in good agreement with published results. Although we trained and tested the 20 and 23 month-old groups 4 or 7 months after the 16 month-old control group, it is not likely that this time difference is responsible for the observed effects, as there are no report in the literature regarding possible seasonal influence on fear conditioning in mice. Furthermore, the critical finding in our study is the reversal of cognitive deficit provided by the drug treatments. Both compounds appeared to significantly decrease the deficits in learning and memory that take place between 16 and 23 months of age, and reverse the increases in brain lipid peroxidation, ROS content, and oxidized guanine occurring during this time period. Chronic treatment with either EUK-189 EUK-207 also significantly reduced the increased cognitive decline that occurred between 20 and 23 months of age. Moreover, our results indicate that oxidative load in aged mice accounts for about 50-60% of the variance in learning ability. Interestingly, while treatment with either EUK-189 or EUK-207 produced almost complete reversal of age-related increase in oxidative load, it decreased decline in performance in fear conditioning by about 50-60%. This result suggests that some factors other than oxidative load account for the remaining of the decline in cognitive function with aging.
Both concentrations of EUK-189 and EUK-207 were equally effective in preventing age-associated cognitive decline and oxidative stress in aged mice. However, previous work done in our laboratory suggested that the effectiveness of theses compounds might decrease at high concentrations. Like in this present study, 1.5 mM of both compounds inhibited age-dependent cognitive deficits and oxidative stress in middle-aged mice (Liu et al., 2003). However, in the previous study, we also showed that at higher concentrations (15 mM), EUK-207 was less effective than at 1.5 mM (Liu et al., 2003). In the current study, in an attempt to eliminate potentially deleterious doses as well as to better characterize dose-dependency of the compounds, we repeated the 1.5 mM dose and added a ten-fold lower dose group. In this study, while not yet identifying a suboptimal dose, we report for the first time in any in vivo system that EUK-189 and EUK-207 are active at a dose of 0.15 mM, that is approximately 15 μg/kg-day.
Our results also indicate that the age-associated deficits in learning and memory we observed might be brought on by oxidative damage to hippocampus, amygdala, or both. Contextual fear conditioning is a learning and memory task that is dependent on both amygdala and hippocampus (Maren et al., 1997; Phillips and LeDoux, 1992), and our data show that increased levels of markers for oxidative stress coincides with deficits in contextual fear conditioning. Furthermore, chronic treatment with either EUK-189 or EUK-207 significantly alleviates these age-associated deficits in contextual fear conditioning. These results are also in agreement with a similar study in middle-aged mice (Liu et al., 2003). While it might have been interesting to determine changes in oxidative load in hippocampus and amygdala, the small size of these structures and the need for relatively large amounts of tissues for all the biochemical assays compelled us to limit our tissue samples to the combined forebrain and midbrain.
This body of work and previous studies by others suggest that antioxidant compounds could prove to be beneficial in treating age-dependent cognitive deficits in humans. However, compounds that mimic SOD and catalase might prove to be even more beneficial in humans because, unlike antioxidants, they do not react on a stoichiometric basis. Earlier work with a carboxyfullerene SOD mimetic showed a dramatic decrease in age-dependent learning and memory deficits and oxidative stress in mice (Quick et al., 2008). The multiple catalytic activities of EUK-189 and EUK-207 might prove to be even more beneficial, and could account for the very low efficacious doses, as compared to that of the carboxyfullerene tested. Thus, a dose of the EUK compounds of about 15 μg/kg/day was as potent as a dose of 10 mg/kg/day of carboxyfullerene. In addition, it is important to note that the carboxyfullerene is only an SOD mimetic whereas the EUK compounds tested can also protect against damage caused by hydrogen peroxide, and through catalase-like mechanisms (Doctrow et al., 2002; Sharpe et al., 2002), reactive nitrogen species.
Chronic treatment with EUK-189 or EUK-207 was initiated at a relatively late stage in the lives of these mice, but our 6 month-long treatments were still able to provide protection against cognitive declines that occurred between 16 months and 23 months of age. Thus, our results suggest that these compounds might prove to be beneficial in preventing further cognitive impairment in relatively old individuals that already exhibit mild cognitive impairment. In addition, while this study was not intended to address chronic toxicity, it is well worth noting that long term, sustained treatment with these compounds was beneficial to the mice without showing any indications of toxicity. Similar observations have been made in other long-term treatment studies, for example, chronic administration of EUK-189 in a mouse Alzheimer’s disease model (Melov et al., 2005).
Previous work done using SOD2 knock-out mice provided indirect evidence that salen-manganese compounds such as EUK-8, EUK-134, or EUK-189 are able to cross the blood brain barrier and to be mito-protective (Melov et al., 2001). EUK-207 is as equally effective as EUK-189 in extending the lifespan of SOD2 knock-out mice (unpublished data), but its ability to alleviate the neurological phenotype has not been characterized.
In conclusion, our data demonstrate that chronic treatment with the superoxide dismutase/catalase mimetics EUK-189 or EUK-207 significantly reduces age-related cognitive impairment and age-associated oxidative stress in mice. These findings add support to the hypothesis that oxidative stress plays a critical role in age-related learning and memory dysfunction. Furthermore, they suggest that EUK-189 or EUK-207 have potential value as a treatment for age-related cognitive dysfunction.
Figure 6. Correlation between performance in the contextual fear conditioning task and brain levels of free radicals and lipid peroxidation.
Individual data for contextual fear conditioning and brain free radical content (A) or lipid peroxidation (B) were plotted for 16-month-old control mice, 23-month-old vehicle control mice, and 23-month-old EUK-189 and EUK-207 treated mice. Free radical content was expressed as percentage of 16-month-old control value (A) and levels of lipid peroxidation were expressed as nmol malondialdehyde equivalent per mg of protein (B). Contextual fear conditioning performance was calculated as percent time the mouse expressed freezing behavior during the 8-min observation period for context minus the percent time the mouse expressed freezing behavior prior to training (A & B). Regression lines were plotted for the control animals and analysis indicated a significant negative correlation between contextual fear conditioning performance and brain free radical content (Pearson’s correlation coefficient ρ= −0.68, p = 0.0054, n =15) (A) and lipid peroxidation levels (Pearson’s correlation coefficient, ρ= −0.76, p = 0.0011, n =15) (B). No significant correlation between performance in the contextual fear conditioning task and brain levels of free radicals or lipid peroxidation was observed in 23-month-old EUK-189 (n =17) and EUK-207 (n =19) treated mice (A & B).
Acknowledgements
This work was supported by National Institute on Aging Grant R41AG012570 to Dr. Susan R. Doctrow. We would like to thank our research specialist Anna Knize for helping out with the survival surgeries and Dr. Peter Z. Qin’s lab for letting us use their fluorimeter.
Footnotes
Disclosure Statement
Sue Doctrow works for Proteome Systems, Inc and Michel Baudry is a shareholder from Proteome Systems, Inc. Aaron Clausen has no involvement with Proteome Systems. To our knowledge, nobody at the University of Southern California has contracts relating to this research through which it or any other organization may stand to gain financially now or in the future. There are no other agreements of the authors or their institutions that could be seen as involving a financial interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol. Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Radical Biol. Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci. Lett. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech. Ageing Develop. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin trapping compound N-tert-butyl-alpha-phenylnitrone. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol. Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- Davis HP, Small SA, Stern Y, Mayeux R, Feldstein SN, Keller FR. Acquisition, recall and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J. Med. Chem. 2002;45:4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S. Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidant defense mechanisms. Ann. NY. Acad. Sci. 2001;928:168–175. doi: 10.1111/j.1749-6632.2001.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. U.S.A. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfroy-Camine B, Doctrow SR. Cyclic salen metal complexes: reactive oxygen species scavengers useful as antioxidants for treatment and prevention of disease. 6,589,948 U.S. Pat. 2003
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Life extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nulliygous mice treated with superoxide dismutase-catalase mimetics. J. Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Wolf N, Strozyk D, Doctrow SR, Bush AI. Mice transgenic for Alzheimer disease beta-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radical Biol. Med. 2005;38:258–261. doi: 10.1016/j.freeradbiomed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neurosci. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- O’Donnell E, Lynch MA. Dietary antioxidant supplementation reverses age-related neuronal changes. Neurobiol. Aging. 1998;19:461–467. doi: 10.1016/s0197-4580(98)00082-7. [DOI] [PubMed] [Google Scholar]
- O’Donnell E, Vereker E, Lynch MA. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. Eur. J. Neurosci. 2000;12:345–352. doi: 10.1046/j.1460-9568.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;59:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, Dugan LL. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol. Aging. 2008;29:117–128. doi: 10.1016/j.neurobiolaging.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Kulkarni SK. Possible antioxidant mechanisms in melatonin reversal of aging and chronic ethanol-induced amnesia in plus-maze and passive avoidance memory tasks. Free Radical Biol. Med. 2001;30:595–602. doi: 10.1016/s0891-5849(00)00447-0. [DOI] [PubMed] [Google Scholar]
- Rao G, Xia E, Richardson A. Effect of age on the expression of antioxidant enzymes in male Fischer F344 rats. Mech. Ageing Develop. 1990;53:49–60. doi: 10.1016/0047-6374(90)90033-c. [DOI] [PubMed] [Google Scholar]
- Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart, and liver during the lifetime of the rat. Mech. Ageing Develop. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem. J. 2002;366(Pt 1):97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira RI, Fochesatto C, Da Silva Torres IL, Dalmaz C, Netto CA. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:27 1–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Develop. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Stoll S, Rostock A, Bartsch R, Korn E, Meichelbock A, Muller WE. The potent free radical scavenger alpha-lipoic acid improves cognition in rodents. Ann. NY. Acad. Sci. 1994;717:122–128. doi: 10.1111/j.1749-6632.1994.tb12079.x. [DOI] [PubMed] [Google Scholar]