Abstract
Background:
Vitamin B12 (B12) deficiency is common in Indians and a major contributor to hyperhomocysteinemia, which may influence fetal growth, risk of type 2 diabetes and cardiovascular disease.
Objective:
To study the effect of physiological doses of B12 and folic acid on plasma total homocysteine (tHcy).
Design:
A cluster randomized, placebo-controlled, double-blind, 2x3 factorial trial, using the family as the randomization unit. Vitamin B12 was given as 2 or 10 μg capsules, with or without 200 μg folic acid, forming six groups (B0F0, B2F0, B10F0, B0F200, B2F200, B10F200). Plasma tHcy was measured before and after 4 and 12 mo of supplementation.
Results:
Three hundred individuals from 119 families in the Pune Maternal Nutrition Study were randomised. There was no interaction between B12 and folic acid (P=0.14) in relation to tHcy change and their effects were analyzed separately: B0 vs. B2 vs. B10; and F0 vs. F200. At 12 mo, tHcy fell by a mean 5.9 (95% CI: −7.8, −4.1) μmol/L in B2, and by 7.1 (95% CI: −8.9, −5.4) μmol/L in B10, compared to non-significant rise of 1.2 (95% CI: −0.5, 2.9) μmol/L in B0. B2 and B10 did not differ significantly. In F200, tHcy fell by 4.8 (95% CI: −6.3, −3.3) μmol/L compared to 2.8 (95% CI: −4.3, −1.2) μmol/L in F0.
Conclusion:
Daily oral supplementation with physiological doses of B12 is an effective community intervention to reduce tHcy. Folic acid (200 μg/d) showed no additional benefit, neither had any unfavourable effects.
Keywords: Cyanocobalamin, folic acid, homocysteine, randomised controlled trial, South Asian Indians, vitamin B12
INTRODUCTION
Hyperhomocysteinemia is a risk factor for cardiovascular disease (CVD) (Wald et al., 2002), psychiatric disorders (dementia and Alzheimer's disease) (Smith, 2008), and in pregnancy for adverse outcomes including early pregnancy loss, birth defects and low birth weight (LBW) (Vollset et al., 2000; Selhub, 2008). Low vitamin B12(B12) status and hyperhomocysteinemia are common among Indians living in India (Refsum et al., 2001; Yajnik et al., 2006), and those migrated abroad (Chambers et al., 2000; Chandalia et al., 2003). This is largely due to B12 deficiency, even with normal folate status, reflecting vegetarian food habits. In recent years, this has been particularly well documented from Pune, India (Refsum et al., 2001; Yajnik et al., 2006 and 2008). In the Pune Maternal Nutrition Study (PMNS) maternal hyperhomocysteinemia predicted LBW (Yajnik et al., 2005), and neurocognitive impairment in children (Bhate et al., 2008), and low maternal B12 with high erythrocyte folate predicted higher adiposity and higher insulin resistance (IR) in the offspring (Yajnik et al., 2008). Based on these results we propose that B12 supplementation in women of child bearing age may be a simple and effective mass measure to lower the incidence of LBW, adiposity and insulin resistance and thus of T2D and CVD, and also improve neurocognitive function of the children.
In an earlier ‘proof of concept’ trial (Yajnik et al., 2007), we demonstrated that high dose oral B12 supplementation (500 μg alternate d, for 6 wk) reduced circulating tHcy concentrations. We report results of a randomized, placebo-controlled trial of B12 supplementation on plasma homocysteine, using physiological doses over 12 mo.
METHODS
Participants
The participants were families from an ‘extended’ cohort of the PMNS. The PMNS methodology has been reported in detail by Rao in 2001 (Rao et al., 2001). In brief, 2675 married women of childbearing age, living in 6 rural villages near Pune city were recruited, and those who became pregnant were followed up. After the main study, we enrolled an additional 153 pregnant women from the same recruited sample, in order to study the early fetal growth. They did not contribute to the main study, and nutritional and blood measurements were not available during pregnancy. Of these, 119 families remain in follow-up, and the child and parents (349 individuals) were invited to take part in the current study.
The study was approved by the KEM Hospital Ethics Committee. Exclusion criteria were: unwillingness to participate, pregnancy, anemia (hemoglobin (Hb) <9 g/dL), already taking supplements containing iron, folic acid and/or B12 for 10 or more days, or on treatment with drugs known to impair the absorption or utilization of folic acid or B12 (e.g. phenytoin, antacids). We obtained informed written consent from the parents and informed written assent from the children (mean age 9 y).
For blood collection (June to November 2006) the families were brought to the Research Centre, the evening before the study. A standard vegetarian dinner was provided, after which they rested. A fasting blood sample was collected in the morning.
Study design and intervention
The trial was double blinded. We planned to test three levels of B12 supplementation (none, 2 and 10 μg) and each of these at two levels of folic acid supplementation (none and 200 μg), forming 6 groups (A = B0F0, B = B2F0, C = B10F0, D = B0F200, E = B2F200, F = B10F200) (Figure 1). Randomization was computer-based. The unit of randomization was the family, making it a cluster randomized trial. We stratified the families by the children's baseline plasma B12 concentrations; those below and above the median value were equally distributed in six groups. Within each group, the statistician randomly allocated codes (A to F) to the participant families. The contents of the capsules were known only to the pharmacist until the end of the trial. The codes were revealed only after data analysis. The study capsules were manufactured in six different colors. The supplements were dispensed monthly in containers labeled with the participant's name and capsule group (A through F). All members of the family received the same colored capsules. The participants were advised to take one capsule orally, daily before breakfast. The number of dispensed capsules and those returned at each monthly home visit were counted to calculate the compliance. At each monthly home visit we recorded adverse events and treatment of intercurrent illnesses, if any. Participants who took medicine containing folic acid and/or B12 for more than 10 days were omitted from data analysis. The duration of supplementation was 12 mo, and took place between April 2007 and March 2008. Laboratory analysis of the study medication at the beginning and end of the study period revealed similar potency of the capsules.
FIGURE 1.
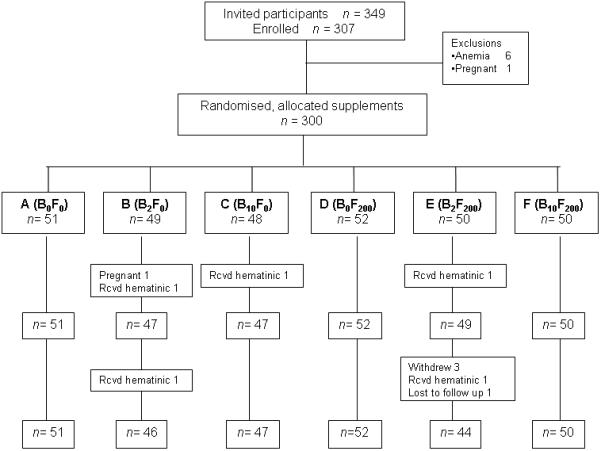
Participant flow and follow up
Measurements
Blood samples were collected at baseline and 4 and 12 mo after supplementation and were measured in separate batches. The samples were collected in EDTA tubes, kept on ice and spun within one h (2500 g × 30 min) and plasma aliquots were stored (−70°C) until further analysis. Hemoglobin was measured within one hour of blood collection on a Beckman Coulter analyser (AC.T diff ™, Miami, Florida). Plasma creatinine was measured on an Alcyon 300 automated analyser (Abbott Laboratories, Abbott Park, IL, USA) using Jaffe's method. Plasma B12and folate were measured by microbiological assay using a colistin sulfate-resistant strain of L. leichmanii (Kelleher et al., 1987, 1991) and a chloramphenicol-resistant strain of L. casei (Horne and Patterson, 1988; Tamura et al., 1990), with inter-batch CV <8% and <7% respectively. Plasma tHcy was measured by fluorescence polarization immunoassay (Abbott, IL, USA; CV <8%) (Shipchandler and Moore, 1995).
Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (CMS Instruments, London, UK), and body weight to the nearest 0.005 kg (Conveigh, Electronic Instruments Ltd., Mumbai, India). Dietary intake of B12 and folate rich foods was recorded by food frequency questionnaire in children at the beginning and end of 12 mo.
Definitions
Compliance was defined as taking ≥ 80% of the dispensed capsules, hyperhomocysteinemia as plasma tHcy concentrations >15 μmol/L (in adults), >10 μmol/L (in children) (Refsum et al., 2004), and B12 and folate deficiency as concentrations <150 pmol/L (Refsum et al., 2001) and <7 nmol/L (Clarke et al., 2004) respectively.
Statistical methods
The data are presented as mean and standard deviation (SD). Though B12 and tHcy concentrations were not normally distributed, we used parametric tests for differences between group means, which are normally distributed as per the central limit theorem. We used ANOVA to test the differences between supplementation groups, adjusting for the cluster design. There was no difference between clustered and un-clustered analysis (statistically insignificant intraclass correlation). The change in the prevalence of hyperhomocysteinemia from baseline was tested by McNemar's test.
The change in plasma tHcy concentrations was adjusted for the baseline tHcy concentrations, age and gender. There was no significant interaction between the effects of B12 and folic acid supplementation on change in plasma tHcy concentration (P=0.14; Figure 1 in supplementary information). Therefore, the effects of 2 μg and 10 μg B12 supplementation were tested against no B12, combining the folic acid supplementation groups (B0= B0F0, B0F200; B2= B2F0, B2F200; B10= B10F0, B10F200) and the effect of 200 μg folic acid was tested against no folic acid by combining the B12 supplementation groups (F0= B0F0, B2F0, B10F0; F200= B0F200, B2F200, B10F200). The relative benefit for hyperhomocysteinemia was calculated by taking ratios of absolute benefit in different supplementation groups against non-supplemented group. The number needed to treat (NNT) was calculated as the reciprocal of the absolute risk reduction (ARR) for hyperhomocysteinemia at the end of 12 mo. All analyses were done using STATA, version 7.0 (STATA Inc. College Station, Texas, USA).
RESULTS
Recruitment and Participant flow (Figure 1)
Of the 119 families (349 individuals) in the extended PMNS cohort, 307 individuals were willing to participate (88% response), seven (1 pregnant, 6 anemic) were excluded and 300 (106 children, 93 fathers and 101 mothers) were randomized. During the intervention, 1 woman became pregnant and 5 participants received B12 containing medication (from family physician) and were excluded from the analysis. Three participants withdrew and one was lost to follow up after collection of the 4 mo sample; they were analysed using the Last Observation Carried Forward (LOCF) method. Thus the final analysis includes 294 participants (106 children, 92 fathers and 96 mothers).
Baseline characteristics
Table 1 shows the basic characteristics of the 300 participants. Seventy two percent fathers, 48% mothers and 27% children were B12 deficient, and 75% fathers, 35% mothers and 47% children were hyperhomocysteinemic. In contrast, only 14% fathers, 8% mothers and 2% children had folate deficiency. Baseline B12, folate and tHcy concentrations were similar in the different supplementation groups.
TABLE 1.
Baseline parameters in children and parents
| Physical and biochemical parameters | Children n= 106 |
Fathers n= 93 |
Mothers n= 101 |
|---|---|---|---|
| Age (y) | 9.0 (0.2) | 36.8 (3.7) | 30.4 (3.1) |
| Weight (kg) | 21.9 (2.9) | 59.2 (10.0) | 47.9 (8.3) |
| Height (cm) | 126.4 (5.4) | 165.6 (7.0) | 155.3 (5.4) |
| BMI (kg/m2) | 13.7 (1.4) | 21.6 (3.3) | 20.4 (3.5) |
| < 18.5 kg/m2 (%) | Boys 42.61 | 21.5 | 37.6 |
| > 25 kg/m2 (%) | Girls 55.81 | 17.2 | 13.9 |
| Hemoglobin (g/dL) | 12.5 (0.9) | 14.3 (1.2) | 12.2 (1.4) |
| Plasma creatinine (mg/dL) | 0.6 (0.1) | 0.9 (0.1) | 0.8 (0.1) |
| Plasma B12 concentration (pmol/L) | 203 (83) | 130 (65) | 161 (77) |
| Plasma vitamin B12 < 150 pmol/L (%) | 26.7 | 72.2 | 48.4 |
| Plasma folate concentration (nmol/L) | 18.9 (6.3) | 16.3 (5.5) | 16.8 (6.8) |
| Plasma folate < 7 nmol/L (%) | 1.9 | 14.4 | 8.3 |
| Plasma tHcy concentration (μmol/L) | 10.7 (3.8) | 31.4 (22.6) | 14.6 (7.8) |
| Plasma tHcy > 15 μmol/L (adults) and > 10 μmol/L (children) (%) |
47 | 75.3 | 34.7 |
All values are mean (SD) unless specified.
% of children < −2 SD of age and gender specific BMI (WHO Reference population).
Compliance
Seventy one percent (n=210) participants returned <20% of the dispensed capsules over 12 mo and were defined as ‘compliers’. Fourteen percent participants consumed 70-80%, 6% consumed 60-70%, another 6% consumed 50-60% and remaining 3% consumed <50% of the dispensed dose. The mean plasma tHcy concentration, fall in plasma tHcy concentration and prevalence of hyperhomocysteinemia at 4 and 12 mo were similar in the compliers (n=210) and non-compliers (n=84) (Table 1 in supplementary information). Overall compliance rates were similar at 4 and 12 mo.
The frequency of consumption of folate and B12 rich foods in children was similar at baseline and after 12 mo.
Plasma vitamin B12 and folate concentrations (Table 2)
TABLE 2.
Mean concentrations of plasma vitamin B12, folate and tHcy in the B0, B2, B10, F0 and F200 groups at baseline, 4 and 12 mo
| B0 n=102 |
B2 n=94 |
B10 n=98 |
F0 n=143 |
F200 n=151 |
|
|---|---|---|---|---|---|
|
Mean compliance at 12 months (%) |
82 | 84 | 87 | 86 | 82 |
| Plasma B12 pmol/L | |||||
| • Baseline | 171 (76) | 168 (85) | 159 (83) | 163 (84) | 169 (79) |
| • 4 mo | 181 (141) | 267 (158)*** | 326 (158)*** | 267 (191)*** | 248 (131)*** |
| • 12 mo | 201 (69)*** | 242 (73)*** | 307 (119)*** | 252 (114)*** | 247 (84)*** |
| Plasma folate nmol/L | |||||
| • Baseline | 13.9 (5.7) | 12.6 (4.3) | 13.5 (3.8) | 13.2 (5.6) | 13.5 (5.6) |
| • 4 mo | 24.9 (15.6)*** | 24.6 (15.7)*** | 24.2 (14.3)*** | 15.5 (6.4)*** | 33.1 (16.1)*** |
| • 12 mo | 23.7 (15.2)*** | 20.2 (11.4)** | 19.7 (11.5) | 14.6 (6.3)*** | 27.8 (14.4)*** |
| Plasma tHcy μmol/L | |||||
| • Baseline | 17.6 (15.3) | 19.7 (19.0) | 18.5 (14.3) | 19.8 (17.3) | 17.5 (15.1) |
| • 4 mo | 18.5 (17.1) | 14.2 (10.6)*** | 12.9 (9.4)*** | 17.2 (14.5)* | 13.4 (11.3)*** |
| • 12 mo | 19.3 (16.8) | 12.9 (7.9)*** | 11.6 (7.4)*** | 16.3 (13.7)** | 13.1 (10.3)*** |
Values in mean (SD);
P < 0.05,
P < 0.01,
P < 0.001 different from baseline concentration
At baseline 48% participants were B12 deficient. Plasma B12 concentrations increased significantly in those who received B12 supplements. At 12 mo the rise was 64% in those who received 2 μg (B2) and 119% in those who received 10 μg (B10). In both groups this was similar to the rise achieved by 4 mo. Plasma B12 concentrations were higher in the B10 compared to the B2 group. After 12 mo of supplementation 6% of the B2 and 2% of the B10 group remained B12 deficient. Participants who did not receive B12 (B0) also showed a rise in plasma B12 concentration (33% above baseline) after 12 mo.
Plasma folate concentrations increased by 112% in those who received folic acid (F200) and by 18.8% in the group who did not (F0). At baseline 8% participants were folate deficient; after 12 mo this reduced to 0% in the supplemented and to 6% in the non-supplemented group.
Plasma total homocysteine (tHcy) concentration
B12 supplementation (Figure 2, Table 2)
FIGURE 2.
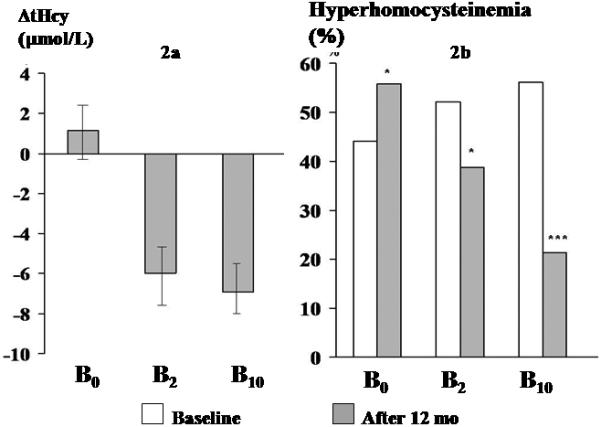
Effect of 12 mo of supplementation with vitamin B12 (B0, B2, B10) on plasma tHcy
2a Change in tHcy (mean and 95% CI) over 12 mo in 3 groups (0 line indicates baseline tHcy concentration); 2b Proportion with hyperhomocysteinemia at baseline and at 12 mo in 3 groups
Plasma tHcy concentrations fell in the B2 and B10 groups, and showed little change in the B0 group. The fall was greater in those with higher baseline concentrations (r = −0.6, P=0.000). We therefore adjusted the change in plasma tHcy concentrations for baseline concentrations. The change in plasma tHcy concentrations was not related to the baseline plasma B12 and folate concentrations. The baseline-adjusted fall was 5.9 (95% CI: −7.8, −4.1) μmol/L in the B2 group and 7.1 (95% CI: −8.9, −5.4) μmol/L in the B10 group (not significantly different). The B0 group showed a non-significant rise of 1.2 (95% CI: −0.5, 2.8) μmol/L. Eighty-two percent of the fall was achieved by 4 mo. After 12 mo, in the B2 group the proportion of hyperhomocysteinemic participants fell from 52% to 39% (P=0.02), in the B10 group from 56% to 21% (P<0.000), and in the B0 group it increased from 44% to 56% (P=0.02).
Folic acid supplementation (Figure 3, Table 2)
FIGURE 3.
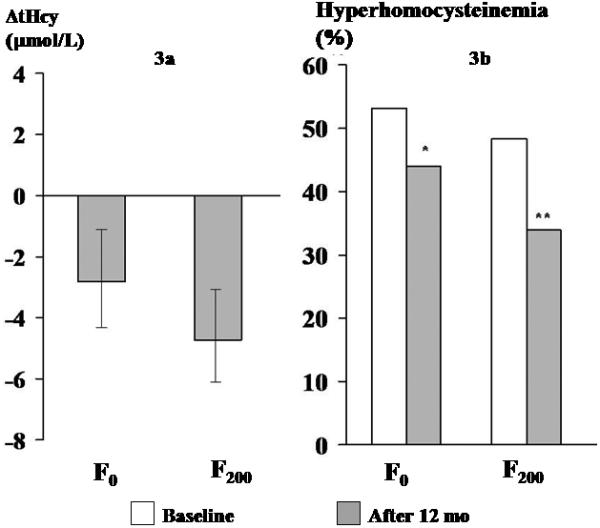
Effect of 12 mo of supplementation with folic acid (F0, F200) on plasma tHcy
3a Change in tHcy (mean and 95% CI) over 12 mo in 2 groups (0 line indicates baseline tHcy concentration), 3b Proportion with hyperhomocysteinemia at baseline and at 12 mo in 2 groups
The F0 and F200 groups showed similar fall in plasma tHcy concentration: F0 2.8 (95% CI: −4.3, −1.2) μmol/L and F200 4.8 (95% CI: −6.3, −3.3) μmol/L. In the F0 group the proportion of hyperhomocysteinemic participants fell from 53% to 44%, P=0.07 and in the F200 group from 48% to 34%, P=0.003.
Table 3 shows the number of hyperhomocysteinemic individuals in different supplementation groups who became normohomocysteinemic (‘responded’) or remained hyperhomocysteinemic (‘not responded’) after 12 mo. The relative benefit of supplementation was similar in the two B12 supplemented groups (B2 and B10), but was higher in the B10 compared to the F200 group. The number needed to treat (NNT) was 4 for B2, 2 for B10 and 10 for F200 group.
TABLE 3.
Relative benefit and number needed to treat (NNT) for hyperhomocysteinemia in different supplementation groups after 12 mo
| Groups | Responded (n) |
Not responded (n) |
Relative benefit (95% CI) |
Number needed to treat (NNT) |
|---|---|---|---|---|
| B0 | 6 | 39 | ||
| B2 | 18 | 29 | 2.87 (1.25, 6.58) | 4 |
| B10 | 39 | 16 | 5.32 (2.48, 11.4) | 2 |
| F0 | 29 | 47 | ||
| F200 | 34 | 37 | 1.25 (0.86, 1.82) | 10 |
(Responded = number of hyperhomocysteinemic participants who became normohomocysteinemic, Not responded = number of hyperhomocysteinemic participants who remained hyperhomocysteinemic at the end of trial).
Side effects
There were 62 responses from 46 participants during the study period. One woman reported an accidental injury requiring hospital admission, which was not attributable to supplementation. Other responses were classified into positive (increased appetite, weight gain, sense of wellbeing; n=40) and negative (abdominal pain and acidity, feeling unwell; n=22). There was no obvious clustering of side effects in any particular intervention group.
DISCUSSION
This is the first community-based randomized trial of B12 supplementation in an Indian population with substantial B12 deficiency due to low dietary intake. We found that both 2 and 10 μg/d of oral B12 (cyanocobalamin) significantly reduced plasma tHcy concentrations in otherwise healthy, free-living, rural participants. Eighty two percent of the effect was achieved by 4 mo. Overall, the two doses of B12 were similarly effective in reducing plasma tHcy concentrations. Folic acid by itself had no additional effect on plasma tHcy reduction, over placebo or in combination with B12 (Table 2 in supplementary information).
The relatively large effect of such small doses of B12 is probably related to the high prevalence of vitamin B12 deficiency and hyperhomocysteinemia in this population (Refsum et al., 2001; Yajnik et al., 2006, 2008). Without B12 supplementation, hyperhomocysteinemia increased by 12% over the 12 mo period. Using the cut-point of 15 μmol/L (adults) and 10 μmol/L (children), we found there was a 13% fall in the hyperhomocysteinemia with 2 μg/d and 35% fall with 10 μg/d of B12 from the baseline. The large effect of supplementation was also evident in the small numbers needed to treat: only 4 hyperhomocysteinemic individuals needed to be treated with 2 μg/d of B12 for 12 mo for 1 to become normohomocysteinemic, and only 2 with 10 μg/d B12. If the relationship of maternal B12 deficiency and hyperhomocysteinemia with fetal outcomes is causal vitamin B12 intervention could translate into a substantial reduction in the incidence of low birth weight, diabetes and CVD in this community as well as improvement in cognitive function based on our previous findings (Yajnik et al., 2005, 2008; Bhate et al., 2008).
Although we knew that B12 deficiency was common in this population (Refsum et al., 2001; Yajnik et al., 2006, 2008), we used a placebo to maintain the scientific rigor and included an arm with only folic acid to test comparative effects, especially in view of proposed food fortification in India. Despite doubling of plasma folate concentrations, folic acid by itself had no effect on circulating tHcy concentrations; neither did it enhance the effect of B12 (Figure 1, supplementary information). This supports our contention that folate deficiency is not common in this population (Refsum et al., 2001; Yajnik et al., 2006, 2008). Although this dose of folic acid was not associated with any adverse effects over 12 mo, the proposal for folic acid fortification in India for prevention of first occurrence neural tube defects (The Flour Fortification Initiative, 2007) needs to be formally investigated, including co-fortification with B12.
The effect of supplementation on plasma tHcy was unrelated to the compliance (Table 1 in supplementary information), perhaps because the overall compliance was good (72%). Another explanation is that the dose of B12 over 4 and 12 mo was more than necessary to achieve the effect. This observation is reassuring for future public health interventions.
Major strengths of our study are that it was community-based and included apparently healthy children and adults, rather than being targeted at high-risk groups or patients. The participation rate was high and compliance was maintained at high levels throughout the 12 mo. The factorial design allowed us to look at independent effects of B12 and folic acid in comparison to their combination and the placebo. We used physiological rather than pharmacological doses of vitamins, with a view to translate our findings into future public health programs. The difficulty in obtaining specially manufactured capsules led to a five months gap between the baseline data collection and commencement of the intervention. However this was distributed similarly in different groups and therefore should not affect our results.
The striking reduction in plasma tHcy concentrations with small doses of 2 and 10 μg of B12 merits discussion. In a recent study we have demonstrated that 3 doses of 2 μg of B12 at 6 hr intervals not only raised plasma B12 concentrations but also caused a significant (though small) fall in tHcy concentrations within 24 hr of the first dose (Bhat et al., 2009). This is perhaps a reflection of a B12 deficient state and high baseline plasma tHcy concentrations. The almost similar effect of 2 and 10 μg doses is perhaps related to characteristics of intestinal B12 absorption, which is predominantly by an active (intrinsic factor-mediated) mechanism which saturates after a 1.5 to 2 μg dose (Carmel, 2008). Only about 1% of absorption is by passive absorption (by diffusion).
In addition to these considerations the duration of supplementation is also an important determinant of the effect. Small doses over a long time might be equally effective as a large dose over a short time (Carmel, 2008). Our previous study (in vegetarian women) used a large dose of oral B12 (500 μg every alternate day for 6 wk) (Yajnik et al., 2007). In 2 wk (total dose 3 mg B12) plasma tHcy concentrations fell from 18.0 to 13.0 μmol/L, which remained static over the next 4 wk (total dose 9 mg B12). In the present study 0.72 mg of B12 (2 μg/d × 12 mo) achieved a similar effect, 82% of which was achieved by 4 mo with 0.24 mg B12.
The majority of published studies of B vitamin supplementation have been in predominantly non-vegetarian western populations, in whom folate deficiency is the main determinant of hyperhomocysteinemia (Selhub, 2008). After folic acid fortification of foods in these populations, the attention has now shifted towards B12 deficient groups like the elderly, in whom B12 deficiency is thought to be due to ‘atrophic gastritis’, rather than dietary deficiency. This causes food cobalamin malabsorption, which could require large doses of B12 to be effective (Eussen et al., 2005), although recent studies have shown efficacy with smaller doses (Bor et al., 2006; Blacher et al., 2007) as well as foods fortified with folic acid, B12 and/or B6 in the elderly (Tucker et al., 2004; Dhonukshe-Rutten et al., 2005; van Vliet et al., 2007; Winkles et al., 2008).
Our trial can be considered a public health scale ‘proof of principle’ study, following on from a high-dose, short-term intervention we reported in a small group of volunteers (Yajnik et al., 2007). The two studies have demonstrated an unequivocal role for B12 deficiency as contributing to hyperhomocysteinemia in our population. It is of interest that our interventions have not reduced the plasma tHcy concentrations to those in age-matched Europids, suggesting that other factors also contribute to hyperhomocysteinemia in this population. Such factors may be protein malnutrition (Ingenbleek et al., 2002), low methionine intake (Elshorbagy et al., 2009), or deficiency of riboflavin (Hustad et al., 2000) or pyridoxine (Selhub J, 1999). However, it is rewarding that we were able to shift the distribution of plasma homocysteine to more favorable concentrations and this might contribute to a better risk reduction in the population than concentrating on the relatively smaller number with hyperhomocysteinemia (Rose G, 1985). There is scope for further investigation to find the etiology of the residual hyperhomocysteinemia, including the role of “tropical sprue-like” conditions.
In the meanwhile, public health specialists may build on our results and plan large-scale community based strategies to improve B12 nutrition of Indians at different stages of the life-cycle. Of particular relevance will be to include B12 along with folic acid in the National Nutritional Anemia Control Program or in the proposed food-fortification.
Supplementary Material
Acknowledgements
We are grateful to the community, in particular the children and their families from PMNS, for taking part in this study and to NuLife Pharmaceuticals Ltd, India for free supply of the study medicine. We thank Dr K J Coyaji, Director, KEM Hospital for providing facilities. We are grateful to Pallavi C Yajnik, the staff at Diabetes Unit and the paramedical field workers for practical assistance during the trial, to Prof Elaine Rush for her comments, and to Carole Johnston for help in laboratory analysis.
Funding source: The study is supported by the Wellcome Trust, London, UK.
Footnotes
TRIAL REGISTRATION: Current Controlled Trials ISRCTN 59289820.
Conflict of interest statement: The authors declare that there is no duality of interest associated with this manuscript.
Supplementary information is available at European Journal of Clinical Nutrition's website.
References
- Bhat DS, Thuse NV, Lubree HG, Joglekar CV, Naik SS, Ramdas LV, et al. Increases in plasma holotranscobalamin can be used to assess vitamin B-12 absorption in individuals with low plasma vitamin B-12. J Nutr. 2009;139:2119–23. doi: 10.3945/jn.109.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate V, Deshpande S, Bhat D, Joshi N, Ladkat R, Watve S, et al. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr Bull. 2008;29:249–254. doi: 10.1177/156482650802900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher J, Czernichow S, Raphael M, Roussel C, Chadefaux-Vekemans B, Morineau G, et al. Very low oral doses of vitamin B-12 increase serum concentrations in elderly subjects with food-bound vitamin B-12 malabsorption. J Nutr. 2007;137:373–378. doi: 10.1093/jn/137.2.373. [DOI] [PubMed] [Google Scholar]
- Bor MV, Lydeking-Olsen E, Moller J, Nexo E. A daily intake of approximately 6 microg vitamin B-12 appears to saturate all the vitamin B-12-related variables in Danish postmenopausal women. Am J Clin Nutr. 2006;83:52–58. doi: 10.1093/ajcn/83.1.52. [DOI] [PubMed] [Google Scholar]
- Carmel R. Efficacy and safety of fortification and supplementation with vitamin B12: biochemical and physiological effects. Food Nutr Bull. 2008;29:S177–87. doi: 10.1177/15648265080292S121. [DOI] [PubMed] [Google Scholar]
- Chambers JC, Obeid OA, Refsum H, Ueland P, Hackett D, Hooper J, et al. Plasma homocysteine concentrations and risk of coronary heart disease in UK Indian Asian and European men. Lancet. 2000;355:523–527. doi: 10.1016/S0140-6736(99)93019-2. [DOI] [PubMed] [Google Scholar]
- Chandalia M, Abate N, Cabo-Chan AVJ, Devaraj S, Jialal I, Grundy SM. Hyperhomocysteinemia in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88:1089–1095. doi: 10.1210/jc.2002-021133. [DOI] [PubMed] [Google Scholar]
- Clarke R, Grimley EJ, Schneede J, Nexo E, Bates C, Fletcher A, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33:34–41. doi: 10.1093/ageing/afg109. [DOI] [PubMed] [Google Scholar]
- Dhonukshe-Rutten RA, van ZM, de Groot LC, Eussen SJ, Blom HJ, van Staveren WA. Effect of supplementation with cobalamin carried either by a milk product or a capsule in mildly cobalamin-deficient elderly Dutch persons. Am J Clin Nutr. 2005;82:568–574. doi: 10.1093/ajcn.82.3.568. [DOI] [PubMed] [Google Scholar]
- Elshorbagy AK, et al. Sulfur amino acids in methionine-restricted rats: Hyperhomocysteinemia. Nutrition. 2009 doi: 10.1016/j.nut.2009.09.017. doi:10.1016/j.nut.2009.09.017 (in press) [DOI] [PubMed] [Google Scholar]
- Eussen SJ, de Groot LC, Clarke R, Schneede J, Ueland PM, Hoefnagels WH, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Arch Intern Med. 2005;165:1167–1172. doi: 10.1001/archinte.165.10.1167. [DOI] [PubMed] [Google Scholar]
- Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–9. [PubMed] [Google Scholar]
- Hustad S, Ueland PM, Vollset SE, Zhang Y, Bjørke-Monsen AL, Schneede J. Riboflavin as a determinant of plasma total homocysteine: effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem. 2000;46:1065–71. [PubMed] [Google Scholar]
- Ingenbleek Y, Hardillier E, Jung L. Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition. 2002;18:40–6. doi: 10.1016/s0899-9007(01)00783-3. [DOI] [PubMed] [Google Scholar]
- Kelleher BP, Broin SD. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J Clin Pathol. 1991;44:592–595. doi: 10.1136/jcp.44.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher BP, Walshe KG, Scott JM, O’Broin SD. Microbiological assay for vitamin B12 with use of a colistin-sulfate-resistant organism. Clin Chem. 1987;33:52–4. [PubMed] [Google Scholar]
- Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131:1217–1224. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–241. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- Rose G. Sick individuals and sick populations. International Journal of Epidemiology. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–46. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Selhub J. Public health significance of elevated homocysteine. Food Nutr Bull. 2008;29:S116–S125. doi: 10.1177/15648265080292S116. [DOI] [PubMed] [Google Scholar]
- Shipchandler M, Moore E. Rapid, fully automated measurement of plasma homocyst(e)ine with the Abbott IMx analyzer. Clin Chem. 1995;41:991–994. [PubMed] [Google Scholar]
- Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008;29:S143–72. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- Tamura T, Freeberg L, Cornwell P. Inhibition of EDTA of growth of Lactobacillus casei in the folate microbiological assay and its reversal by added manganese or iron. Clin Chem. 1990;36:1993. [PubMed] [Google Scholar]
- The Flour Fortification Initiative website Meeting on flour fortification in India. http://www.sph.emory.edu/wheatflour/IndiaMeeting/ (accessed 19th March 2009)
- Tucker KL, Olson B, Bakun P, Dallal GE, Selhub J, Rosenberg IH. Breakfast cereal fortified with folic acid, vitamin B-6, and vitamin B-12 increases vitamin concentrations and reduces homocysteine concentrations: a randomized trial. Am J Clin Nutr. 2004;79:805–811. doi: 10.1093/ajcn/79.5.805. [DOI] [PubMed] [Google Scholar]
- van VT, Jacobs RG, de DE, van den Berg H, de BA, van der Put NM. Effect of fortified spread on homocysteine concentration in apparently healthy volunteers. Eur J Clin Nutr. 2007;61:769–778. doi: 10.1038/sj.ejcn.1602570. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71:962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkels RM, Brouwer IA, Clarke R, Katan MB, Verhoef P. Bread cofortified with folic acid and vitamin B-12 improves the folate and vitamin B-12 status of healthy older people: a randomized controlled trial. Am J Clin Nutr. 2008;88:348–355. doi: 10.1093/ajcn/88.2.348. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, Uradey BS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–782. [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. doi: 10.1007/s00125-007-0793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajnik CS, Deshpande SS, Panchanadikar AV, Naik SS, Deshpande JA, Coyaji KJ, et al. Maternal total homocysteine concentration and neonatal size in India. Asia Pac J Clin Nutr. 2005;14:179–181. [PubMed] [Google Scholar]
- Yajnik CS, Lubree HG, Thuse NV, Ramdas LV, Deshpande SS, Deshpande VU, et al. Oral vitamin B12 supplementation reduces plasma total homocysteine concentration in women in India. Asia Pac J Clin Nutr. 2007;16:103–109. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


