Abstract
Both in vivo and in vitro, neurofilaments (NFs) are among the most highly phosphorylated proteins known. The majority of the NF phosphorylation sites reside on the carboxyl-terminal tails of the proteins. We have isolated and characterized an effector-independent neurofilament-specific protein kinase from bovine spinal cord that is associated with the NF complex and exhibits a marked substrate specificity for NF-H, the largest subunit of the NF triplet. This kinase activity emerges from a NF-conjugated affinity column coincident with a 67-kDa doublet on NaDodSO4/polyacrylamide gels and has a purity of greater than 90%. The purified enzyme exclusively phosphorylates NF-H tails and is dependent on prior phosphorylation of this molecule. The enzyme is also not autophosphorylated. While the molecular properties and substrate specificities of the NF kinase distinguish it from cAMP-dependent protein kinase, protein kinase C, Ca2+/calmodulin kinase, and casein kinases I and II, it exhibits certain properties similar to, but different from, the growth-associated histone H1 kinase. The molecular properties and specific sequence requirements of the NF kinase suggest that this enzyme could play a pivotal role in the phosphorylation of NFs in normal and pathological states such as Alzheimer disease, where NFs are hyperphosphorylated.
Full text
PDF
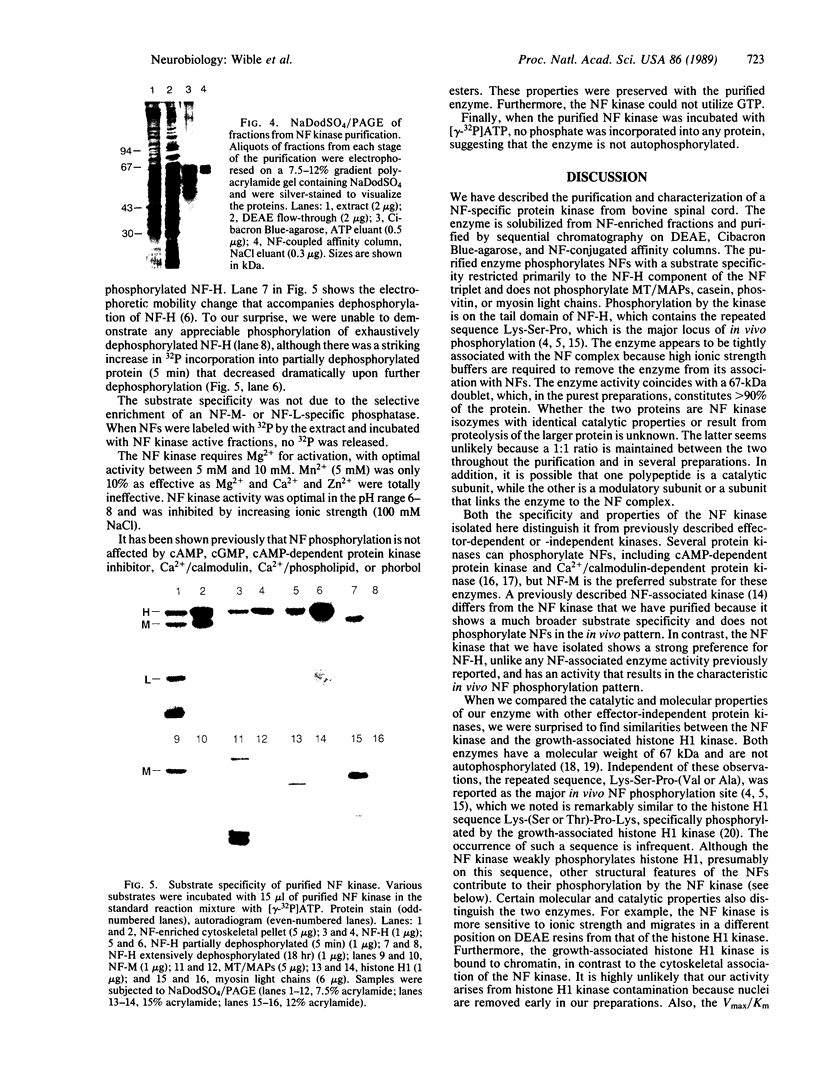

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Castellani L., Cohen C. Myosin rod phosphorylation and the catch state of molluscan muscles. Science. 1987 Jan 16;235(4786):334–337. doi: 10.1126/science.3026049. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Geisler N., Vandekerckhove J., Weber K. Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 1987 Sep 14;221(2):403–407. doi: 10.1016/0014-5793(87)80964-x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J. 1988 Jan;7(1):15–20. doi: 10.1002/j.1460-2075.1988.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987 Aug 13;328(6131):649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983 Mar 25;258(6):4019–4025. [PubMed] [Google Scholar]
- Kaufmann E., Geisler N., Weber K. SDS-PAGE strongly overestimates the molecular masses of the neurofilament proteins. FEBS Lett. 1984 May 7;170(1):81–84. doi: 10.1016/0014-5793(84)81373-3. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Phosphatase and carbocyanine dye binding define different types of phosphate groups in mammalian neurofilaments. J Neurosci. 1987 Nov;7(11):3554–3560. doi: 10.1523/JNEUROSCI.07-11-03554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Preferential phosphorylation of the 150,000 molecular weight component of neurofilaments by a cyclic AMP-dependent, microtubule-associated protein kinase. J Cell Biol. 1981 Sep;90(3):755–760. doi: 10.1083/jcb.90.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano E. W., Chin S. S., Colman D. R., Liem R. K. Complete amino acid sequence and in vitro expression of rat NF-M, the middle molecular weight neurofilament protein. J Neurosci. 1987 Aug;7(8):2590–2599. [PMC free article] [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E. Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J Biol Chem. 1986 Dec 15;261(35):16298–16301. [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E., Marotta C. A. Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J Neurosci. 1987 Apr;7(4):1145–1158. doi: 10.1523/JNEUROSCI.07-04-01145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987 May;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Quirin-Stricker C. Histone H1 kinase from mouse plasmacytoma. Further characterization and molecular structure. Eur J Biochem. 1984 Jul 16;142(2):317–322. doi: 10.1111/j.1432-1033.1984.tb08288.x. [DOI] [PubMed] [Google Scholar]
- Robinson P. A., Wion D., Anderton B. H. Isolation of a cDNA for the rat heavy neurofilament polypeptide (NF-H). FEBS Lett. 1986 Dec 15;209(2):203–205. doi: 10.1016/0014-5793(86)81111-5. [DOI] [PubMed] [Google Scholar]
- Scott D., Smith K. E., O'Brien B. J., Angelides K. J. Characterization of mammalian neurofilament triplet proteins. Subunit stoichiometry and morphology of native and reconstituted filaments. J Biol Chem. 1985 Sep 5;260(19):10736–10747. [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toru-Delbauffe D., Pierre M., Osty J., Chantoux F., Francon J. Properties of neurofilament protein kinase. Biochem J. 1986 Apr 1;235(1):283–289. doi: 10.1042/bj2350283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallano M. L., Buckholz T. M., DeLorenzo R. J. Phosphorylation of neurofilament proteins by endogenous calcium/calmodulin-dependent protein kinase. Biochem Biophys Res Commun. 1985 Aug 15;130(3):957–963. doi: 10.1016/0006-291x(85)91708-5. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Wong J., Hutchison S. B., Liem R. K. An isoelectric variant of the 150,000-dalton neurofilament polypeptide. Evidence that phosphorylation state affects its association with the filament. J Biol Chem. 1984 Sep 10;259(17):10867–10874. [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford T. A., Pardee A. B. Histone H1 kinase in exponential and synchronous populations of Chinese hamster fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4669–4676. [PubMed] [Google Scholar]