Abstract
We sought to investigate whether APOE genotype is associated with unique profiles of cognitive functioning during early life. School-aged children (N = 147) received standardized achievement tests, the Rey-Osterrieth Complex Figure Test (Copy Condition; RCFT-CC), assessment of hand dominance for writing, and buccal swab testing to determine their APOE genotype. Significant differences were found on the RCFT-CC, with ε2-positive children performing worse on this measure relative to both ε3/3 (p = .032) and ε4-positive children (p = .018). Further, a higher prevalence of left-hand dominance for writing was observed among ε2-positive children (29.2%) relative to ε3/3 (8.9%) and ε4-positive children (6.1%; p = .012), although this finding did not account for the observed group differences on the RCFT-CC. Findings raise the possibility that in childhood, the ε2 allele may be associated with (a) decreased functioning in certain cognitive domains; and (b) factors associated with atypical hemispheric dominance. Results may be consistent with the theory of antagonistic pleiotropy, which suggests that APOE may have different protective effects at different developmental stages.
Keywords: Cognitive aging, neurodevelopment, apolipoprotein E, developmental neuropsychology, dementia, Alzheimer’s disease
1. Introduction
Alzheimer’s disease (AD) is associated with a number of risk factors, with the most prominent being possession of one or more ε4 alleles of the apolipoprotein E (APOE) gene (Corder et al., 1993). In addition to this established genetic risk factor, there is also evidence that the development of AD may be associated with a number of early-life risk factors, including poor perinatal conditions, sub-optimal early-life brain development and body growth, poor early-life socioeconomic conditions, and decreased cognitive reserve, including lower educational attainment (for review see Borenstein et al., 2006). The fact that these two sets of risk factors have in common an association with the development of AD raises the possibility that APOE-ε4 itself could be associated with the presence or absence of one or more of these early-life variables (Richards and Sacker, 2003). In other words, are individuals with the APOE-ε4 genotype at risk for both early-life and late-life cognitive compromise?
Efforts to address this question in children have produced intriguing and somewhat counterintuitive results. A small number of developmental studies have found evidence for protective effects of the ε4 allele during human prenatal, perinatal, and infancy periods of life, characterized by higher survival rates and better cognitive functioning in the face of illness and toxic exposure (Oria et al., 2005; Wright et al., 2003; Zetterberg et al., 2002). Furthermore, one study found evidence for a detrimental effect of the ε2 allele, characterized by its over-representation in a Scottish cohort of perinatal deaths (Becher et al., 2006). This finding seems contrary to expectations given that the ε2 allele has been shown to have protective properties against the development of AD later in life (Farrer et al., 1997). On the basis of these findings, it has been proposed that APOE may be an example of a gene that exhibits antagonistic pleiotropy (Wright et al., 2003), which is a theory that suggests, in part, that some genes may have different effects at different life stages (Albin, 1993; Williams, 1957).
In contrast to these findings, several studies have failed to find APOE-related differences in brain and cognitive functioning among children beyond infancy (Deary et al., 2002; Plomin et al., 1995; Turic et al., 2001). However, these analyses were generally restricted to investigation of general intellectual ability (i.e., IQ), with specific domains of cognition not examined. Therefore, especially in light of evidence of early life APOE-related differences in pre- and perinatal survival rates (Becher et al., 2006; Zetterberg et al., 2002), susceptibility to the adverse effects of illness and toxic exposure on cognition (Oria et al., 2005; Wright et al., 2003), brain functional differences using EEG (Alexander et al., 2007), and region-specific cortical morphology (Shaw et al., 2007), the question remains as to whether or not APOE genotype influences development of cognitive functions in children.
The aim of the current study was to further explore this question by examining achievement and visuospatial test performances in a sample of school-aged children and adolescents genotyped for APOE. In addition, APOE-related differences in hand dominance, which is often considered an indicator of early atypical brain and/or cognitive development (Satz, 1973), were assessed. Based on several studies that have found decreased cognitive functioning among ε4-positive adults (for review see Small et al., 2004) and the relative absence of cognitive decline among ε2-positive adults, we would predict that a similar pattern would be observed among our sample of school-aged children (i.e., worse performance among ε4-positive children relative to ε2-positive children). However, the studies reviewed above reporting an advantageous effect of the ε4 allele very early in human development and possibly a detrimental effect of the ε2 allele, suggest that the opposite hypothesis was also plausible (i.e., better performance among ε4-positive children relative to ε2-positive children).
2. Methods
The study was approved by the Institutional Review Boards of the University of California, San Diego and San Diego State University. Informed consent was obtained from a parent of each participant, and informed assent was obtained from each participant.
2.1 Subjects
Recruitment
Participants were recruited from a group of San Diego area charter middle schools and high schools. An email message was sent to parents of prospective children, and classroom presentations were made by the first author to explain the study. An “informational booth” was then set up outside the school during after-school hours. During this time, parents and students could obtain additional information about the study and sign an informed consent agreement to participate if they chose to do so, which included a release of information providing access to the child’s standardized group achievement test records.
Screening
Typically-developing children between the ages of 11 and 16 years were included in the study. Parents of participants were asked to complete an online demographic questionnaire pertaining to their child’s developmental, medical, educational, psychiatric, and family medical history. Exclusion criteria consisted of the following: First language learned was not English, color blindness, uncorrected visual impairment, upper extremity motor disability that may affect test performance on visual-motor tasks, genetic disorder known to affect central nervous system functioning (e.g., Fragile X), history of head injury with loss of consciousness for greater than 10 minutes, and a diagnosed seizure disorder. A history of learning and/or attentional problems was not exclusionary. In addition, due to the fact that the children were tested in their classroom groups (see Procedures below), it often occurred that a child was tested prior to their parent(s) completing the screening questionnaire. However, if it was determined, after a child was tested, that he or she did not meet inclusion criteria, their case was removed from further analyses. Overall, screening questionnaires were completed for approximately 80% of the sample. In the case where two or more siblings enrolled in the study, if applicable, either the male sibling and/or the sibling with achievement test data available were included.
2.2 Procedures
Once a number of students from a particular class had signed up to participate in the study, a lunchtime testing session was arranged with the teacher of that particular class. On the specified date, children participating in the study remained in their classroom during lunch and were administered the RCFT-CC (Osterrieth, 1944) in the group setting. Following administration of this measure, DNA samples were obtained from each child using a buccal swab technique (i.e., a mild brushing of the inside of the cheek). Then, as a group, the children received a complimentary pizza lunch for their participation (e.g., see Brown et al., 2005). Finally, standardized group achievement test records were requested from the school for each participant. This procedure was repeated several times over the course of a five-month period during which all data were collected.
DNA samples
As previously stated, DNA samples were obtained using a buccal swab technique. This is a very simple, noninvasive, and widely used procedure. The samples were genotyped for APOE allele type using a polymerase chain reaction based method (Corder et al., 1993).
Achievement tests
Results from the California Achievement Test, Sixth Edition (CTB/McGraw-Hill, 2001) were requested from each participant’s respective school. For some children the school did not have records on file; in these cases, copies of test results were requested from parents. The California Achievement Test, Sixth Edition (CAT-6) is a standardized, multiple-choice, group achievement test that assesses basic skills in four broad domains, including Language, Reading, Spelling, and Mathematics. The Language subtest assesses skills related to vocabulary, grammar, usage, and literary analysis. The Reading subtest assesses basic reading skills, and the Spelling subtest assesses basic spelling skills. The Mathematics subtest assesses basic math skills, including computation and problem solving. The CAT-6 has been used in previous neurocognitive research (Barbaresi et al., 2007; Esquivel and Lopez, 1988); for example, it has been used to assess the effects of chronic antiepileptic drug therapy on academic achievement (Tennison et al., 1998). Standardized Normal Curve Equivalent (NCE) scores provided for each subtest of the CAT-6 were used in the current study. NCE scores have a mean of 50 and a standard deviation of 21.06.
Visuospatial test
Standardized group achievement tests, including the CAT-6, are generally considered to be verbally-loaded tests. Therefore, in addition to examination of CAT-6 subtest scores, the Copy Condition of the Rey-Osterrieth Complex Figure Test (RCFT-CC) was administered to each child in the group setting and scored using the Taylor Scoring Criteria (Kolb and Whishaw, 1990; Waber and Holmes, 1985). The person who administered and scored the RCFT-CC (C.S.B.) was blind to subjects’ APOE genotype. A z-score was calculated for each child, which was then transformed to a NCE score in order to facilitate direct comparisons with CAT-6 subtest scores. The RCFT-CC has been widely used to assess visuospatial perception and construction in adults and children (Fischer and Loring, 2004).
Handedness
At the same time children completed the RCFT-CC, they were asked to report their handedness for writing. Because a small number of children neglected to report their handedness, their parents were contacted via email in order to obtain this information.
2.3 Statistical analyses
All statistical analyses were conducted using SPSS statistical software. RCFT-CC and CAT-6 subtest scores were found to be generally normally distributed and variances among the genotype groups were observed to be roughly equal. Data were also screened for the presence of extreme cases.
Univariate ANOVA was employed to examine differences in mean RCFT-CC and CAT-6 subtest scores as a function of APOE genotype and as a function of gender. ANOVA is robust, even to moderate departures from homogeneity of variance (Box, 1954), which is noted given that standard deviations for each of the genotype groups are unequal (see Table 2). Gender was included in the analysis given that there are differential prevalence rates among boys and girls with respect to a number of neurodevelopmental disorders that impact cognition and are thought to have a genetic component (e.g., specific language impairment). In addition, there is some evidence that the presence of the ε4 allele appears to confer a greater risk for cognitive decline (Hyman et al., 1996) and AD (Farrer et al., 1997; Martinez et al., 1998; Payami et al., 1996) on adult women relative to men. Significant main effects of APOE genotype group (i.e., differences between ε2-positive children versus ε3/3 homozygotes versus ε4-postive children) were followed up by testing all pairwise comparisons utilizing Tukey’s HSD procedure. It should be noted that in this case, where group sample sizes are unequal, requesting Tukey’s HSD procedure in SPSS will actually produce the Tukey-Kramer test, which is based on the harmonic mean and appropriate for unequal group sizes (Toothacker, 1993). Chi-square tests were used to examine prevalence rates of left- and right-hand dominance for writing as a function of APOE genotype, and differences in test scores as a function of handedness were investigated using Mann-Whitney U tests. Given that investigation of these research questions in a sample of school-aged children can be considered exploratory, an alpha level of 0.05 was used in the interpretation of all results.
Table 2.
Mean Cognitive and Achievement Test Performance by APOE Genotype
| Test | ε2+ | ε3/3 | ε4+ | pc |
|---|---|---|---|---|
| RCFT-CCa | 45.32 (16.19) | 52.58 (12.32) | 54.56 (8.74) | .024* |
| Mathb | 67.67 (17.78) | 62.86 (20.15) | 61.43 (19.48) | .536 |
| Languageb | 70.86 (16.46) | 66.02 (21.80) | 64.79 (19.71) | .574 |
| Readingb | 69.62 (18.31) | 65.86 (19.88) | 65.25 (15.06) | .727 |
| Spellingb | 66.52 (15.60) | 63.19 (19.11) | 64.96 (24.95) | .692 |
Based on n = 145; data presented as mean (standard deviation).
Based on n = 134; data presented as mean (standard deviation).
Represents main effect of APOE genotype.
2.4 Sample size and characteristics
A total of 196 school-aged children and adolescents were enrolled into the study. Of those, 177 participated in a lunchtime testing session, which included completion of the RCFT-CC and buccal swab testing. APOE genotype results were obtained for 163 of these subjects. Four female participants were excluded because each of them already had a sibling who was in the study, and 8 children were excluded because they did not meet inclusion criteria. Also, because the aim of this study was to assess the independent effects of the APOE-ε4 allele in the absence of the potential confounding effects of the APOE-ε2 allele, a total of 4 ε2/4 heterozygotes were excluded from the analyses. Thus, the study included a total of 147 subjects for whom basic demographic data, APOE genotype, RCFT-CC, and handedness results were obtained.
In addition, there were 2 subjects (i.e., 1 ε3/3 homozygote and 1 ε3/4 heterozygote) for whom RCFT-CC scores fell more than three standard deviations below the mean and were thus considered to be outliers. These two cases were removed from all subsequent analyses of RCFT-CC scores only. Further, because 13 subjects who otherwise met inclusion criteria either had no group achievement test data available, or had group achievement test reports available from a test other than the CAT-6 (e.g., the Stanford Achievement Test, Ninth Edition), the study included a subset of 134 subjects for whom group achievement test data were available and analyzed.
3. Results
3.1 Basic demographics
APOE allelic frequencies and other basic demographic data presented as a function of genotype can be found in Table 1. The reader should note that the ε2-positive group consisted entirely of subjects who were ε2/3 heterozygotes, while the ε4-positive group included all ε3/4 heterozygotes with the exception of one subject who was an ε4/4 homozygote. With respect to the sample as a whole, ages ranged from 11.32 years to 16.84 years (M = 13.34, SD = 1.26), and there was a total of 63 boys (42.9%) and 84 girls (57.1%). The breakdown of the sample with regard to ethnicity was as follows: Asian, n = 10 (6.8%); African American, n = 9 (6.1%); Caucasian, n = 97 (66.0%); Filipino, n = 6 (4.1%); Hispanic, n = 23 (15.6%); and Other, n = 2 (1.4%). Parental educational attainment was assessed via parent-report, and these data were available for approximately 76.9% of the sample. Based on the information provided, years of education were determined according to widely-accepted criteria (Heaton et al., 2004). Maternal years of education ranged from 12 to 20 (M = 16.38, SD = 2.10), and paternal years of education ranged from 10 to 20 (M = 16.22, SD = 2.45).
Table 1.
Basic Demographic Information by Genotype
| ε2+ | ε3/3 | ε4+ | ||
|---|---|---|---|---|
| n = 24 | n = 90 | n = 33 | p | |
| Allele Frequency | 0.163 | 0.612 | 0.224 | n/a |
| Age at Exama | 13.54 (1.08) | 13.28 (1.30) | 13.34 (1.32) | .665b |
| Male/Female | 13/11 | 35/55 | 15/18 | .382c |
| Ethnicity | .185c | |||
| Asian | 1 | 8 | 1 | |
| African American | 2 | 2 | 5 | |
| Caucasian | 17 | 60 | 20 | |
| Filipino | 2 | 4 | 0 | |
| Hispanic | 2 | 14 | 7 | |
| Other | 0 | 2 | 0 | |
| Handedness (R/L) | 17/7 | 82/8 | 31/2 | .012*c |
| Parent Education in Yearsa | ||||
| Motherd | 16.65 (1.93) | 16.28 (2.15) | 16.46 (2.17) | .769b |
| Fathere | 16.75 (2.34) | 16.07 (2.44) | 16.21 (2.60) | .559b |
Data are presented as mean (standard deviation).
One-way ANOVA used to test group differences.
Chi-square used to test group differences.
Based on n = 113.
Based on n = 112.
3.2 Handedness
With respect to the whole sample, there were a total of 17 children (11.6%) who identified themselves as left-hand dominant for writing, which is slightly higher than adult population estimates (i.e., studies generally estimate that 5 to 10 percent of adults are left-handed), but consistent with previous estimates among children and adolescents, which suggest that 10 to 14 percent are left-handed (Annett, 2002; Briggs and Nebes, 1975). None of the children identified themselves as ambidextrous for writing.
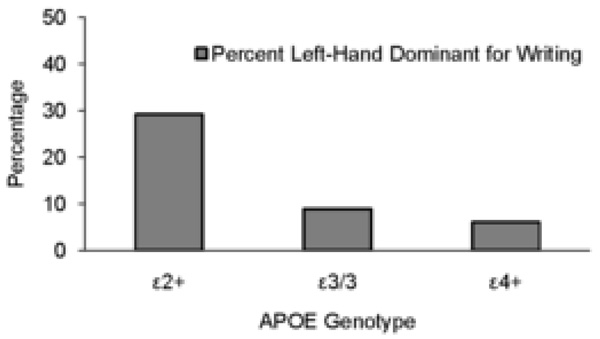
Analysis of prevalence rates of hand dominance for writing as a function of APOE genotype revealed a significantly higher percentage of left-handedness among the ε2-positive children, χ2(2) = 8.878, p = .012 (see Table 1). Specifically, 7 out of 24 subjects (29.2%) were left-handed in the ε2-positive group, 8 out of 90 subjects were left-handed (8.9%) in the ε3/3 group, and 2 out of 33 subjects were left-handed (6.1%) in the ε4-positive group (Figure 1). Because of this observed difference in prevalence rates of left-hand dominance as a function of APOE genotype and because there is evidence that cognitive test performance can be influenced by hand dominance (Gordon and Kravetz, 1991), separate follow-up analyses were conducted comparing test performance as a function of APOE genotype for the right-handed children only, and test performance as a function of handedness for the ε2-positive children only.
Figure 1. APOE Genotype and Left-Hand Dominance in School-Aged Children.
Depiction of the percentage of school-aged children within each APOE genotype group who self-report left-hand dominance for writing.
3.3 Cognitive test performance
Achievement test results
Analysis of CAT-6 scores failed to find a significant main effect of genotype (Table 2) or gender, or a significant interaction between genotype and gender with respect to any of the achievement subtests, including Reading, Math, Language, or Spelling, which was contrary to predictions.
Visuospatial test results
Analysis of RCFT-CC scores, however, revealed a significant main effect of genotype (F(2, 139) = 3.825, p = .024; ηp2 = .052; Table 2), and Tukey HSD post hoc tests found that ε2-positive subjects had significantly lower scores relative to both ε3/3 homozygotes (p = .032) and ε4-positive subjects (p = .018; Figure 2). The extent to which gender or the interaction between gender and genotype predicted RCFT-CC scores was also examined, but neither effect was significant.
Figure 2. APOE Genotype and Visuospatial Functioning in School-Aged Children.
Depiction of mean RCFT-CC scores as a function of APOE genotype among a sample of school-aged children. Error bars represent 95% confidence interval.
Cognitive test performance in left- versus right-handed subjects
Given the relatively large proportion of left-handed children in the ε2-positive group (29.2%), as well as the fact that differences in cognitive test performance as a function of handedness have been demonstrated (e.g., Gordon and Kravetz, 1991), analysis of test performance was repeated after excluding left-handed subjects. Consistent with results presented above, analysis of achievement test scores failed to find any significant differences between the APOE genotype groups (data not shown). Similarly, analysis of visuospatial test scores after excluding left-handed subjects also showed findings consistent with those presented above. Specifically, results with this smaller subset of children (i.e., n = 128) indicated a main effect of genotype that approached significance (F(2, 122) = 2.607, p = .078; ηp2 = .041). The potential influence of hand dominance among the ε2-positive subjects was also explored (Table 3). However, as shown, results of Mann-Whitney U tests failed to find any significant differences in test performances between ε2-positive left- and right-handed children. Overall, follow-up analyses to explore the influence of hand dominance on test performance among the genotype groups showed minimal, if any, influence of this potential confound.
Table 3.
Mean Cognitive Test Performance in Right- Versus Left-Handed ε2-positive Children
| ε2+ | |||
|---|---|---|---|
| Test | Right | Left | pc |
| RCFT-CCa | 46.33 (16.69) | 42.87 (15.88) | .567 |
| Mathb | 64.27 (16.73) | 76.17 (18.97) | .170 |
| Languageb | 73.07 (16.49) | 65.33 (16.46) | .310 |
| Readingb | 66.33 (17.56) | 77.83 (19.07) | .242 |
| Spellingb | 67.40 (16.56) | 64.33 (14.02) | .724 |
Based on n = 17 right-handed children and n = 7 left-handed children; data presented as mean (standard deviation).
Based on n = 15 right-handed children and n = 6 left-handed children; data presented as mean (standard deviation).
Mann-Whitney U tests used test differences between right- and left-handed subjects.
4. Discussion
The aim of the current study was to examine the extent to which APOE genotype influences cognitive functioning in childhood by investigating cognitive test performance and hand dominance in a sample of school-aged children and adolescents genotyped for APOE. Two primary findings emerged from this study. First, a higher prevalence of left-hand dominance for writing was observed among ε2-positive children (29.2%) relative to ε3/3 homozygotes (8.9%) and ε4-positive children (6.1%). Second, significant group differences as a function of APOE genotype were observed on a measure of visuospatial functioning (i.e., the RCFT-CC), with ε2-positive children performing significantly worse on this measure relative to both ε3/3 homozygotes and ε4-positive children. However, despite these APOE genotype group differences with respect to handedness and performance on the RCFT-CC, mean scores of all the genotype groups on both the RCFT-CC and each of the CAT-6 achievement subtests were within the average range. That is, at least with respect to the limited number and types of tests used in the current study, APOE is not associated with impaired test performance (i.e., NCE scores are greater than 1 standard deviation below average) for any of the genotype groups in this sample of school-aged children. This is generally consistent with previous findings that were restricted to general cognitive ability (e.g., Turic et al., 2001).
The finding of an increased prevalence of left-hand dominance for writing among ε2-positive children raises the possibility that the ε2 allele may influence or be associated with factors that give rise to atypical hemispheric dominance. In an effort to investigate this finding further, we examined prevalence rates of left-hand dominance among 650 elderly subjects identified from a larger pool of subjects participating in longitudinal studies of aging at the University of California, San Diego. Results from this sample of convenience showed small but statistically significant differences as a function of APOE genotype (χ2(2) = 8.878, p = .012) with 6 out of 51 subjects (11.8%) either left-hand dominant or ambidextrous in the ε2-positive group versus only 14 out of 293 subjects (4.8%) in the ε3/3 homozygote group and 16 out of 288 subjects (5.6%) in the ε4-positive group (Bloss, 2007). These findings are also consistent with those from previous studies that have reported a lower incidence of left-handedness compared to population norms among individuals with AD (de Leon et al., 1986; Doody et al., 1999).
While there are some possible explanations for the association between APOE genotype and hand dominance that are worth mentioning, at this juncture, these theories are quite speculative and not particularly compelling or empirically grounded. For instance, given that handedness is generally thought to be genetically determined (Annett, 2002), one possibility is that APOE is in linkage disequilibrium (LD) with genetic variants that may have some role in regulating brain asymmetry. Another possibility is that APOE itself could play a role with respect to the development of this phenotype given known differences in structure between the different APOE isoforms (Hatters et al., 2006), which have been shown to produce different functional consequences that may impact neurodevelopment (Weisgraber et al., 1982). Another interpretation of the association between the APOE-ε2 allele and left-hand dominance stems from evidence that left-handedness can also result from early trauma (e.g., adverse pre- or perinatal event), a concept known as “pathological left-handedness” (Satz, 1972). Somewhat consistent with the findings of Becher and colleagues (2006), this scenario would suggest that the ε2 allele would be associated with detrimental effects during the human pre- or perinatal period. This explanation, however, is quite speculative given that it is not known whether some proportion of children in the current study (e.g., the left-handed ε2-positive children) are pathologically left-handed, given that family history of left-handedness was not assessed. Overall, the role of APOE, if any, in regulating brain asymmetry, is likely subtle and involves complex interactions involving other genes, neural connectivity, and plasticity (i.e., gene-gene and gene-environment interactions).
These findings raise other questions and possibilities regarding the impact of APOE genotype across the human lifespan. For instance, how should the current findings of decreased cognitive test performance and increased prevalence of left-handedness among ε2-positive children be interpreted within the context of evidence suggesting protective effects associated with this allele during late life? Similarly, and consistent with our findings, other studies have found evidence for protective effects of the ε4 allele during early life, which certainly runs counter to very strong evidence of an association between APOE-ε4 and the development of AD later in life. To explain these seemingly incongruent findings, it has been proposed that APOE may be an example of a gene that exhibits antagonistic pleiotropy (Wright et al., 2003). This theory posits that genes can have different effects at different developmental stages, and that natural selection will favor genes conferring short-term benefits to an organism at the cost of detrimental effects that may occur during the post-reproductive years (Albin, 1993; Finch and Sapolsky, 1999; Williams, 1957). It may be that natural selection selects against the ε2 allele during the perinatal and infancy periods of life in favor of the ε4 allele, which may confer beneficial effects during this time. This early advantage would occur at the expense of negative effects of APOE-ε4 in late life. Thus, with respect to the current findings, if only the strongest or “fittest” ε2-positive children adapt well and survive their early years (i.e., the period during which this genetic variant may be associated with detrimental effects), factors associated with this survival, such as increased brain and/or cognitive reserve, confer later advantages such as relative protection from the onset of pathological aging (e.g., AD).
The current findings also suggest other theories with respect to the impact of APOE genotype across the human lifespan. First, it is possible that an increased prevalence of left-handedness in ε2-positive individuals may, more directly, contribute to the mechanism by which this allele confers protection against the development of AD. That is, because cognitive abilities are more widely distributed in the brains of some left-handed persons, these individuals may be less susceptible to the onset of AD pathology and/or the clinical expression of AD. Second, an association with left-handedness also suggests the notion that this genotype may serve as a risk factor for certain disorders (e.g., developmental learning disorders, immune disorders) found to be more prevalent in left-handed individuals (Geschwind and Behan, 1982). Third, an association between the ε2 allele and risk for adverse event(s) during infancy or early childhood could also theoretically contribute to its relatively low prevalence in the population, and similarly, a net benefit during neurodevelopment may keep the ε4 allele relatively common.
Results of a recent study of cortical morphology in children and adolescents with different APOE genotypes (Shaw et al., 2007) also have implications for the present findings. These investigators found APOE-related differences in cortical thickness in left entorhinal regions such that ε4-positive children had the thinnest cortex, ε2-positive children the thickest, and with ε3/3 homozygotes occupying an intermediate position (Shaw et al., 2007). Importantly, these authors concluded that the thinner cortex in ε4-positive children could represent a neural endophenotype that renders carriers more susceptible to neurodegenerative changes later in life, and that the thicker cortex in ε2-positive children may contribute to the so-called protective effects observed in adults with this variant. While a very plausible interpretation with respect to the late-life effects of this observed difference, it is also possible that there are immediate consequences (i.e., in childhood) of these region-specific differences in cortical thickness, including the possibility that the increased thickness associated with the ε2 allele may actually be detrimental during neurodevelopment. Evidence in support of this stems from studies of typically developing children that have found thicker cortex to be associated with decreased performance on tests of verbal learning and verbal intellectual functioning (Sowell et al., 2001; Sowell et al., 2004), and another more recent study (i.e., also with typically developing children) that found thicker cortex in large areas of the frontal, parietal, and temporal lobes to be associated with worse performance on the copy condition of the Rey Complex Figure test (Sowell et al., 2008), which was the same measure used in the current study. Thus, the finding of thicker cortex in ε2-positive children and adolescents could be quite consistent with the current findings, as well as the idea that APOE is an example of antagonistic pleiotropy. This idea is further supported by another recent study that found an association between better episodic memory performance and more efficient use of neural resources during functional neuroimaging among young adults with the APOE-ε4 allele (Mondadori et al., 2007).
Certainly, the results from the present study are considered exploratory and require validation in future studies, particularly given the following limitations. First, although this was not a significantly underpowered study, statistical power was not optimal for detecting the small effect sizes observed; for example, the main effect of APOE genotype group on RCFT-CC scores was small (ηp2 = .052), also raising the issue of clinical versus statistical significance. In addition, some characteristics of the sampling method (e.g., the majority of parents who were contacted regarding the study elected not to participate), could have biased the sample in some way. Another limitation is that the method used to assess handedness (i.e., child- or parent-report) was not ideal (i.e., the use of a thorough and standardized assessment of handedness would have been preferable), and handedness was only assessed for writing. Finally, to some extent the test measures used in this study represent tests of convenience (e.g., existing group achievement test data). In light of the fact that significant APOE genotype group differences emerged, individual administration of a comprehensive test battery that assessed a greater number of cognitive domains may have been more sensitive to other subtle differences in cognitive functioning between the genotype groups.
In conclusion, results of the current study failed to find lower mean test performance among ε4-positive children relative to ε2-positive children, as generally predicted. However, APOE genotype group differences emerged in the opposite direction, which was not entirely unexpected, and in fact suggest that the ε2-allele may be a risk factor for both lower cognitive test performance in childhood, as well as left-handedness. These findings are generally consistent with a small number of developmental studies suggesting that the presence of the ε4 allele may be beneficial early in life, and that alternatively, possession of one or more ε2 alleles may be detrimental during this phase. If it is the case that the ε2 allele is associated with poorer cognitive functioning in childhood, additional studies are needed to determine the nature and extent of this association. For example, the current study suggests that only certain cognitive domains are affected (i.e., no APOE genotype group differences were observed on verbally loaded tests of achievement). In addition, longitudinal cognitive assessment would be needed to determine at what age a “switch” may occur and the protective effects of APOE-ε2 coupled with the deleterious effects of APOE-ε4 may emerge.
Acknowledgements
Our deepest gratitude is extended to the children, families, and teachers who gave of their time to participate in this study. This work was supported, in part, by grants from the National Institutes of Health (K24 AG026431, R01 AG12674, and P50 AG05131) and by a Merit Review Research Program from the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement for Authors
None of the authors have any actual or potential conflicts of interest to disclose.
References
- Albin RL. Antagonistic pleiotropy, mutation accumulation, and human genetic disease. Genetica. 1993;91:279–286. doi: 10.1007/BF01436004. [DOI] [PubMed] [Google Scholar]
- Alexander DM, Williams LM, Gatt JM, Dobson-Stone C, Kuan SA, Todd EG, Schofield PR, Cooper NJ, Gordon E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol Psychol. 2007;75:229–238. doi: 10.1016/j.biopsycho.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Annett M. Handedness and Brain Asymmetry: The Right Shift Theory. New York: Taylor and Francis/Psychology Press; 2002. [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28:265–273. doi: 10.1097/DBP.0b013e31811ff87d. [DOI] [PubMed] [Google Scholar]
- Becher JC, Keeling JW, McIntosh N, Wyatt B, Bell J. The distribution of apolipoprotein E alleles in Scottish perinatal deaths. J Med Genet. 2006;43:414–418. doi: 10.1136/jmg.2005.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS. APOE genotype and cognitive functioning in school-aged children: A risk factor for decreased cognitive reserve or an example of antagonistic pleiotropy? Dissertation Abstracts International. 2007;68B (UMI No. 3258531) [Google Scholar]
- Borenstein AR, Copenhaver CI, Mortimer JA. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:63–72. doi: 10.1097/01.wad.0000201854.62116.d7. [DOI] [PubMed] [Google Scholar]
- Box G. Some theorems on quadratic forms applied in the study of analysis of variance problems. Annals of Statistics. 1954;25:290–302. [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex. 1975;11:230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Brown SA, Anderson KG, Schulte MT, Sintov ND, Frissell KC. Facilitating youth self-change through school-based intervention. Addict Behav. 2005;30:1797–1810. doi: 10.1016/j.addbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- CTB/McGraw-Hill. TerraNova. Second Edition. Monterey, CA: CTB/McGraw-Hill; 2001. [Google Scholar]
- de Leon MJ, la Regina ME, Ferris SH, Gentes CI, Miller JD. Reduced incidence of left-handedness in clinically diagnosed dementia of the Alzheimer type. Neurobiol Aging. 1986;7:161–164. doi: 10.1016/0197-4580(86)90037-0. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- Doody RS, Vacca JL, Massman PJ, Liao TY. The influence of handedness on the clinical presentation and neuropsychology of Alzheimer disease. Arch Neurol. 1999;56:1133–1137. doi: 10.1001/archneur.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Esquivel GB, Lopez E. Correlations among measures of cognitive ability, creativity, and academic achievement for gifted minority children. Percept Mot Skills. 1988;67:395–398. doi: 10.2466/pms.1988.67.2.395. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Finch CE, Sapolsky RM. The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Fischer JS, Loring DW. Construction. In: Lezak MD, Howieson DW, Loring DW, editors. Neuropsychological Assessment. New York, pp: Oxford University Press, Inc; 2004. pp. 531–568. [Google Scholar]
- Geschwind N, Behan P. Left-handedness: association with immune disease, migraine, and developmental learning disorder. Proc Natl Acad Sci U S A. 1982;79:5097–5100. doi: 10.1073/pnas.79.16.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW, Kravetz S. The influence of gender, handedness, and performance level on specialized cognitive functioning. Brain Cogn. 1991;15:37–61. doi: 10.1016/0278-2626(91)90014-y. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Hyman BT, Gomez-Isla T, Briggs M, Chung H, Nichols S, Kohout F, Wallace R. Apolipoprotein E and cognitive change in an elderly population. Ann Neurol. 1996;40:55–66. doi: 10.1002/ana.410400111. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw I. Fundamentals of Human Neuropsychology. New York: W.H. Freeman and Co; 1990. [Google Scholar]
- Martinez M, Campion D, Brice A, Hannequin D, Dubois B, Didierjean O, Michon A, Thomas-Anterion C, Puel M, Frebourg T, Agid Y, Clerget-Darpoux F. Apolipoprotein E epsilon4 allele and familial aggregation of Alzheimer disease. Arch Neurol. 1998;55:810–816. doi: 10.1001/archneur.55.6.810. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Boesiger P, Hock C, Nitsch RM, Papassotiropoulos A, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cereb Cortex. 2007;17:1934–1947. doi: 10.1093/cercor/bhl103. [DOI] [PubMed] [Google Scholar]
- Oria RB, Patrick PD, Zhang H, Lorntz B, de Castro Costa CM, Brito GA, Barrett LJ, Lima AA, Guerrant RL. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Contribution a l'etude de la perception et de la memoire. Archives de Psychologie. 1944;30:206–353. [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Yu CE, Wijsman EM, Heston LL, Litt M, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- Plomin R, McClearn GE, Smith DL, Skuder P, Vignetti S, Chorney MJ, Chorney K, Kasarda K, Kasarda S, Thompson LA, Detterman DK, Petrill SA, Daniels J, Owen MJ, McGuffin P. Allelic associations between 100 DNA markers and high versus low IQ. Intelligence. 1995;21:31–48. [Google Scholar]
- Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25:614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- Satz P. Pathological left-handedness: an explanatory model. Cortex. 1972;8:121–135. doi: 10.1016/s0010-9452(72)80013-3. [DOI] [PubMed] [Google Scholar]
- Satz P. Left-handedness and early brain insult: an explanation. Neuropsychologia. 1973;11:115–117. doi: 10.1016/0028-3932(73)90071-7. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennison M, Kankirawatana P, Bowman MR, Greenwood R, Lewis D, Burchinal M. Effect of chronic antiepileptic drug therapy on california achievement test scores. Journal of Epilepsy. 1998;11:208–214. [Google Scholar]
- Toothacker L. Multiple Comparisons Procedures. Thousand Oaks, CA: Sage Publications; 1993. [Google Scholar]
- Turic D, Fisher PJ, Plomin R, Owen MJ. No association between apolipoprotein E polymorphisms and general cognitive ability in children. Neurosci Lett. 2001;299:97–100. doi: 10.1016/s0304-3940(00)01789-4. [DOI] [PubMed] [Google Scholar]
- Waber DP, Holmes JM. Assessing children's copy productions of the Rey-Osterrieth Complex Figure. J Clin Exp Neuropsychol. 1985;7:264–280. doi: 10.1080/01688638508401259. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–2521. [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandez-Avila M. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr Res. 2003;54:819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Palmer M, Ricksten A, Poirier J, Palmqvist L, Rymo L, Zafiropoulos A, Arvanitis DA, Spandidos DA, Blennow K. Influence of the apolipoprotein E epsilon4 allele on human embryonic development. Neurosci Lett. 2002;324:189–192. doi: 10.1016/s0304-3940(02)00198-2. [DOI] [PubMed] [Google Scholar]