Abstract
The body senses “danger” from “damaged self” molecules through members of the same receptor superfamily it uses for microbial “non-self”, triggering canonical signaling pathways that lead to the generation of acute inflammatory responses. For this reason, the biology of normal tissue responses to moderate and clinically relevant doses of radiation is inextricably connected to innate immunity. The complex sequence of inflammatory events that ensues causes further cell and tissue damage to eliminate potential invaders but also leads to cytoprotective responses that limit the spread of damage and to wound healing through tissue regeneration or replacement. These sequential processes are orchestrated through multiple feedback control mechanisms involving cyclical production of free radicals and cytokines that are common to both radiation and immune signaling. This requires a concerted effort by resident tissue and inflammatory cell types, with macrophages apparently leading the way. The initial response to moderate doses of radiation therefore feeds into a pro-inflammatory paradigm whose eventual outcome is critically dependent upon the properties of the immune cells that are involved in tissue damage, regeneration and repair and that are in part under genetic influence. Importantly, these canonical pathways provide targets for interventions aimed at modifying normal tissue radiation responses. In this review, we examine areas of intersection between innate immunity and normal tissue radiobiology.
INTRODUCTION
Mammals have evolved complex mechanisms to maintain tissue homeostasis in the face of a vast array of challenges. More than any other category of agent, microbes have shaped host immunity during evolution. Responses to other, more eclectic challenges like those posed by moderate doses of ionizing radiation are not tailored but are dealt with through the same canonical pathways that orchestrate host defense and maintenance of tissue homeostasis. The biology of normal tissue responses to radiation therefore involves the immune system in multiple ways. This review aims to cover those specific aspects of tissue inflammation and immunity that we believe overlap and inform most on processes relevant to normal tissue radiobiology.
PATTERN RECOGNITION RECEPTORS AND THE IMMUNE SYSTEM
A major recent development in innate immunity is the realization that microbial “non-self” and “damaged self” are recognized by a shared system of receptors. Acute inflammation is generated to deal with the “danger” inherent in such situations. Surprisingly, as will be discussed later, non-pathogenic commensal microbes that are tolerated by the body are recognized by the same system, suggesting regulatory control that is dictated by the nature of the challenge. Janeway (1) first fully recognized that innate immune cells must have evolved a system for recognizing conserved “non-self” microbial products through pattern recognition receptors (PRRs). His group later identified a Toll-like receptor (TLR) as a key PRR capable of activating innate immune responses to bacterial lipopolysaccharide (LPS) (2). Recently, the spectrum of PRRs has been widened to encompass intracellular nucleotide binding oligomerization domain (NOD)-like and retinoic acid inducible gene (Rig)-like receptors (3) and C-type lectins (4).
PRRs were originally proposed as a recognition system for exogenous (microbial) pathogen-associated molecular patterns (PAMPS). However, the same receptor superfamily was later found to recognize endogenous damage-associated molecular patterns (DAMPS), also known as “alarmins” (5, 6). This suggests that PRRs may have a role in maintaining tissue homeostasis, wound healing and tissue regeneration after damage, for which there is some evidence (7). It would therefore be surprising if they were not major players in radiation-induced normal tissue damage and repair.
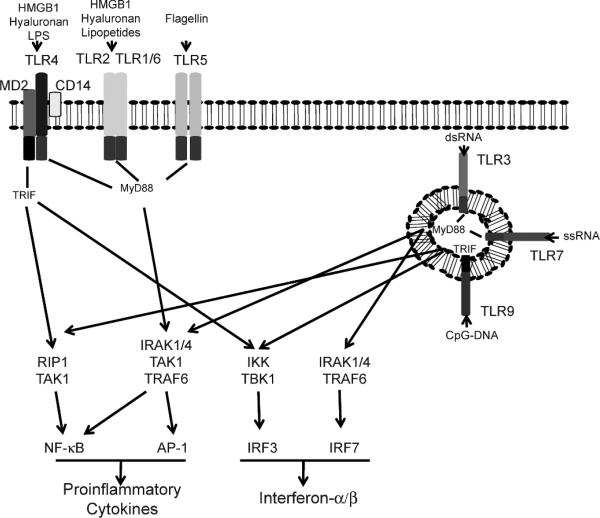
Most of what we know about PRRs today concerns TLRs, and we will focus on these as a model for how PRRs interface with our microbial world and with tissue damage to maintain homeostasis. TLRs are evolutionary ancient sensors that lie at the heart of our innate immune system. They are members of a superfamily of receptors that has homology to the drosophila Toll protein, but also to IL-1RI; all members share a TIR (Toll-IL-1 receptor) domain. There are currently about 12 members of the TLR subfamily, with some species variation (8). The ligands for some TLRs have yet to be identified, but TLR4/MD2 dimers are particularly important in the response to LPS, a process that also involves CD14 in the formation of an activation cluster and sends a stronger signal than other TLRs. Of the other TLRs, TLR2 can form heterodimers with TLR1 or TLR6 and responds to lipopeptide components of gram-positive and -negative bacteria, and TLR5 recognizes bacterial flagellins. In contrast to these cell surface dimers, TLR3, TLR7 and TLR9 are intracellular receptors that sense mainly microbial RNA and DNA, as do NOD- and RIG-like receptors (Fig. 1) [reviewed in ref. (7)].
FIG. 1.
The Toll-like receptor system. DAMP and PAMP ligands activate dTLRs in the plasma membrane (TLR4/2/1/6/5) or in lysosomal vesicles (TLR3/7/9) through MyD88 or TRIF adapter proteins. NF-κB, AP1 or IRF3/7 transcription factors result in production of pro-inflammatory cytokines and type I interferons.
DAMPS that we know are recognized by TLRs include the high-mobility-group box 1 (HMGB1) proteins. These are abundant chromatin-binding proteins that bind within the minor DNA groove and are released from damaged and activated cells. They share with LPS the ability to activate TLR4/MD2 but may also activate TLR2 (9). Other DAMPS include heat-shock proteins, degradation products of extracellular matrix (surfactant protein A, fibronectin extradomain A and hyaluronan fragments), and other damage-associated proteins, such as beta defensin, uric acid, S100, minimally modified LDL and possibly proteins damaged by reactive oxygen species (ROS) (5). As is the case with microbial nucleotides, endogenous DNA and DNA-activated autoantigens activate cells through TLR9, and the role of TLRs in various human autoimmune diseases is therefore an area of intense research (10). The exact requirements for a molecule to act as a DAMP is not clear, but primarily only TLR2 and TLR4/MD2 seem to act as receptors. Presumably this restricts the response that can be made, as may the fact that a high concentration of DAMP is needed for TLR activation. Such control mechanisms must exist to ensure that the need for the response outweighs the damage that inflammation might cause.
In the real world, multiple TLRs will respond simultaneously to a challenge and the cellular distribution of the TLRs, their co-receptors and accessory proteins, and their downstream adaptor molecules form a mosaic signal that orchestrates the response. In general, immune cells express varying TLR profiles and were thought for a while to be the only players. But recently, epithelial, fibroblast and other cells also have been found to express TLRs, and although they may have a more restricted profile, they appear to be functionally important (see below). In spite of their complexity, all TLRs essentially signal through the adapter proteins, MyD88 and/or TRIF (11), to activate primarily the transcription factors NF-κB and AP-1 and interferon regulatory factor (IRF) 3 or 7 (Fig. 1). Although this is an oversimplification, it is certainly true that the pathways are restricted and the target genes for NF-κB and AP-1 activation are pro-inflammatory cytokines, while IRFs signal type I interferon production. In fact, a spectrum of cytokines is produced in keeping with the need to eliminate viruses and intracellular bacteria on the one hand and deal with extracellular microbes on the other. How these polarized responses are orchestrated is not clear, but understanding the mechanism(s) will be important if we are to manipulate such responses for therapeutic benefit. In particular, while we know that radiation generates pro-inflammatory cytokines, the MyD88/TRIF dependence of the profile in different tissues has not yet been defined.
A compelling aspect of PRR signaling is that PAMPS and DAMPS can be classified as “danger” signals (12) that link inflammation to antigen-specific immunity (5, 13). The signals that are generated can license immature dendritic cells (DCs), which normally maintain peripheral immune tolerance, to mature into potent antigen presenting cells that initiate antigen-specific immunity (14, 15). For DAMPS, this must be carefully controlled since autoimmunity is the flip side of this coin.
PRRs in Radiation and Immunity
The importance of PRR signaling in radiation responses has yet to be fully explored, but there are compelling hints as to its relevance. In retrospect, the older findings that LPS and IL-1 protect mice against lethal whole-body irradiation (16) might now be seen as implicating the Toll-IL-1R superfamily. In fact, radiation has been shown to affect expression of TLR-related molecules. Shan et al. (17) reported that 5 cGy to 2 Gy increased TLR4/MD2 and CD14 expression on mouse macrophages as well as elevating intracellular levels of MyD88, and this was thought to be responsible for their radiation-enhanced secretion of IL-12 and IL-18. More recently, a homolog of TLR4, but lacking the TIR domain, called radioprotective 105 (RP105) has been discovered in mouse B cells (18). Its co-receptor, MD1, is a homolog of MD2. An antibody to RP105 caused B cells to proliferate and protected them against radiation-induced apoptosis. RP105 is also expressed in myeloid cells and, at least in some systems, it serves as a negative regulator of LPS/TLR4 signaling and cytokine production (19). Thus, in the lung, while TLR4–/–/TLR2–/– and MyD88–/– mice are more sensitive to bleomycin-induced epithelial injury and have decreased survival, the opposite seems to be true for RP105–/– mice (19). Further work is needed to elucidate the situations in which TLR4 or RP105 is dominant, but clearly these mutually antagonistic signaling pathways may dictate pro-inflammatory cytokine production in response to radiation and TLRs may serve as useful targets for radiotherapeutic intervention.
The most compelling emerging concept is that radiation-induced DAMP signaling through TLR4 and TLR2 might affect the outcome of cancer treatment. Apetoh et al. have shown that radiation releases HMGB1 from dying tumor cells and that HMGB1 is mandatory for host DCs to become licensed to present tumor antigens and generate tumor-specific immunity (9). Intriguingly, patients with breast cancer who carry a TLR4 loss-of-function allele, which prevents HMGB1 binding, relapse more quickly after radiotherapy and chemotherapy than those carrying the normal TLR4 allele (9).
The relationship between infection and cancer regression has a long history and has prompted many attempts to use microbial products for cancer immunotherapy, as with Coley's toxins in the early 20th century. Pathologists have frequently shown inflammation to correlate with the outcome of cancer treatment, for example in colorectal cancer (20), and this response may in fact be a little-recognized factor in conventional treatment success (21). It seems likely that the discovery of TLRs will herald a new era of investigation into how the host balances anti-tumor reactivity and normal tissue damage after radiation therapy and how to rebalance this equation to encourage a favorable outcome. In fact, we have known since the beginning of radiation therapy that it has a pro-inflammatory component, and numerous studies have explored the subsequent dialogue between the immune system and the mesenchymal and epithelial components that is required for successful tissue repair. The recent discovery that PRRs are not the sole property of immune cells but are expressed on other lineages, including epithelial cells (22, 23), and are key players in this dialogue forces a reassessment of these lines of communication in irradiated tissues.
PRRS ON EPITHELIAL SURFACES
The fact that DAMPS and PAMPS can signal through TLRs suggests that sterile and non-sterile inflammatory stimuli share canonical pathways, at least in part. In reality, after radiation exposure, epithelial barrier function is often compromised and microbial invasion is facilitated, so this distinction may be moot at times. However, it is true that the colon, for example, shows considerable tolerance to the >1012 commensal microbes it contains. Although it has long been known that there is a dynamic interaction between the host immune system and these commensals and that they are a critical element in shaping the repertoire of potential immune responses (24), the discovery that epithelial cells can express PRRs and are involved in barrier maintenance and regeneration of epithelial tissue (7) challenges our concepts of how the host immune system deals with barrier challenges and has profound implications for normal tissue radiobiology.
PRRs, Epithelial Barriers and Radiation Sensitivity
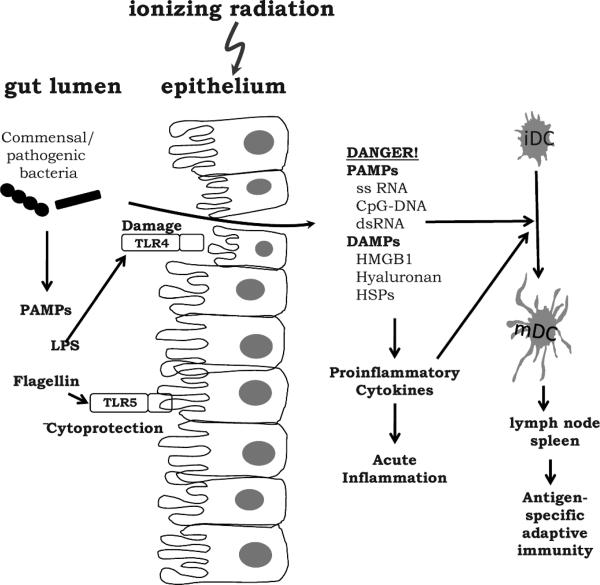
While the array of PRRs on epithelial cells (Fig. 2) and their sometimes mutually antagonistic actions present a complex picture, PRRs clearly act at the barrier level to modulate responses to microbial and other potentially damaging challenges (23, 25). Thus, in the gut, TLR4 can mediate internalization of E. coli by enterocytes and their translocation to mesenteric lymph nodes (26) and exacerbate the development of colitis in response to gram-negative organisms (27). Probiotic bacteria, in contrast, inhibit TLR4/NF-κB signaling and the associated pro-inflammatory cytokine production that causes colitis (28), suggesting that they trigger counteractive signaling pathways. Somewhat paradoxically, TLR4 protected against dextran sulfate-induced colitis (29), as it did in the lung response to bleomycin (19). PRRs therefore seem able to respond differently depending on the challenge. Since TLR ligands are present on both non-pathogenic and pathogenic bacteria, it is unclear how the host remains tolerant to one while responding to the other. The most likely explanation is that under normal conditions the system is under negative regulation, and this has to be overcome to trigger cytokine production. Recently, a Toll-IL-1R member, SIGIRR, was found that negatively regulated TLR activation in colon epithelial cells (30). Since it is commonly believed that chronic production of pro-inflammatory cytokines is responsible not only for colitis but also for neoplastic transformation of colon epithelial cells (31), understanding these regulatory mechanisms is of paramount importance. It would be surprising if the response of the gut to radiation was not determined in part by the microbiota and host PRR profiles.
FIG. 2.
The Toll-like receptor system in the intestine. TLR signaling at the epithelial surface can be protective or harmful to barrier function or can assist bacterial translocation to lymph nodes. Microbial products (PAMPS) that pass the barrier stimulate an acute inflammatory response with the release of pro-inflammatory cytokines, DAMPS and PAMPS that cause more inflammation and that license dendritic cells to acquire the ability to present antigens to the adaptive immune system in the lymph nodes and spleen.
In fact, we already know this to be the case and, furthermore, that direct targeting of PRRs can be a useful strategy for ameliorating radiation-induced damage. The field is still in its infancy, but the TLR5 ligand flagellin has been found to protect enterocytes against radiation-induced apoptosis (32) and mice against whole-body irradiation (33, 34). Also, in the lung, flagellin initiates an early TLR5-dependent inflammatory response that has been ascribed to stimulation of radiation-resistant epithelial cells, although macrophages cannot be excluded (35). Interestingly, radiobiological literature dating back to the 1960s shows that the basal intestinal crypt turnover rate depends upon the strength of the microbial stimulus, being slower in germ-free than conventionally housed mice (36, 37). It would be interesting to know the contribution of PRRs in such a model.
Radiobiologists are keenly aware of the contribution of epithelial cell regeneration to normal tissue radiation damage, in particular in a tissue that has rapid turnover like the gut. It is therefore of interest that TLR4/TLR2/MyD88 signaling has been shown to stimulate the proliferation of epithelial progenitor cells in the gut in response to injury (38). This regenerative response is transmitted through macrophages (38), but it requires TLR expression by epithelial cells; similar pathways may mediate crypt regeneration after radiation exposure. Because of the focus of PRRs on microbes, their role in human radiation-induced colitis clearly requires further investigation. Clinical interventions with TLR agonists or probiotics (39) might be of value in prevention or mitigation of radiation-induced intestinal damage and its repair.
In disease situations, it is often difficult to extricate the role of PRRs on epithelial cells from those of lymphoid cells in subepithelial and systemic compartments and, in reality, responses by both compartments are important. Under normal conditions, the continuing host-microbe interplay maintains the delicate balance between responding to pathogens and maintaining tolerance to harmless commensals and incoming antigens, such as food. This delicate equation may be set in part by the outcome of PRR signaling at the epithelial barrier, but it also involves continuing interplay between immune cells producing pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) as well as regulatory cytokines such as IL-4, IL-10 and transforming growth factor beta (TGFβ). The importance of regulatory cytokines in intestinal responses is vividly seen in IL-10 knockout mice, which spontaneously develop colitis at 2–3 months of age (40, 41). Initiation of this response is antigen-specific and is dependent on the generation of T-regulatory cells (Treg), which are a major source of regulatory cytokines. The effector mechanism is non-antigen-specific, a phenomenon known as antigen-driven bystander suppression. The “antigen” in this model is most likely enteric bacteria (42), and in several models, colitis has failed to develop if mice are housed under germ-free conditions (43). Intestinal microbes or their products that cross a damaged epithelial barrier can also have profound systemic immunological effects. For example, they can seriously aggravate graft-versus-host disease in an allogeneic bone marrow transplant setting (44), although in a syngeneic setting, they may cause “rebound” recovery in host immune cells by stimulating their proliferation.
Overall, there is compelling evidence that PRRs can control the initiation of inflammatory responses through recognition of PAMPS and DAMPS by immune and non-immune cells. This sets in motion a remarkable sequence of events that influences the outcome of a radiation challenge.
INFLAMMATION
The philosophy of the innate immune system in dealing with “danger” situations is to surround, entrap, eliminate and repair the lesion. Since it does not distinguish greatly between “self” damage and microbial challenge, the processes are quite stereotyped. An initial invasion of polymorphonuclear leukocytes (PMNs) into the inflammatory site is followed by monocytes/macrophages, with the presence of large numbers of lymphocytes often being diagnostic of a more chronic condition. No attempt will be made to cover inflammation in any detail, but certain “textbook” concepts that are relevant to radiation responses will be mentioned briefly.
The “surround” stage of an inflammatory process is exemplified in the cardinal signs of inflammation described 2000 years ago by Celsus as redness and swelling with heat and pain that clearly implicate the vasculature in the initial tissue response. Local release of nitric oxide, prostacyclin, complement and other vasoactive mediators in the damaged area cause endothelial cells to swell and retract, dramatically increasing blood flow to the affected area and making the vessels leaky for fluids and cells. This process is amplified by changes in cell adhesion molecules and integrins on the endothelial surface, trapping platelets that are activated to release histamine, serotonin, fibrin and other mediators, including the cytokines platelet-derived growth factor (PDGF) and TGFβ.
As a result of these vascular changes, fluid exudes and cells, initiallylargely PMNs, activelyflowintothedamaged site down a concentration gradient of bioactive molecules. Monocytes appear later and transform into classically activated macrophages under the influence of bacterial products such as LPS and cytokines such as interferon gamma (IFN-γ), TNF-α and GM-CSF (45); lymphocytes and mast cells also often participate. The whole response is orchestrated in large part by the key pro-inflammatory cytokines TNF-α and IL-1, aided by chemokines that primarily direct the cellular infiltrate. Importantly, ionizing radiation is a pro-inflammatory signal, fully capable of causing all these effects [see ref. (46)].
Inflammation in Radiation Biology
Historically, the low power output of the first X-ray machines used in therapy necessitated prolonged exposures and gave relatively high skin doses and erythema. To circumvent these problems, clinicians fractionated the dose, something from which tissues other than the skin were later found to derive benefit. It therefore could be said that inflammation has been a deciding factor in shaping the strategies used in radiation therapy to minimize normal tissue damage and increase the therapeutic benefit. Strangely, lower doses per fraction and lower total doses of radiation can have anti-inflammatory effects (47, 48) and can be used to treat chronic inflammatory conditions (49). A mechanism has been proposed for this, but a full explanation is not currently available (50).
Part of our current understanding involves the fact that, just as there is a DNA damage response to radiation, there is also a cellular damage response that is independent of DNA damage, and these pathways must be integrated to determine the final outcome. The first response is made within minutes of exposure and is largely pro-inflammatory. Radiation induces cells to express immediate early genes such as c-fos, c-myc, c-jun and beta-actin (51) as well as TNF-α, GM-CSF, COX2 and ICAM-1. This occurs primarily through post-transcriptional mRNA stabilization (52–54) and often requires AU-rich 3′-untranslated regions that are common to many pro-inflammatory mRNAs (55). Rapid radiation-induced activation of receptor tyrosine pathways and mitochondrial-associated responses may also contribute. The timing of this immediate early response suggests that it precedes DAMP signaling. Its role is uncertain, however, because the initial flurry tends to fade before major transcriptionally initiated pro-inflammatory responses are made. However, since inflammatory responses are amplified and propagated through feedback control loops in a recurring fashion, they probably play at least a modulatory role.
Numerous inflammatory molecules are involved at many levels in radiation responses, but the TNFR family perhaps best exemplifies some of the interactions that exist. This family has in excess of 27 members that have partial homology in their cysteine-rich extracellular domains. Based on their intracellular sequences, TNFRs can be subdivided into three groups. Members of the first group [TNF-R1, Fas (CD95), TRAIL-R1 (DR4), TRAIL-R2 (DR5), TRAIL-R4 (DcR2) and TRAMP (DR3)] have so-called intracellular death domains that can recruit adaptor proteins to cause cell death by caspase 8-dependent apoptosis, although p53-, caspase 9-dependent death (56) and non-apoptotic p53- and caspase-independent death (57) have also been reported. It has been known for many years that radiation up-regulates TNF ligands and TNF receptor expression in vitro and in vivo in many cells and tissues, as well as many other pro-inflammatory genes2 (58–61). This can result in radiation-induced cell death by a mechanism that is independent of direct unrepairable DNA damage. Remarkably, the same TNFR family members can also act through NF-κB-dependent pathways to enhance survival of some cell types (62). Under normal circumstances, most normal cells survive and may even proliferate in response to TNFR activation, although some are sensitive.
This critical balance between death and survival pathways is part of the yin-yang tradeoff inherent in inflammatory responses. It is not clear how it functions, but receptor-interacting protein (RIP) 1, which is downstream of TNFR, can act as an NF-κB-independent dual-function switch molecule early in TNF signaling that can mediate survival or death depending on its ubiquitination state (63). In addition, TNFRs that do not have death domains or soluble or decoy receptors may protect some cells against radiation damage (64). Knockout or knockdown experiments with TNFR family members clearly indicate their role in radiation-induced responses in the intestine (65–67), lymphoid tissues (67), brain (64, 67, 68), liver (69) and lung (70). In some cases the protective role of TNFR signaling in cells is seen, in others its detrimental side.
The well-known vascular effects of TNF suggest that many radiation-induced vascular changes may be mediated through pro-inflammatory cytokines and that at least some can be inhibited by anti-TNF antibody (71). It important to note that not only are the TNFR pathways cell- and tissue-dependent (72), they are also genetically determined, as is the pro-apoptosis tendency of normal tissues (73). The relevance of this variation to radiation-induced normal tissue radiation responses is not clear at this time, but caution should be exercised when drawing broad conclusions.
TNF is used here simply to illustrate the range of radiation-induced responses that can be modified through acute inflammation, and many other examples could have been presented of other molecules that have been shown to be radiobiologically relevant. Remarkably, the pathways that perpetuate acute inflammation are in fact limited and similar to those triggered by PRRs, leading one to ask questions regarding the origins of inflammation, how it is controlled when it is clearly harmful to the body, and how it is down-regulated to allow damage resolution. Without a doubt, free radicals are major ancestral players that have been harnessed as effectors in inflammation, which is another point of intersection between radiation and immunity.
Free Radicals, Inflammation and Radiation
The biology of inflammation and ionizing radiation is intimately intertwined with that of free reactive oxygen and nitrogen species (ROS and RNS), such as superoxide, hydroxyl radicals, hydrogen peroxide, nitric oxide, peroxynitrite and their derived products (74). During the elimination phase of an inflammatory lesion, phagocytic cells are activated to express more integrins, extravasate and migrate to the lesion where they generate yet more inflammatory mediators and become even more phagocytic. Although microbes are eliminated by multiple mechanisms, ROS are critical for this and for many other aspects of inflammation. For example, during phagocytosis, PMNs increase their oxygen uptake, sometimes more than 50-fold in a respiratory burst, and large amounts of free radicals are generated through a membrane-bound NADPH oxidase system. This is unassembled in resting cells but, when activated, produces superoxide (O2·–), hydrogen peroxide (H2O2) via dismutase and hydroxyl radicals and hydroxide-halide (HOCl) radicals via myeloperoxidase (75); about 10 nmol O2·– min/106 cells may be produced (76). These ROS, some more so than others, play a direct role in microbial killing, although the half-life of these killing machines is short and indeed is actively curtailed. The PMNs own death, and the death of surrounding cells contributes to antimicrobial killing, through yet more ROS production, neutrophil extra-cellular traps (NETs) that inhibit bacterial spread (77), and release of considerable quantities of other bioactive agents, including DAMPS and PAMPS that perpetuate the inflammatory response.
The role of nitric oxide (NO) and the RNS formed from the reaction of NO either with oxygen or super-oxide (78) extends well beyond the scope of this review. In inflammation, however, classically activated macrophages produce high levels of inducible nitric oxide synthetase (NOS2) that makes NO in a NADPH-dependent process from l-arginine (79). Radiation is not a strong stimulus for NOS2 induction, and low doses may even inhibit production (47, 48), but RNS can efficiently radiosensitize tumor cells (80, 81), even under hypoxia (80). The evidence for a role of RNS in cell death and survival is therefore more confusing than for ROS. In some circumstances, RNS have been found to mediate cell death through inhibition of NF-κB (82), whereas in other models, RNS inhibited cell death by inactivating caspases (83).
As pro-inflammatory cytokine levels increase, more and more ROS are generated. The link between pro-inflammatory cytokines and ROS is most evident in that TNF-α-elevated ROS levels are required for pathogen killing by phagocytes, for TNF-α-induced apoptosis, and for TNF signaling (57, 84, 85). In turn, ROS activate NF-κB, leading to further TNF-α production. Of interest to radiobiologists is the finding of the several cysteine-rich modules in the extracellular domain of TNFRs that can directly promote TNFR oligomerization and downstream NF-κB activation in response to oxidative stress (57, 86), and this may be a mechanism by which radiation directly stimulates this pathway. ROS and RNS act as second messengers, regulating numerous cellular processes, including cell proliferation through activating EGFR, PDGR and other kinases (87, 88), inactivating phosphatases such as Cdc25, and affecting ion channels (89). Many of the molecules involved are directly redox-sensitive. The spectrum of such molecules has yet to be fully defined, but it includes c-jun, c-fos, junB, TP53, NF-κB, EGFR, PDGFR (90), TGFβ (91) and the 26S proteasome (92), all of which link free radicals and inflammation to radiation responses.
While it is true that ionizing radiation can generate ROS directly, the main sources of ROS in cells are the mitochondrial respiratory chain and oxygen metabolizing enzymes, such as NADPH oxidases, myeloperoxidases, cyclo-oxygenase and lipoxygenase, and hypoxanthine/xanthine, so that radiation-induced ROS production is likely to be largely indirect. For example, the prodigious amount of ROS generated by phagocytes, amplified and perpetuated through inflammation, is far greater than what would be produced directly by moderate doses of radiation. Therefore, radiation-induced oxidative damage most likely stems from many sources within a cell and is perpetuated in vivo by feedback control signaling through pro-inflammatory cytokines and cell death to last long beyond the initial radiation-induced burst. These ongoing radiation-induced inflammatory responses contribute to molecular and cellular responses in tissues and, in all likelihood, to genomic instability and cancer and radiation-induced late effects.
Lesion Resolution
1. Focal responses
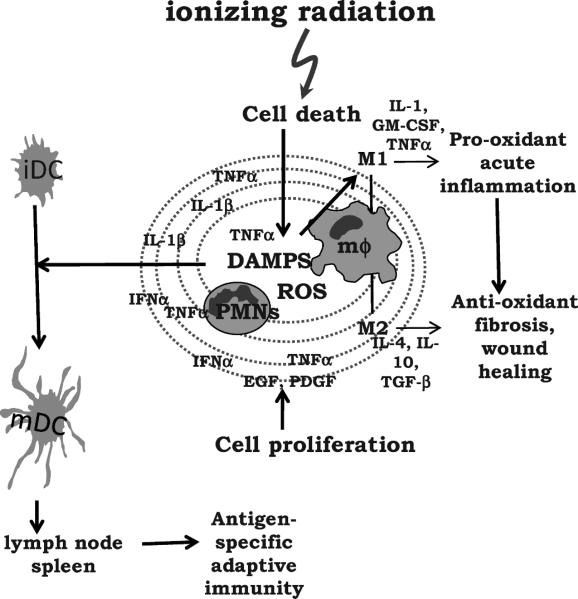
Inflammation is obviously a two-edged sword. On the one hand, its purpose is to eliminate invaders and repair tissue damage. On the other hand, the free radicals, hydrolytic/proteolytic enzymes, cytokines and other mediators that are generated for this purpose are toxic and inevitably cause focal tissue destruction. The spread of cytotoxic mediators outside the immediate vicinity of a lesion can cause a lacuna of cell death (93), while further down the concentration gradient, cytoprotective and proliferative responses are generated that limit the extent of damage and the spread of potential pathogens (Fig. 3). ROS/RNS, prostaglandins and cytokines often show concentration-dependent, opposing effects on cell death and proliferation that may account for such reactions, but the ligand-receptor interactions that are responsible have yet to be fully identified. Clearly, DAMPS released after irradiation are likely to play a role.
FIG. 3.
Acute inflammation and its evolution after radiation exposure. Cell death caused by radiation or by the pro-inflammatory cytokines and ROS that are generated cause infiltration of PMNs and macrophages (mϕ) that expand the extent of damage. DAMPS are released that have the ability to generate antigen-specific responses. This may occur for tumor-associated and possibly even self antigens released from damaged normal tissue. Further away from the center of the lesion, cells become protected, angiogenesis is initiated, and a proliferative response ensues with the aim of healing the lesion. The acute inflammatory response transitions to a wound-healing phase accompanied by a decrease in pro-oxidant cytokines and an increase in the antioxidant profile with production of a collagen matrix. This is orchestrated in part by a change in the macrophage phenotype from M1 to M2.
For a radiobiologist, it is tempting to draw parallels between spatially oriented inflammatory responses and radiation-induced “non-targeted” effects, which are often referred to as “bystander” and “adaptive” responses. There is no real evidence to support such an extrapolation; however, inflammation forms a useful paradigm for considering such responses. For example, there is confusion in the literature as to whether non-target radiation-induced responses are damaging, protective or adaptive, and multiple end points have been used. If one considers damage as focal, various responses are possible depending on the spatial distance from an irradiated site. It follows that bystander effects and adaptive responses may reflect various aspects of an inflammatory response to radiation-induced stress and injury with a variety of the manifestations, as has been pointed out by others (94).
Obvious examples of focal irradiation are brachytherapy, inhaled or injected radionuclides, and stereotactic radiosurgery or therapy, and it is worth considering possible radiobiological differences between these and standard large-field, homogeneous radiation delivery. For example, high local doses might be expected to generate more “danger” signals and to be more likely to generate anti-tumor immunity (46). As clinical radiotherapy moves toward more focal delivery of radiation, the radiobiological concepts involved are likely to diverge from those for conventional regimens. However, it is interesting to note that even when the radiation field had been designed to be homogeneous, many radiation-induced late tissue complications seem to be expressed, or initiated, focally: for example, white matter necrosis or certain cases of lung fibrosis. This is consistent with a role for inflammatory foci in triggering parenchymal cell death. The volume of tissue will also play a major role in dictating the extent of inflammatory damage and what can be tolerated, irrespective of the extent of clonogen deletion.
2. Proliferative and cytoprotective responses
Epithelial cells, endothelial cells and fibroblasts participate in angiogenesis, fibrogenesis and epithelialization to limit the damage to an inflamed tissue and initiate tissue repair. The triggers for proliferative responses include free radicals (89), TLRs (38) and growth factors such as fibroblast (FGF), epithelial (EGF), vascular endothelial (VEGF) and platelet-derived growth factors (PDGF) as well as cytokines like TNF-α and IL-1, with macrophages being a likely major source. When cells are activated in this way, they appear to be protected and may be more resistant to radiation.
The relationship between cell death and proliferation is of particular interest to radiobiologists, although the link to cytoprotection may not be readily apparent. In fact, there is ample evidence that cells can gain radioresistance as a consequence of cytokine and growth factor stimulation (95). This is the rationale driving combined cancer radiation therapy with growth factor inhibitors (96). The fact that mitogen-stimulated cells switch their metabolism to aerobic glycolysis (97) with mitochondrial uncoupling (98) and decreased ROS production (100) may be one mechanism associated with radioresistance. In this respect, mitogen-driven responses seem different from steady-state proliferation, which is often associated with a high apoptosis index and where cells tend to be more radiation sensitive.
A radiation-related concern in tissues stimulated to proliferate in this way is that this canonical inflammatory framework is being superimposed on cells that may have potentially lethally damaged DNA. Induced proliferation could therefore result in mitotic cell death or, in the worst case, carcinogenesis. Indeed, waves of induced cell death are one possible reason for the waves of pro-inflammatory cytokine expression seen in many tissues after irradiation (100). This begs the question as to whether such inflammation-induced cytoprotection/proliferation after radiation exposure would be of benefit. It could contribute to the development of resistance during a fractionated course, or it could precipitate tissue damage or even carcinogenesis.
It should be noted that proliferative responses during inflammation are not necessarily regenerative. Some may be, but in tissues with limited regenerative potential, such as most late-responding tissues, healing is often by fibrotic replacement of damaged tissue. Fibrin leakage through damaged vasculature is an early event in inflammation, and its deposition within an extracellular matrix (ECM) serves as a scaffold for ensuing events. Angiogenesis, epithelialization and organ recovery are normally associated with fibrogenesis or, in the central nervous system, with gliosis and must proceed in a controlled way to result in tissue restoration. This requires some ECM remodeling, which can continue for a long time. In fact, most tissues in the body have a limited capability for regeneration and frequently resort to replacement strategies that involve secretion of immature collagen by myofibroblasts stimulated by cytokines such as TNF-α, TGFβ and PDGF. All too often, scarring, fibrosis, fistula formation, stenosis, atherosclerosis or other conditions result. There is still controversy as to the sources of myofibroblasts in these responses; some of the possibilities include preexisting fibroblasts, bone marrow-derived mesenchymal stem cells, macrophages or epithelial-mesenchymal transition (101, 102).
3. Resolution of inflammation
The transition from acute inflammation to the remodeling phase with angiogenesis and fibrogenesis is critical for tissue repair. It requires conversion of a pro-inflammatory, pro-oxidant state into an anti-inflammatory, antioxidant one, which involves changing key transcriptionally activated genetic programs. While NF-κB is a key transcription regulator for acute inflammation, others such as NF-E2-related factor 2 (Nrf2) may be important in down-regulating inflammation and initiating healing. Nrf2 produces cytoprotective antioxidants such as manganese-superoxide dismutase (MnSOD), glutathione, heme oxygenase 1 (HO-1), thioredoxin and peroxiredoxin (103, 104) and is therefore able to restore the redox balance. Related cytoprotective proteins that are up-regulated include members of the Bcl-2 and inhibitor of apoptosis (IAP) families (105). The role of the Nrf2 pathway in inflammation is evident in the increase in NF-κB and pro-inflammatory cytokine levels in Nrf2 knockout mice (106), and there is some evidence that the Nrf2 pathway can attenuate NF-κB signaling (107). The timing of these responses must be critical and highly signal-dependent because inflammation must be maintained long enough to eliminate any invader but short enough to minimize damage. In this respect, it is of interest that radiation induces Nrf2 expression by cells in vitro only some days after exposure (McDonald, unpublished results).
Macrophage subsets must play a major role in the transition from pro-inflammatory, pro-oxidant to anti-inflammatory, antioxidant conditions (Fig. 3). In general, pro-inflammatory cytokines (TNF-α, GM-CSF, IFN-γ) and LPS generate classically activated macrophages (M1) that mediate acute inflammation, kill intracellular microbes, and hinder tumor growth (45). However, IL-4, IL-13, IL-10, TGFβ or immune complexes generate various subsets of alternatively activated macrophages (M2) (108), which promote angiogenesis and fibrogenesis and are effective at encapsulating parasites but contribute to tumor progression. M2 cells are generally immune suppressive and have high arginase levels, producing ornithine and polyamines rather than NO from arginine. These classes of macrophages mirror their reciprocity with Th subsets. M1 cells cooperate with Th1 cells, whereas M2 cells participate in Th2 and Treg reactions.
The M1 phenotype predominates in the acute inflammatory phase, and this switches to M2 during angiogenesis and tissue repair (109). The outcome of radiation exposure may therefore depend upon the involvement of these subsets, which can be determined genetically. For example, C3H/HeN and C57BL/6 mice show little difference in their initial pro-inflammatory cytokine profiles up to 1–2 months after lung irradiation, but the former appear to lack the ability to switch from an M1 to an M2 pattern and die of fulminating pneumonitis after high radiation doses. In contrast, C57BL/6 mice down-regulate pro-inflammatory cytokine production during the subacute phase after lung irradiation and develop radiation fibrosis (110). This provides a cautionary tale in that acute pro-inflammatory cytokine responses are unlikely to predict the final outcome.
The impact of radiation on macrophage subset function requires further study, but in one model, tumor irradiation appeared to stimulate a predominantly M2 intratumoral phenotype that promoted tumor growth (111). Irradiation of fibroblasts also has been shown to cause premature terminal differentiation of progenitor fibroblasts to post-mitotic fibrocytes through a TGFβ pathway leading to increased collagen deposition (112), which is likely to encourage replacement of radiation-damaged tissue with fibrotic masses. M2 cells may promote such a response in vivo. An important issue here is whether the development of a wound healing profile hinders organ regeneration. If it does, then later fibrogenesis is a bone fide target for intervention. On the other hand, if inhibition of the later phase simply allows acute inflammation to proceed unhindered, the result may be less than beneficial.
SUMMARY
The general thesis we have presented is that normal tissue radiation biology is joined at the hip to inflammation and immunity through PRRs, “danger” signaling and ROS generation. Similar canonical pathways are activated, leading to acute inflammation, and these provide targets for intervention in normal tissue radiation protection and in cancer therapy. However, inflammation is a two-edged sword that segues through many phases, and care must be taken to understand the effects of radiation on the different phases if intervention is going to be successful. Further, an individual molecule may be involved at several different times and influence several different cell lineages in several different ways. Identifying the critical target of any intervention may not be easy, and false conclusions are possible based on limited knowledge.
Radiation-induced inflammatory cytokine production is generally considerably greater at higher radiation doses, and it seems likely that dose fractionation may minimize the damage that results from this source. Cell death after irradiation is often a slow process, and fractionation may minimize the extent of cytokine-induced damage and allow tissue repair by many possible mechanisms. Since the delivery of high single doses or a small number of fractions of radiation has re-emerged as a force driving clinical practice, the radiobiology behind its effectiveness may be very different from that underlying conventional treatment. Although likely to generate more inflammation, focal irradiation with ablative doses may be superior at generating “danger” signaling and rapid cell death and promoting the generation of tumor-specific immune responses (113). A deciding factor in determining the outcome of many tissue radiation responses may be the host macrophage that is generated, whether it is of the acute inflammatory (M1) or the wound healing (M2) immunosuppressive phenotype. In a broader sense, the genetics that dictate the nature of the host inflammatory response after radiation treatment may prove critical in predicting tumor response, the nature of late complications, the extent of persistent oxidative stress, and the incidence of second cancers.
ACKNOWLEDGMENTS
NIH grants U19-AI067769 and RC1 AI-081287 (WMcB) and DOD grants W81XWH-07-1-0135 (WMcB) and DOD W81XWH-87-1-0014 (DS) supported this work.
Footnotes
W. H. McBride and T. Hussain, Effects of irradiation on monocyte function. Presented at the Thirty-seventh Annual Meeting of the Radiation Research Society, Seattle, WA, 1989.
REFERENCES
- 1.Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 3.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat. Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 5.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 6.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 7.Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. 2009;87:125–138. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 2008;226:10–18. doi: 10.1111/j.1600-065X.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Miyake K. Nucleic acid-sensing Toll-like receptors: beyond ligand search. Adv. Drug Deliv. Rev. 2008;60:782–785. doi: 10.1016/j.addr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Akira S. TLR signaling. Curr. Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 12.Matzinger P. An innate sense of danger. Ann. NY Acad. Sci. 2002;961:341–342. doi: 10.1111/j.1749-6632.2002.tb03118.x. [DOI] [PubMed] [Google Scholar]
- 13.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 16.Neta R, Douches S, Oppenheim JJ. Interleukin 1 is a radioprotector. J. Immunol. 1986;136:2483–2485. [PubMed] [Google Scholar]
- 17.Shan YX, Jin SZ, Liu XD, Liu Y, Liu SZ. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose–response relationship, mechanisms and implications. Radiat. Environ. Biophys. 2007;46:21–29. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- 18.Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J. Immunol. 1995;154:3333–3340. [PubMed] [Google Scholar]
- 19.Divanovic S, Trompette A, Petiniot LK, Allen JL, Flick LM, Belkaid Y, Madan R, Haky JJ, Karp CL. Regulation of TLR4 signaling and the host interface with pathogens and danger: the role of RP105. J. Leukoc. Biol. 2007;82:265–271. doi: 10.1189/jlb.0107021. [DOI] [PubMed] [Google Scholar]
- 20.Debucquoy A, Libbrecht L, Roobrouck V, Goethals L, McBride W, Haustermans K. Morphological features and molecular markers in rectal cancer from 95 patients included in the European Organisation for Research and Treatment of Cancer 22921 trial: prognostic value and effects of preoperative radio (chemo) therapy. Eur. J. Cancer. 2008;44:791–797. doi: 10.1016/j.ejca.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 23.Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol. Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 25.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J. Leukoc. Biol. 2008;83:493–498. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 26.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, Li J, Cetin S, Ford H, Hackam DJ. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 2006;176:3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn YT, Lim KS, Huh CS, Kim DH. Lactobacillus suntoryeus inhibits pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB activation in experimental colitis. Int. J. Colorectal Dis. 2009;24:231–237. doi: 10.1007/s00384-008-0618-6. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am. J. Pathol. 2006;169:1686–1700. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 2008;180:8280–8285. [Google Scholar]
- 35.Janot L, Sirard JC, Secher T, Noulin N, Fick L, Akira S, Uematsu S, Didierlaurent A, Hussell T, Erard F. Radioresistant cells expressing TLR5 control the respiratory epithelium's innate immune responses to flagellin. Eur. J. Immunol. 2009;39:1587–1596. doi: 10.1002/eji.200838907. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin MM, Dacquisto MP, Jacobus DP, Horowitz RE. Effects of the germfree state on responses of mice to whole-body irradiation. Radiat. Res. 1964;23:333–349. [PubMed] [Google Scholar]
- 37.Jervis HR, McLaughlin MM, Johnson MC. Effect of neutron-gamma radiation on the morphology of the mucosa of the small intestine of germfree and conventional mice. Radiat. Res. 1971;45:613–628. [PubMed] [Google Scholar]
- 38.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciorba MA, Stenson WF. Probiotic therapy in radiation-induced intestinal injury and repair. Ann. NY Acad. Sci. 2009;1165:190–194. doi: 10.1111/j.1749-6632.2009.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay JO, Hodgson HJ. Review article: the immunoregulatory cytokine interleukin-10—a therapy for Crohn's disease? Aliment Pharmacol. Ther. 2001;15:1709–1716. doi: 10.1046/j.1365-2036.2001.01093.x. [DOI] [PubMed] [Google Scholar]
- 42.Camerini V, Sydora BC, Aranda R, Nguyen C, MacLean C, McBride WH, Kronenberg M. Generation of intestinal mucosal lymphocytes in SCID mice reconstituted with mature, thymus-derived T cells. J. Immunol. 1998;160:2608–2618. [PubMed] [Google Scholar]
- 43.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill GR. Inflammation and bone marrow transplantation. Biol. Blood Marrow Transplant. 2008;15:139–141. doi: 10.1016/j.bbmt.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 46.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiat. Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 47.Hildebrandt G, Seed MP, Freemantle CN, Alam CA, Colville-Nash PR, Trott KR. Effects of low dose ionizing radiation on murine chronic granulomatous tissue. Strahlenther. Onkol. 1998;174:580–588. doi: 10.1007/BF03038296. [DOI] [PubMed] [Google Scholar]
- 48.Schaue D, Jahns J, Hildebrandt G, Trott KR. Radiation treatment of acute inflammation in mice. Int. J. Radiat. Biol. 2005;81:657–667. doi: 10.1080/09553000500385556. [DOI] [PubMed] [Google Scholar]
- 49.Trott KR, Kamprad F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother. Oncol. 1999;51:197–203. doi: 10.1016/s0167-8140(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 50.McBride WH, Pajonk F, Chiang CS, Sun JR. NF-kappa B, cytokines, proteasomes, and low-dose radiation exposure. Mil Med. 2002;167:66–67. [PubMed] [Google Scholar]
- 51.Hong J-H, Chiang C-S, Sun J-R, Withers HR, McBride WH. Induction of c-fos and junB mRNA following in vivo brain irradiation. Mol. Brain Res. 1997;48:223–228. doi: 10.1016/s0169-328x(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 52.Akashi M, Hachiya M, Koeffler HP, Suzuki G. Irradiation increases levels of GM-CSF through RNA stabilization which requires an AU-rich region in cancer cells. Biochem. Biophys. Res. Commun. 1992;189:986–993. doi: 10.1016/0006-291x(92)92301-d. [DOI] [PubMed] [Google Scholar]
- 53.Laroia G, Sarkar B, Schneider RJ. Ubiquitin-dependent mechanism regulates rapid turnover of AU-rich cytokine mRNAs. Proc. Natl. Acad. Sci. USA. 2002;99:1842–1846. doi: 10.1073/pnas.042575699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwamoto KS, Barber CL. Radiation-induced post-transcriptional control of M6P/IGF2r expression in breast cancer cell lines. Mol. Carcinog. 2007;46:497–502. doi: 10.1002/mc.20303. [DOI] [PubMed] [Google Scholar]
- 55.Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem. Soc. Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- 56.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr., el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998;58:1593–1598. [PubMed] [Google Scholar]
- 57.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 58.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor a mRNA after cellular exposure to ionizing radiation. Proc. Natl. Acad. Sci. USA. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang CS, McBride WH. Radiation enhances tumor necrosis factor alpha production by murine brain cells. Brain Res. 1991;566:265–269. doi: 10.1016/0006-8993(91)91707-8. [DOI] [PubMed] [Google Scholar]
- 60.Chiang CS, McBride WH, Withers HR. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother. Oncol. 1993;29:60–68. doi: 10.1016/0167-8140(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 61.Akca H, Yenisoy S, Yanikoglu A, Ozes ON. Tumor necrosis factor-alpha-induced accumulation of tumor suppressor protein p53 and cyclin-dependent protein kinase inhibitory protein p21 is inhibited by insulin in ME-180S cells. Clin. Chem. Lab. Med. 2002;40:764–768. doi: 10.1515/CCLM.2002.131. [DOI] [PubMed] [Google Scholar]
- 62.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 63.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr. Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61:8859–8865. [PubMed] [Google Scholar]
- 65.Inagaki-Ohara K, Yada S, Takamura N, Reaves M, Yu X, Liu E, Rooney I, Nicholas S, Castro A, Lin T. p53-dependent radiation-induced crypt intestinal epithelial cells apoptosis is mediated in part through TNF-TNFR1 system. Oncogene. 2001;20:812–818. doi: 10.1038/sj.onc.1204172. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Meng A, Lang H, Brown SA, Konopa JL, Kindy MS, Schmiedt RA, Thompson JS, Zhou D. Activation of nuclear factor kappaB in vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res. 2004;64:6240–6246. doi: 10.1158/0008-5472.CAN-04-0591. [DOI] [PubMed] [Google Scholar]
- 67.Finnberg N, Gruber JJ, Fei P, Rudolph D, Bric A, Kim SH, Burns TF, Ajuha H, Page R, El-Deiry WS. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol. Cell. Biol. 2005;25:2000–2013. doi: 10.1128/MCB.25.5.2000-2013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang XW, Yang J, Dragovic AF, Zhang H, Lawrence TS, Zhang M. Antisense oligonucleotide inhibition of tumor necrosis factor receptor 1 protects the liver from radiation-induced apoptosis. Clin. Cancer Res. 2006;12:2849–2855. doi: 10.1158/1078-0432.CCR-06-0360. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M, Qian J, Xing X, Kong FM, Zhao L, Chen M, Lawrence TS. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin. Cancer Res. 2008;14:1868–1876. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 71.Ansari R, Gaber MW, Wang B, Pattillo CB, Miyamoto C, Kiani MF. Anti-TNFA (TNF-alpha) treatment abrogates radiation-induced changes in vascular density and tissue oxygenation. Radiat. Res. 2007;167:80–86. doi: 10.1667/rr0616.1. [DOI] [PubMed] [Google Scholar]
- 72.Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–4612. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 73.Lindsay KJ, Coates PJ, Lorimore SA, Wright EG. The genetic basis of tissue responses to ionizing radiation. Br. J. Radiol. 2007;80:S2–S6. doi: 10.1259/bjr/60507340. Spec. No. 1. [DOI] [PubMed] [Google Scholar]
- 74.Bubici C, Papa S, Dean K, Franzoso G. Mutual crosstalk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 75.Babior BM. The respiratory burst of phagocytes. J. Clin. Invest. 1984;73:599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inanami O, Johnson JL, McAdara JK, Benna JE, Faust LR, Newburger PE, Babior BM. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 77.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 78.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 79.Hobbs AJ, Fukuto JM, Ignarro LJ. Formation of free nitric oxide from l-arginine by nitric oxide synthase: direct enhancement of generation by superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1994;91:10992–10996. doi: 10.1073/pnas.91.23.10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell JB, DeGraff W, Kim S, Cook JA, Gamson J, Christodoulou D, Feelisch M, Wink DA. Redox generation of nitric oxide to radiosensitize hypoxic cells. Int. J. Radiat. Oncol. Biol. Phys. 1998;42:795–798. doi: 10.1016/s0360-3016(98)00327-7. [DOI] [PubMed] [Google Scholar]
- 81.Singh S, Cowen RL, Chinje EC, Stratford IJ. The impact of intracellular generation of nitric oxide on the radiation response of human tumor cells. Radiat. Res. 2009;171:572–580. doi: 10.1667/RR1640.1. [DOI] [PubMed] [Google Scholar]
- 82.Garban HJ, Bonavida B. Nitric oxide disrupts H2O2-dependent activation of nuclear factor kappa B. Role in sensitization of human tumor cells to tumor necrosis factor-alpha-induced cytotoxicity. J. Biol. Chem. 2001;276:8918–8923. doi: 10.1074/jbc.M008471200. [DOI] [PubMed] [Google Scholar]
- 83.Kim YM, Kim TH, Chung HT, Talanian RV, Yin XM, Billiar TR. Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- 84.Jones GR. Free radicals in immunological killing: the case of tumor necrotising factor (TNF). Med. Hypotheses. 1986;21:267–271. doi: 10.1016/0306-9877(86)90019-8. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe N, Niitsu Y, Neda H, Sone H, Yamauchi N, Maeda M, Urushizaki I. Cytocidal mechanism of TNF: effects of lysosomal enzyme and hydroxyl radical inhibitors on cytotoxicity. Immunopharmacol. Immunotoxicol. 1988;10:109–116. doi: 10.3109/08923978809014405. [DOI] [PubMed] [Google Scholar]
- 86.Ozsoy HZ, Sivasubramanian N, Wieder ED, Pedersen S, Mann DL. Oxidative stress promotes ligand-independent and enhanced ligand-dependent tumor necrosis factor receptor signaling. J. Biol. Chem. 2008;283:23419–23428. doi: 10.1074/jbc.M802967200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 88.Joo CK, Kim HS, Park JY, Seomun Y, Son MJ, Kim JT. Ligand release-independent transactivation of epidermal growth factor receptor by transforming growth factor-beta involves multiple signaling pathways. Oncogene. 2008;27:614–628. doi: 10.1038/sj.onc.1210649. [DOI] [PubMed] [Google Scholar]
- 89.Sarsour E, Kumar MG, Chaudhuri L, Kalen AL, Goswami P. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 91.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 92.Pervan M, Iwamoto KS, McBride WH. Proteasome structures affected by ionizing radiation. Mol. Cancer Res. 2005;3:381–390. doi: 10.1158/1541-7786.MCR-05-0032. [DOI] [PubMed] [Google Scholar]
- 93.Ernst C, Christie BR. Temporally specific proliferation events are induced in the hippocampus following acute focal injury. J. Neurosci. Res. 2006;83:349–361. doi: 10.1002/jnr.20724. [DOI] [PubMed] [Google Scholar]
- 94.Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- 95.McBride WH, Dougherty GJ. Radiotherapy for genes that cause cancer. Nat. Med. 1995;1:1215–1217. doi: 10.1038/nm1195-1215. [DOI] [PubMed] [Google Scholar]
- 96.Bonner JA, Raisch KP, Trummell HQ, Robert F, Meredith RF, Spencer SA, Buchsbaum DJ, Saleh MN, Stackhouse MA, Waksal HW. Enhanced apoptosis with combination C225/radiation treatment serves as the impetus for clinical investigation in head and neck cancers. J. Clin. Oncol. 2000;18:47S–53S. [PubMed] [Google Scholar]
- 97.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- 100.Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int. J. Radiat. Biol. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- 101.Inumaru J, Nagano O, Takahashi E, Ishimoto T, Nakamura S, Suzuki Y, Niwa SI, Umezawa K, Tanihara H, Saya H. Molecular mechanisms regulating dissociation of cell-cell junction of epithelial cells by oxidative stress. Genes Cells. 2009;14:703–716. doi: 10.1111/j.1365-2443.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 102.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir. Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Banning A, Brigelius-Flohe R. NF-kappaB, Nrf2, and HO-1 interplay in redox-regulated VCAM-1 expression. Antioxid. Redox Signal. 2005;7:889–899. doi: 10.1089/ars.2005.7.889. [DOI] [PubMed] [Google Scholar]
- 104.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl.):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 106.Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, Yang X, Chen G, Ji Y. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, pro-inflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008;2008:725174. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu S, Kong AN. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J. Clin. Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, Norwood C, Wang E, Marincola FM, Stroncek DF. Gene expression profiling of cutaneous wound healing. J. Trans. Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. Compartmental responses after thoracic irradiation of mice: strain differences. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 111.Tsai CS, Chen FH, Wang CC, Huang HL, Jung SM, Wu CJ, Lee CC, McBride WH, Chiang CS, Hong JH. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 112.Herskind C, Rodemann HP. Spontaneous and radiation-induced differentiation of fibroblasts. Exp. Gerontol. 2000;35:747–755. doi: 10.1016/s0531-5565(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 113.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int. J. Radiat. Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]