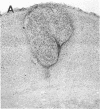
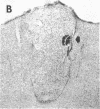
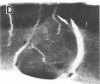
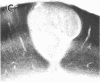
Abstract
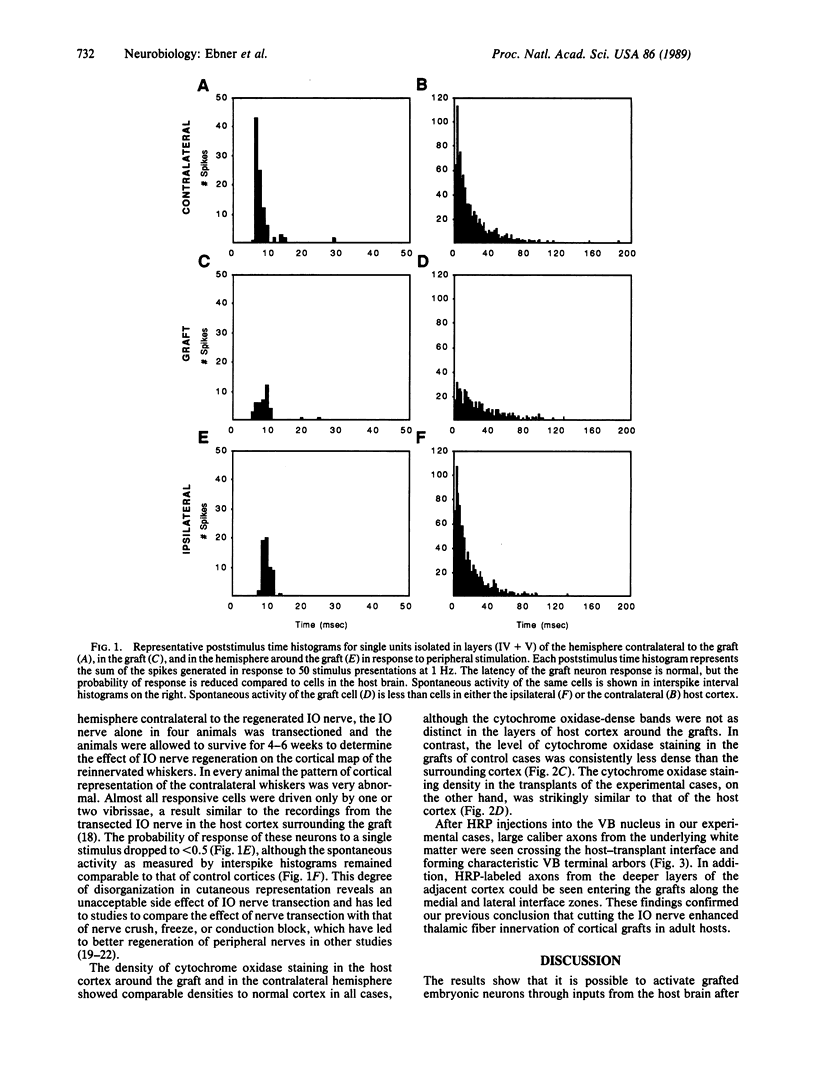
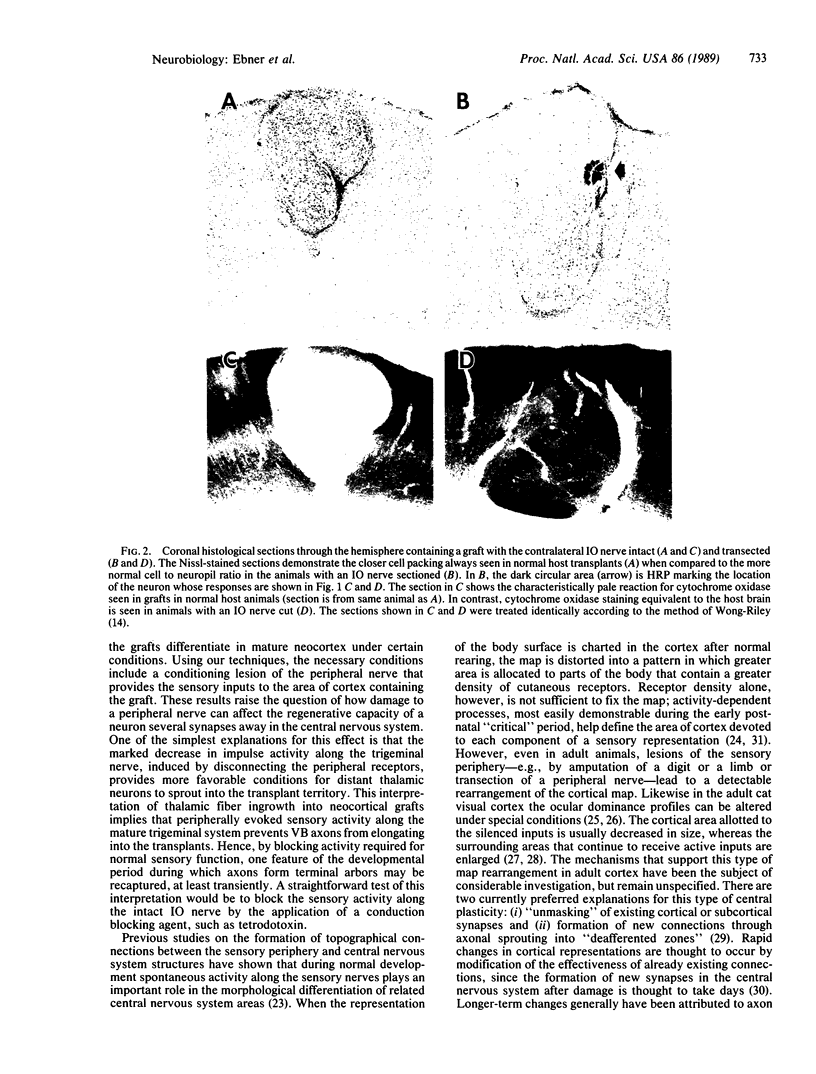
The neural pathways that relay information from cutaneous receptors to the cortex provide the somatic sensory information needed for cortical function. The last sensory relay neurons in this pathway have cell bodies in the thalamus and axons that synapse on neurons in the somatosensory cortex. After cortical lesions that damage mature thalamocortical fibers in the somatosensory cortex, we have attempted to reestablish somatosensory cortical function by grafting embryonic neocortical cells into the lesioned area. Such grafts survive in adult host animals but are not innervated by thalamic neurons, and consequently the grafted neurons show little if any spontaneous activity and no responses to cutaneous stimuli. We have reported that transection of peripheral sensory nerves prior to grafting "conditions" or "primes" the thalamic neurons in the ventrobasal complex so that they extend axons into grafts subsequently placed in the cortical domain of the cut nerve. In this report we present evidence that the ingrowth of ventrobasal fibers leads to graft neurons that become functionally integrated into the sensory circuitry of the host brain. Specifically, the conditioning lesions made prior to grafting produce graft neurons that are spontaneously active and can be driven by natural activation of cutaneous receptors or electrical stimulation of the transected nerve after it regenerates. Furthermore, oxidative metabolism in these grafts reaches levels that are comparable to normal cortex, whereas without prior nerve cut, oxidative metabolism is abnormally low in neocortical grafts. We conclude that damage to the sensory periphery transsynaptically stimulates reorganization of sensory pathways through mechanisms that include axonal elongation and functional synaptogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong-James M., Caan A. W., Fox K. Threshold effects of N-methyl D-aspartate (NMDA) and 2-amino 5-phosphono valeric acid (2APV) on the spontaneous activity of neocortical single neurones in the urethane anaesthetised rat. Exp Brain Res. 1985;60(1):209–213. doi: 10.1007/BF00237036. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Spatiotemporal convergence and divergence in the rat S1 "barrel" cortex. J Comp Neurol. 1987 Sep 8;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Millar J. Carbon fibre microelectrodes. J Neurosci Methods. 1979 Oct;1(3):279–287. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M. The functional status and columnar organization of single cells responding to cutaneous stimulation in neonatal rat somatosensory cortex S1. J Physiol. 1975 Apr;246(3):501–538. doi: 10.1113/jphysiol.1975.sp010902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford G. R., Killackey H. P. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol. 1980 Sep 15;193(2):335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Ebner F. F., Olschowka J. A., Jacobowitz D. M. The development of peptide-containing neurons within neocortical transplants in adult mice. Peptides. 1984 Jan-Feb;5(1):103–113. doi: 10.1016/0196-9781(84)90059-7. [DOI] [PubMed] [Google Scholar]
- Erzurumlu R. S., Ebner F. F. Peripheral nerve transection induces innervation of embryonic neocortical transplants by specific thalamic fibers in adult mice. J Comp Neurol. 1988 Jun 22;272(4):536–544. doi: 10.1002/cne.902720407. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Seo M. L. Avoidance of neonatal cortical lesions by developing somatosensory barrels. Nature. 1983 Feb 17;301(5901):600–602. doi: 10.1038/301600a0. [DOI] [PubMed] [Google Scholar]
- Itoh K., Konishi A., Nomura S., Mizuno N., Nakamura Y., Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979 Oct 19;175(2):341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Merzenich M. M., Killackey H. P. The reorganization of somatosensory cortex following peripheral nerve damage in adult and developing mammals. Annu Rev Neurosci. 1983;6:325–356. doi: 10.1146/annurev.ne.06.030183.001545. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T., Pettigrew J. D., Ary M. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol. 1979 May 1;185(1):163–181. doi: 10.1002/cne.901850110. [DOI] [PubMed] [Google Scholar]
- Lee K. J., Woolsey T. A. A proportional relationship between peripheral innervation density and cortical neuron number in the somatosensory system of the mouse. Brain Res. 1975 Dec 5;99(2):349–353. doi: 10.1016/0006-8993(75)90035-9. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983 Jan;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Ostberg A. J., Raisman G., Field P. M., Iversen L. L., Zigmond R. E. A quantitative comparison of the formation of synapses in the rat superior cervical sympathetic ganglion by its own and by foreign nerve fibres. Brain Res. 1976 May 14;107(3):445–470. doi: 10.1016/0006-8993(76)90137-2. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M. Peripheral injury enhances central regeneration of primary sensory neurones. 1984 Jun 28-Jul 4Nature. 309(5971):791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Verge V. M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987 May 19;411(2):406–408. doi: 10.1016/0006-8993(87)91096-1. [DOI] [PubMed] [Google Scholar]
- Simons D. J., Land P. W. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987 Apr 16;326(6114):694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Simons D. J. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978 May;41(3):798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Singer W., Yinon U., Tretter F. Inverted monocular vision prevents ocular dominance shift in kittens and impairs the functional state of visual cortex in adult cats. Brain Res. 1979 Mar 23;164:294–299. doi: 10.1016/0006-8993(79)90024-6. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verley R., Onnen I. Somatotopic organization of the tactile thalamus in normal adult and developing mice and in adult mice dewhiskered since birth. Exp Neurol. 1981 May;72(2):462–474. doi: 10.1016/0014-4886(81)90236-3. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol. 1984 Jul;352:425–445. doi: 10.1113/jphysiol.1984.sp015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol. 1984 Jul;352:425–445. doi: 10.1113/jphysiol.1984.sp015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. T., Cusick C. G. Cutaneous responsiveness in primary somatosensory (S-I) hindpaw cortex before and after partial hindpaw deafferentation in adult rats. J Neurosci. 1984 Jun;4(6):1499–1515. doi: 10.1523/JNEUROSCI.04-06-01499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. T., Cusick C. G. The representation of peripheral nerve inputs in the S-I hindpaw cortex of rats raised with incompletely innervated hindpaws. J Neurosci. 1986 Apr;6(4):1129–1147. doi: 10.1523/JNEUROSCI.06-04-01129.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. T., Felleman D. J., Kaas J. H. Recovery of normal topography in the somatosensory cortex of monkeys after nerve crush and regeneration. Science. 1983 Aug 19;221(4612):771–773. doi: 10.1126/science.6879175. [DOI] [PubMed] [Google Scholar]
- Wall J. T., Kaas J. H., Sur M., Nelson R. J., Felleman D. J., Merzenich M. M. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationships to sensory recovery in humans. J Neurosci. 1986 Jan;6(1):218–233. doi: 10.1523/JNEUROSCI.06-01-00218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]