Abstract
Peptidyl-glycine alpha-amidating monooxygenase (PAM; EC 1.14.17.3) catalyzes the conversion of a variety of glycine-extended peptides into biologically active alpha-amidated product peptides in a reaction dependent on copper, ascorbate, and molecular oxygen. We have isolated and sequenced cDNAs representing the two major classes of PAM mRNA in the adult rat heart atrium. The two types of cDNA, rPAM-1 and rPAM-2, are identical except for the deletion of a 315-base-pair segment within the protein coding region in rPAM-2, suggesting that rPAM-1 and rPAM-2 arise by alternative splicing. Northern analysis using a cDNA probe derived from within the 315-base-pair region deleted in rPAM-2 visualized the larger of the PAM mRNAs in adult rat atrium and not the smaller, indicating that the presence or absence of this 315-nucleotide segment is a major feature distinguishing the two size forms of PAM mRNA. The 105 amino acid segment that distinguishes the two forms of atrial PAM contains a consensus N-glycosylation site and a paired basic amino acid site of potential importance in endoproteolytic processing. Comparison of the nucleotide sequences of rat, frog, and bovine PAM cDNAs reveals an extremely well conserved segment in the 3' untranslated region. The high degree of conservation in amino acid sequence throughout the catalytic, intragranular, and cytoplasmic domains of rat atrium, bovine pituitary, and frog skin PAM suggests that both the catalytic and noncatalytic domains of the protein subserve important functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Gallego M. E., Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Enzyme-catalysed peptide amidation. Isolation of a stable intermediate formed by reaction of the amidating enzyme with an imino acid. Eur J Biochem. 1987 Dec 15;169(3):579–584. doi: 10.1111/j.1432-1033.1987.tb13648.x. [DOI] [PubMed] [Google Scholar]
- Capasso O., Bleecker G. C., Heintz N. Sequences controlling histone H4 mRNA abundance. EMBO J. 1987 Jun;6(6):1825–1831. doi: 10.1002/j.1460-2075.1987.tb02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dickey L. F., Wang Y. H., Shull G. E., Wortman I. A., 3rd, Theil E. C. The importance of the 3'-untranslated region in the translational control of ferritin mRNA. J Biol Chem. 1988 Mar 5;263(7):3071–3074. [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Peptide alpha-amidation. Annu Rev Physiol. 1988;50:333–344. doi: 10.1146/annurev.ph.50.030188.002001. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., May V., Braas K. M. Membrane-associated peptidylglycine alpha-amidating monooxygenase in the heart. J Biol Chem. 1988 Jun 15;263(17):8371–8379. [PubMed] [Google Scholar]
- Eipper B. A., Myers A. C., Mains R. E. Peptidyl-glycine alpha-amidation activity in tissues and serum of the adult rat. Endocrinology. 1985 Jun;116(6):2497–2504. doi: 10.1210/endo-116-6-2497. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Park L. P., Dickerson I. M., Keutmann H. T., Thiele E. A., Rodriguez H., Schofield P. R., Mains R. E. Structure of the precursor to an enzyme mediating COOH-terminal amidation in peptide biosynthesis. Mol Endocrinol. 1987 Nov;1(11):777–790. doi: 10.1210/mend-1-11-777. [DOI] [PubMed] [Google Scholar]
- Gainer H., Russell J. T., Loh Y. P. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology. 1985 Feb;40(2):171–184. doi: 10.1159/000124070. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kruys V., Wathelet M., Poupart P., Contreras R., Fiers W., Content J., Huez G. The 3' untranslated region of the human interferon-beta mRNA has an inhibitory effect on translation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6030–6034. doi: 10.1073/pnas.84.17.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Secretion and regulation of two biosynthetic enzyme activities, peptidyl-glycine alpha-amidating monooxygenase and a carboxypeptidase, by mouse pituitary corticotropic tumor cells. Endocrinology. 1984 Nov;115(5):1683–1690. doi: 10.1210/endo-115-5-1683. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Myers A. C., Eipper B. A. Hormonal, drug, and dietary factors affecting peptidyl glycine alpha-amidating monooxygenase activity in various tissues of the adult male rat. Endocrinology. 1985 Jun;116(6):2505–2515. doi: 10.1210/endo-116-6-2505. [DOI] [PubMed] [Google Scholar]
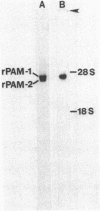
- May V., Cullen E. I., Braas K. M., Eipper B. A. Membrane-associated forms of peptidylglycine alpha-amidating monooxygenase activity in rat pituitary. Tissue specificity. J Biol Chem. 1988 Jun 5;263(16):7550–7554. [PubMed] [Google Scholar]
- Mehta N. M., Gilligan J. P., Jones B. N., Bertelsen A. H., Roos B. A., Birnbaum R. S. Purification of a peptidylglycine alpha-amidating enzyme from transplantable rat medullary thyroid carcinomas. Arch Biochem Biophys. 1988 Feb 15;261(1):44–54. doi: 10.1016/0003-9861(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Ohsuye K., Wada Y., Fuchimura K., Tanaka S., Matsuo H. Cloning and sequence of cDNA encoding a peptide C-terminal alpha-amidating enzyme from Xenopus laevis. Biochem Biophys Res Commun. 1987 Oct 29;148(2):546–552. doi: 10.1016/0006-291x(87)90911-9. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Sakata J., Kojima M., Kangawa K., Matsuo H. Peptide C-terminal alpha-amidating enzyme purified to homogeneity from Xenopus laevis skin. Biochem Biophys Res Commun. 1986 Jun 30;137(3):984–991. doi: 10.1016/0006-291x(86)90322-0. [DOI] [PubMed] [Google Scholar]
- Murthy A. S., Mains R. E., Eipper B. A. Purification and characterization of peptidylglycine alpha-amidating monooxygenase from bovine neurointermediate pituitary. J Biol Chem. 1986 Feb 5;261(4):1815–1822. [PubMed] [Google Scholar]
- Needleman P., Greenwald J. E. Atriopeptin: a cardiac hormone intimately involved in fluid, electrolyte, and blood-pressure homeostasis. N Engl J Med. 1986 Mar 27;314(13):828–834. doi: 10.1056/NEJM198603273141306. [DOI] [PubMed] [Google Scholar]
- Ohsuye K., Kitano K., Wada Y., Fuchimura K., Tanaka S., Mizuno K., Matsuo H. Cloning of cDNA encoding a new peptide C-terminal alpha-amidating enzyme having a putative membrane-spanning domain from Xenopus laevis skin. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1275–1281. doi: 10.1016/0006-291x(88)90767-x. [DOI] [PubMed] [Google Scholar]
- Sakata J., Mizuno K., Matsuo H. Tissue distribution and characterization of peptide C-terminal alpha-amidating activity in rat. Biochem Biophys Res Commun. 1986 Oct 15;140(1):230–236. doi: 10.1016/0006-291x(86)91080-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- de Bold A. J. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985 Nov 15;230(4727):767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]