Abstract
Aim
The goal was to examine the relationship between a risk factor for poor cognitive control and a health outcome of growing public significance – an excess body mass – among adolescents.
Methods
To this end, 109 adolescents aged 14–20 years were recruited and assigned to 1 of 4 groups defined by the crossing of the absence versus presence of a parental history (PH) of externalizing disorders with a body mass index (BMI) percentile (BMIP) <85 versus ≥85. The principal measure estimating cognitive control was the P300 event-related electroencephalographic response recorded during the Stroop task.
Results
The analyses revealed a synergistic interaction between BMIP rank, PH and trial type: the increase in P300 latency and the decrease in response accuracy, elicited by the presence of interfering information, were markedly greater in high-BMIP subjects with a PH of externalizing disorders than in the other subject groups. Analyses of a later component, the N450, previously associated with the Stroop interference effect, revealed no effect of BMI or PH.
Conclusions
We conclude that subjects with both a PH of externalizing disorders and an excess BMI constitute a unique group that is less able to resolve cognitive conflict than others. The excessive delay in P300 evoked by conflicting response demands in these subjects may be a marker of a heritable factor that increases risk for both excess body mass and substance use disorders.
Key Words: P300, Evoked potentials, Overweight, Family history, Externalizing disorders, Obesity, Adolescents, Stroop effect
Introduction
The cognitive control of behavior has been studied in many experimental paradigms of human information processing. A popular paradigm is the task developed by Stroop [1]. During his task, color names are presented in compatible or incompatible colors with the instruction to ignore the prepotent inclination to read the name and, instead, name the color in which it is printed. The challenge to cognitive control occurs when the inclination and the required response are in conflict (e.g. the word ‘blue’ presented in a red font). The recognition and resolution of the conflict is controlled, in part, by neural systems within the prefrontal cortex and anterior cingulate [2,3,4,5].
Using the Stroop task, investigators have demonstrated impaired cognitive control among groups of patients afflicted with any one of a wide assortment of psychiatric or medical disorders [6,7,8,9,10]. The group of current interest is adolescents at risk for obesity. The Stroop task is germane to these subjects because impairments in aspects of cognitive control, including the ability to delay rewards [11], judge portion and meal size [12], and use satiety signals to inhibit further eating [13], are viewed [14,15] as seminal to their disorder. Obese or overweight subjects have repeatedly been shown to differ from normal-weight control groups in other, related aspects. They exhibit more impulsivity [14,15,16,17,18,19], impatience [20], aggressiveness [21], affect lability [19] and executive cognitive dysfunction [22,23,24,25].
Our interest in the cognitive and neural factors that motivate or maintain an elevated body mass index (BMI) during adolescence is timely in the context of the current threat posed by obesity to the health of our youth [26]. It is a special concern for adolescents from racial/ethnic minority groups: for example, the prevalence of an overweight BMI among African-American adolescent girls is twice the rate seen in their Caucasian peers [27]. One goal of the present study was to document an association between BMI percentile (BMIP) and impaired cognitive control in this high-risk, minority population. Indeed, if a simple and inexpensive laboratory measure of control were found to correlate with an elevated BMI, it might later serve, in combination with other variables, as a means for identifying subgroups at the greatest risk for obesity, its serious medical complications and/or a poor treatment response.
The second study goal was to evaluate a related risk factor for poor cognitive control and impulsivity: a family history of substance dependence or antisocial personality disorder, i.e. externalizing disorders. The importance of family history as a factor influencing cognitive control has been demonstrated in adults. For example, Lovallo et al. [28], as well as Silveri et al. [29], conducted analyses of Stroop task performance in the biological offspring of substance-dependent parents and in control groups without this family history. In both studies, a positive family history was associated with a performance impairment. Notably, a positive family history also contributes to obesity risk. In a 10-year prospective study of a superset of the adolescents in the present sample, we found that it was a strong positive predictor of an excess BMI in adulthood.
The third major goal was to evaluate neurophysiological differences associated with excess body mass and family history, as well as their independent or interactive associations with cognitive control. Because neurophysiological differences are covert and objective, they may constitute a more sensitive index of disordered control less affected by demand characteristics, observer bias and measurement error. The differences were expected to involve P300 and N450 event-related potentials (ERP), which have been shown to be delayed in latency or altered in amplitude by the introduction of response conflict [9,10,30,31] during the Stroop task. Admittedly, the association between these ERP components and response conflict is not consistent across all studies. The variable findings may be related to the small numbers of subjects evaluated in some of the published works and the accompanying risk of both type I and type II errors.
It was hypothesized that the combination of an elevated BMI with a parental history (PH) of externalizing disorders would be associated with the greatest disruption in cognitive control. In theory, at least three mechanisms could explain this result: (1) an elevated BMI and a PH of externalizing disorders may be expressions of different dispositional factors that independently contribute to impaired cognitive control; (2) an elevated BMI and a PH of externalizing disorders may be expressions of a shared predisposition that also affects cognitive control, and (3) disruptions in cognitive control may result from gene by environment interplay (interaction), wherein, for example, familial/genetic risk for externalizing disorders increases the duration or severity of weight problems, which, in turn, enhances the accumulation of white matter abnormalities [32,33] in brain regions responsible for cognitive control. To evaluate these alternative conceptual models, alternative path analysis models were tested.
Patients and Methods
Subject Recruitment
338 subjects, aged 14–20 years, were recruited between 1993 and 1997 for a study examining the effects of parental substance dependence and childhood conduct problems on neuropsychological test performance and the development of substance use disorders. Recruitment methods included advertisements and presentations directed at parents or adolescents. A potential recruit or his/her parents were invited to telephone a research assistant for additional information about the study.
Intake Procedures and Group Assignment
If the information obtained during the telephone call suggested eligibility, then the recruit and his/her parent were invited to the laboratory for separate interviews. At the beginning of the interview, a consent agreement form, approved by the University of Connecticut Health Center's Institutional Review Board, was reviewed and signed.
The family's psychiatric history was obtained from the parent via the Family History Assessment Module [34]. The parent and the adolescent subject also summarized their personal psychiatric histories, as well as their demographic characteristics and medical histories. This information was obtained with the Semi-Structured Assessment for the Genetics of Alcoholism [35], which surveyed the major psychiatric disorders defined in the DSM-III-R and DSM-IV.
Recruits were excluded from the study if they reported a history of pregnancy, head injury (e.g. loss of consciousness longer than 5 min), seizures, life-threatening disease, regular psychoactive medication use, alcohol or other drug dependence (except nicotine), schizophrenia or bipolar disorder, neurosurgery, major medical disorders (including diabetes, hypertension, renal disease, lupus and cardiac disorders), or uncorrected visual or auditory deficits. Caucasian subjects were eliminated from the analysis to bring the focus more clearly upon racial/ethnic minorities, who are at greater risk for obesity and its complications. Caucasian subjects were also eliminated because a wealth of data already exists describing the association between their neuropsychological abilities and body mass [22,23,24,25].
The 109 subjects retained for analysis were assigned to 1 of 4 mutually exclusive groups formed by the factorial combination of BMIP (<85 vs. ≥85) with the absence versus presence of a PH of alcohol or drug dependence or antisocial personality disorder. The number of subjects assigned to each group is listed in table 1.
Table 1.
Background characteristics of study subjects
| Age years | Education years | Females % | Conduct disorder symptoms, n | Parental diagnosis of depression, bipolar disorder or schizophrenia, % | Alcohol problems n | Daily cigarette use, % | |
|---|---|---|---|---|---|---|---|
| PH–, BMIP <85 (n = 43) | 16.37 ± 1.52 | 9.46 ± 1.56 | 53.5 | 2.60 ± 2.32 | 2.3 | 2.19 ± 4.93 | 16.3 |
| PH+, BMIP <85 (n = 25) | 16.32 ± 1.49 | 9.68 ± 1.37 | 44.0 | 3.04 ± 1.92 | 8.0 | 1.40 ± 2.87 | 20.0 |
| PH-, BMIP ≥85 (n = 26) | 16.38 ± 1.44 | 9.92 ± 1.64 | 50.0 | 1.80 ± 1.57 | 11.5 | 1.44 ± 2.34 | 11.5 |
| PH+, BMIP ≥85 (n = 15) | 15.73 ± 1.57 | 9.06 ± 1.09 | 66.7 | 2.86 ± 2.69 | 20.0 | 0.69 ± 1.43 | 26.7 |
| F3, 108 or χ2d.f. = 3 | 0.75 | 1.1 | 2.0 | 1.6 | 5.0 | 0.65 | 1.5 |
Values are percentages or means ± SD.
ERP Procedures
Electroencephalograms (EEG) were recorded from adolescent subjects with tin electrodes applied to 31 scalp sites (ElectroCap International, Eaton, Ohio, USA). A reference electrode was taped over the bridge of the nose. The ground electrode was applied to the middle of the forehead. For the detection of eyeblink and eye movement artifacts, a pair of electrodes was placed diagonally above and below the left eye. Interelectrode impedances were maintained below 5 kΩ.
After the electrodes were applied, the subjects were escorted into a sound-shielded chamber and seated in a comfortable chair. The chair faced a 14-inch computer monitor used for the presentation of visual stimuli. A set of shielded stereo headphones, used for auditory stimulus presentation, and other devices (response keys) were located in the immediate proximity of the chair.
The subjects then performed a discreet trials version [10,36] of the Stroop test, in which the stimuli, i.e. the words RED, BLUE or TOWN, were presented in a red or blue font on the computer monitor. The 300 word stimuli were presented with equal probability at a rate of 1 stimulus every 2.3 s for 200 ms each. The subjects were asked to indicate the color of the font by pressing 1 of 2 response keys within a response deadline of 1,500 ms. They were instructed to ignore the word.
The EEG was recorded throughout the task. The 31 channels of the EEG and 1 channel of eye movement (electrooculogram, EOG) activity were appropriately amplified (EEG gain: 20 K; EOG gain: 2 K) and filtered (bandpass: 0.01–12 Hz) using an amplification system by Grass Instruments. Along with markers indicating stimulus and response onsets, the EEG and EOG channels were routed to an A/D converter, and sampled at a rate of 500 Hz for 50 ms preceding and 950 ms following the onset of each stimulus.
During off-line computations, single trial data were sorted by trial type: compatible font color-word combinations (e.g. the word BLUE displayed in blue font), incompatible color-word combinations (e.g. the word BLUE displayed in red font, also known as the ‘Stroop stimulus’) and unrelated combinations (e.g. the word TOWN displayed in blue font). Before averaging, trials containing an eye movement deviation of >50 μV were deleted. Trials with A/D converter overflow and incorrect responses were also deleted.
Time-point-averaged waveforms were then created from a minimum of 20 accepted trials of the incompatible and compatible color-word trial types. The operator then searched the waveform for the most positive voltage between 250 and 600 ms at the Pz electrode and labeled it as P300. P300 latency was the time difference between this peak and stimulus onset. P300 amplitude was measured separately at each electrode at the Pz latency and expressed as the microvolt difference from the average voltage during the prestimulus period.
Using the methods described by Liotti et al. [31], the amplitude of a slow potential, N450, was also scored. It was measured within 3 different poststimulus epochs: 500–600, 600–700 and 700–800 ms. A computer algorithm calculated the average area of the N450 potential at the Fz, Cz and Pz electrode sites.
Task performance indices were recorded during the task and summarized off-line. The average reaction time and the percentage of trials with correct responses were sorted by trial type and preserved for analysis.
Data Analysis Procedures
Initially, the 4 subject groups were compared on demographic, psychological and other features to verify that the group comparisons in the primary analysis were not confounded by a background characteristic. Pearson's χ2 test with 3 degrees of freedom was used to evaluate group equivalence on categorical variables. A one-way ANOVA with 3 numerator degrees of freedom was used to evaluate equivalence on continuous variables. The threshold for statistical significance was p < 0.05.
Subsequently, the groups were compared on P300 latency and amplitude, N450 amplitude and task performance. Each 2-factor (BMIP × PH) ANOVA included trial type as a third factor. The P300 and N450 amplitude analyses also included a fourth factor, anterior-posterior scalp location, with 3 levels, i.e. Fz, Cz and Pz. The N450 analysis specified one additional factor, epoch window, with 3 levels. The degrees of freedom for the P300 and N450 amplitude analyses were adjusted where appropriate, using the Greenhouse-Geisser formula to compensate for violations of the sphericity assumption inherent in repeated measures analyses.
The next analysis was directed toward identifying the neuroanatomical location of the Stroop effect among those subjects (BMIP ≥85 and PH+) who were expected to be most challenged by the task. Using the Curry© software package (version 5.0.8; Compumedics Neuroscan Inc.) and the group-averaged ERP voltage within the P300 search window of 250–600 ms, we searched for the best-fitting, fixed, single dipole source. The search was constrained by a boundary element method model, wherein the volume conduction of the source potential through the liquor, skull and skin compartments was calculated using MRI data from a healthy, 17-year-old subject.
The final set of analyses employed path analytic methods to illuminate and clarify the statistically significant relationships revealed in the ANOVA of BMIP rank, PH and P300 during the incompatible condition. Using Mplus©, we constructed and tested the 3 alternative models described in the Introduction. The first model hypothesized independent associations of BMIP with the number of externalizing disorders (alcohol dependence + drug dependence + antisocial personality disorder) present in the biological parents with P300. The second model viewed BMI and the number of parental externalizing disorders as overlapping expressions of a common mechanism that also affects cognitive control (P300). The final model viewed PH as a factor that alters the course (severity or duration) of an excess BMI and, thereby, alters brain white matter function and cognitive control (P300). Conventional measures of model fit, i.e. discrepancy (χ2), root mean square error of approximation (RMSEA) and comparative fit index (CFI), as well as standardized path coefficients, were computed [37].
Results
Background Characteristics
Table 1 summarizes the background characteristics of the 4 subject groups. These analyses revealed no statistically significant differences between the 4 groups in their age, gender composition, number of alcohol problems reported on the Michigan Alcoholism Screening Test [38], or the percentage of members who reported daily cigarette use. In addition, they did not differ in relation to the number of DSM-III-R childhood conduct disorder problems or in their PH of depression, bipolar disorder or schizophrenia, i.e. nonexternalizing disorders.
Stroop Task
The analyses of ERP component amplitudes revealed significant changes associated with electrode site [P300: F(1.7, 159.6) = 88.2, p < 0.01; N450: F(1.7, 179.1) = 32.9, p < 0.01] and trial type [P300: F(1, 210) = 10.1, p < 0.01]. P300 and N450 amplitudes were more positive at posterior versus anterior electrode sites (fig. 1; bottom versus top panel). P300 was also more positive in amplitude on compatible versus incompatible trials. N450 was likewise more positive in amplitude on compatible versus incompatible trials, but only within the 500–600 ms epoch [trial type × epoch: F(1.6, 174) = 10.8, p < 0.01] proximal to the P300 peak. The ANOVAs of P300 and N450 amplitudes revealed no significant effects of the grouping factors. In addition, they revealed no significant interactions.
Fig. 1.
Group-averaged ERP waveforms at Fz, Cz and Pz electrodes for each trial type.
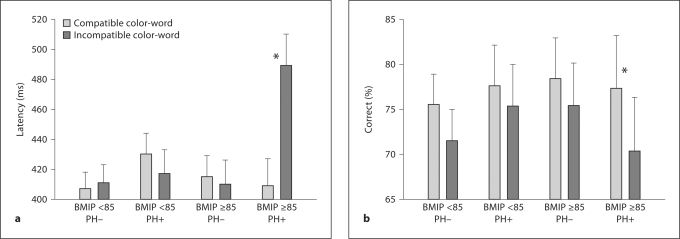
The analyses of P300 latency revealed several significant and interesting findings. In addition to a significant slowing of P300 on incompatible versus compatible trials [F(1, 210) = 4.3, p < 0.05], greater slowing, i.e. a larger Stroop effect, was seen in subjects with an elevated BMI and a positive PH [BMIP × PH × trial type: F(1, 210) = 6.8, p < 0.01]. This significant 3-way interaction is illustrated in figure 2a. The 2-way interaction of BMIP × trial type was also significant [F(1, 210) = 4.5, p < 0.04]. However, it cannot be interpreted independently of the 3-way interaction.
Fig. 2.
P300 latency (a) and response accuracy (b) (+1 SEM) plotted as a function of BMIP rank (<85 vs. ≥85), PH of externalizing disorders and trial type (compatible and incompatible color-word combinations). * p < 0.05.
The analyses of task performance revealed the expected changes. Response accuracy was lower [F(1, 210) = 28.54, p < 0.01], and response times were slower [F(1, 210) = 9.62, p < 0.01], on incompatible versus compatible trials. Response accuracy was also sensitive to the 3-way interaction of BMIP × PH × trial type [F(1, 210) = 3.9, p < 0.05]. As shown in figure 2b and reported in table 2, the pattern of the interaction was the same as was seen in the analysis of P300 latency: the excess BMI PH+ group was more sensitive to the Stroop effect (i.e. showed a greater difference between incompatible and compatible trials) than the other groups.
Table 2.
Stroop task results
| P300 latency1 compatible ms | P300 latency1 incompatible ms | P300 amplitude1 compatible μV | P300 amplitude1 incompatible μV | Reaction time compatible s | Reaction time incompatible s | Correct compatible % | Correct incompatible % | |
|---|---|---|---|---|---|---|---|---|
| PH-, BMIP <85 (n = 43) | 407.76 ± 11.16 | 411.62 ± 12.62 | 11.49 ± 1.01 | 11.32 ± 0.88 | 0.493 ± 0.01 | 0.515 ± 0.01 | 75.5 ± 3.4 | 71.5 ± 3.5 |
| PH+, BMIP <85 (n = 25) | 430.00 ± 14.64 | 417.92 ± 16.55 | 12.76 ± 1.33 | 11.28 ± 1.16 | 0.469 ± 0.01 | 0.484 ± 0.02 | 77.6 ± 4.5 | 75.3 ± 4.7 |
| PH-, BMIP ≥85 (n = 26) | 415.92 ± 14.35 | 410.15 ± 16.23 | 13.72 ± 1.30 | 11.92 ± 1.14 | 0.493 ± 0.01 | 0.512 ± 0.01 | 78.4 ± 4.5 | 75.4 ± 4.7 |
| PH+, BMIP ≥85 (n = 15) | 409.33 ± 18.90 | 489.46 ± 21.372 | 12.30 ± 1.71 | 10.76 ± 1.50 | 0.506 ± 0.02 | 0.519 ± 0.02 | 77.3 ± 5.9 | 70.3 ± 6.02 |
Values denote means ± SE.
P300 amplitude is averaged across Fz, Cz and Pz electrode locations.
BMIP × PH × trial type, p < 0.05.
Dipole Localization
Figure 3 shows the estimated location of the best-fitting dipole source. The localization algorithm yielded a solution with a signal-to-noise ratio of 6.1 and a residual variance of 22.9%. The dipole was located in the anterior cingulate close to the midline.
Fig. 3.

Anatomical location of the probable source of ERP activity between 300 and 600 ms after the presentation of the Stroop stimulus among subjects with both an excess body mass and a PH of externalizing disorders. This single dipole is located within the anterior cingulate.
Path Analysis
Figure 3 shows the results of 3 path analyses wherein the number of parental externalizing disorders, BMIP and P300 latency in the incompatible condition are entered in different configurations to test the conceptual models described in the Introduction. The analyses of each model revealed that only model 2 provided an adequate fit to the data: its 0.54 χ2 (d.f. = 1, p = 0.46) and 0.00 (p = 0.52) RMSEA indices were not statistically significant and, therefore, did not justify a rejection of the model. Furthermore, the 1.0 CFI was greater than the 0.95 criterion. Both of the standardized path coefficients in model 2 were significant (p < 0.05): β1 = 0.19 and β2 = 0.18.
In contrast, model 1 and model 3 were less ideal. Their χ2 (model 1: 3.32, p = 0.06; model 3: 2.7, p = 0.09) and RMSEA (model 1: 0.15, p = 0.11; model 3: 0.12, p = 0.14) indices were larger. Their CFIs were both 0.00. The standardized path coefficients were not statistically significant.
Discussion
The results of the present study confirmed the major hypothesis: the combination of a BMIP ≥85 with a PH of externalizing disorders was associated with a greater disruption of cognitive control (i.e. increased error rate and P300 latency) on incompatible trials than was seen among adolescents with either or neither attribute. The results also confirmed a secondary hypothesis: an elevated body mass, defined as a BMIP ≥85, is not uncommon. Thirty-seven percent (41/109) of our subjects were either overweight (>95th percentile) or at risk for overweight (≥85th percentile) by the CDC definition. Although we do not have a nationally representative sample, it is nonetheless remarkable that this rate from 1993 to 1997 is minimally different from the 43–45% prevalence rates reported for racial/ethnic minorities in 2002 [39].
An elevated BMI is a medical condition of potential significance. It may, in some cases, also be an indicator of a personality type characterized by high levels of impulsivity [14], affect lability [21] or impatience [20], and/or an indicator of an externalizing disorder [40]. It is for this reason that the overlap and interaction between BMIP and a PH of externalizing disorders was hypothesized. In other studies, both independent variables have been associated with these personality or psychiatric features in adolescent [41,42,43,44] and adult [20,45,46] samples. It is also for this reason that an elevated BMI and a PH of externalizing disorders were hypothesized to impair cognitive control: when confronted with conflicting information (i.e. the Stroop stimulus), subjects with both risk factors for an impulsive personality should experience more difficulty than other subjects. They would, for example, be expected to display an increased number of inaccurate responses (fig. 2b; right bar), and disproportionately slow their stimulus evaluation and response selection time to improve performance, resulting in a delay in P300 (fig. 2a; right bar).
It is noteworthy that the present study did not reveal an association between either P300 or N450 amplitude and the grouping factors. The absence of a change in amplitude may suggest that BMIP and PH are less reliably associated with gray matter dysfunction, indexed primarily by ERP amplitude, than with white matter dysfunction, indexed primarily by ERP latency [47]. Indeed, white matter abnormalities do appear to be the predominant and earliest sign of the neuropathology of obesity [33]. It is presently unclear whether white or gray matter abnormalities predominate among individuals with a PH of externalizing disorders.
The present study was also unique in testing three conceptual models in an attempt to clarify how parental externalizing disorders, P300 latency and adolescent BMI may relate to one another. The best-fitting path analysis model (model 2 in fig. 4) conceptualized the P300 delay during the conflict condition, presumably reflecting dysfunction of P300 generators within the anterior cingulate (fig. 3) or prefrontal cortex [2,3,4,5], as a step that mediates the relation between family history and BMI. The connection of this family history variable to both the Stroop interference effect and BMI raises the common question whether it measures a genetic or environmental effect. The present study cannot provide a definitive answer to the question. It would be interesting to evaluate whether the candidate genes (e.g. GABRA2[48], CHRM2[49] and MAOA[50]) that have been implicated in familial risk for externalizing disorders might play a role in affecting both cognitive control and BMI in adolescents. A future study will examine this question.
Fig. 4.
Alternative path models relating BMIP, parental externalizing disorders, and P300 latency following the Stroop stimulus. Standardized path coefficients are reported (* p < 0.05). Model 2 was a superior fit to the data.
In conclusion, we believe that the present results add to our current understanding of the correlates and possible predictors of an excess body mass. Whereas much of the attention within psychiatry has focused on the hypothesized role of internalizing disorders in promoting bulimia and binge eating, these disorders do not always predict an excess BMI. Indeed, bulimic patients will, by definition, engage in compensatory behaviors (i.e. purging) that uncouple the connection between their energy intake and BMI. Therefore, the correlates of eating disorders and an excess BMI may be different. The present results suggest that externalizing disorders may be more important than the internalizing disorders in predicting BMI, particularly among adolescents with an early age of onset of weight problems. Future studies should also evaluate externalizing disorders as potential modifiers of weight loss therapy outcome.
Limitations
Although the present results are intriguing, they are not without limitations. For example, the present study was focused on the correlates of the BMI during adolescence. As such, the design precludes one from identifying correlates that may emerge after years of living with an elevated BMI, its stigma or its medical complications.
The recruitment source is another limitation. The present sample of 109 volunteers was not drawn from a clinic population characterized by serious psychological impairment and exposure to psychotropic medications. The sample was instead drawn from a healthier group with the constraint that a large percentage of subjects were the biological offspring of a father with a lifetime diagnosis of alcohol, cocaine or heroin dependence.
A third limitation is the age range. Adolescence is a reasonable starting point for examining the emergence and development of a substance use disorder. However, it is not necessarily optimal for studying the emergence of weight problems and obesity. An optimal study would begin at an earlier age and follow the children over time. In this manner, one could address an obvious limitation of our path analysis, in which the variables were measured simultaneously, and better determine the temporal relationship between P300 impairments and BMI.
Acknowledgment
This research was supported by Public Health Service grants P60AA03510, M01RR06192, R01DA017666 and R01DA08598.
References
- 1.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 2.Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- 3.Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic versus evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerns JG. Anterior cingulate and prefrontal cortex activity in an fMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura K, Wada-Isoe K, Kitayama M, Nakashima K. Executive dysfunction in non-demented Parkinson's disease patients with hallucinations. Acta Neurol Scand. 2008;117:255–259. doi: 10.1111/j.1600-0404.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- 7.Verdelho A, Madureira S, Ferro JM, Basile AM, Chabriat H, Erkinjuntti T, Fazekas F, Hennerici M, O'Brien J, Pantoni L, Salvadori E, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Inzitari D. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78:1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer LO. The effects of HIV on P300 are moderated by familial risk for substance dependence: implications for a theory of brain reserve. Drug Alcohol Depend. 2008;94:92–100. doi: 10.1016/j.drugalcdep.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the Stroop test. Neuropsychopharmacology. 1999;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 11.Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Burger KS, Kern M, Coleman KJ. Characteristics of self-selected portion size in young adults. J Am Diet Assoc. 2007;107:611–618. doi: 10.1016/j.jada.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Barkeling B, Ekman S, Rossner S. Eating behaviour in obese and normal weight 11-year-old children. Int J Obes Relat Metab Disord. 1992;16:355–360. [PubMed] [Google Scholar]
- 14.Braet C, Claus L, Verbeken S, van Vlierberghe L. Impulsivity in overweight children. Eur Child Adolesc Psychiatry. 2007;16:473–483. doi: 10.1007/s00787-007-0623-2. [DOI] [PubMed] [Google Scholar]
- 15.Nederkoorn C, Braet C, van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: the role of impulsivity. Eat Behav. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Fassino S, Leombruni P, Piero A, Abbate-Daga G, Giacomo Rovera G. Mood, eating attitudes, and anger in obese women with and without binge eating disorder. J Psychosom Res. 2003;54:559–566. doi: 10.1016/s0022-3999(02)00462-2. [DOI] [PubMed] [Google Scholar]
- 17.Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45:1071–1075. doi: 10.1016/j.brat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite. 2006;47:253–256. doi: 10.1016/j.appet.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67:1–8. doi: 10.1097/01.psy.0000151489.36347.18. [DOI] [PubMed] [Google Scholar]
- 20.Bauer LO. Psychiatric and neurophysiological predictors of obesity in HIV/AIDS. Psychophysiology. 2008;45:1055–1063. doi: 10.1111/j.1469-8986.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasler G, Pine DS, Gamma A, Milos G, Ajdacic V, Eich D, Rossler W, Angst J. The associations between psychopathology and being overweight: a 20-year prospective study. Psychol Med. 2004;34:1047–1057. doi: 10.1017/s0033291703001697. [DOI] [PubMed] [Google Scholar]
- 22.Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol. 2008;23:467–474. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham Heart Study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 24.Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiatry. 2007;48:57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 27.Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison J, Biro FM, Daniels SR, Striegel-Moore RH. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2007;150:18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann NY Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- 30.West R, Jakubek K, Wymbs N, Perry M, Moore K. Neural correlates of conflict processing. Exp Brain Res. 2005;167:38–48. doi: 10.1007/s00221-005-2366-y. [DOI] [PubMed] [Google Scholar]
- 31.Liotti M, Woldorff MG, Perez R, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38:701–711. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 32.Haltia LT, Viljanen A, Parkkola R, Kemppainen N, Rinne JO, Nuutila P, Kaasinen V. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284. doi: 10.1210/jc.2006-2495. [DOI] [PubMed] [Google Scholar]
- 33.Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 35.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 36.Duncan-Johnson CC, Kopell BS. The Stroop effect: brain potentials localize the source of interference. Science. 1981;214:938–940. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- 37.Kline RB. Principles and Practice of Structural Equation Modeling. New York: Guilford; 2004. [Google Scholar]
- 38.Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 39.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 40.Anderson SE, Cohen P, Naumova EN, Must A. Relationship of childhood behavior disorders to weight gain from childhood into adulthood. Ambul Pediatr. 2006;6:297–301. doi: 10.1016/j.ambp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Decaluwe V, Braet C, Moens E, van Vlierberghe L. The association of parental characteristics and psychological problems in obese youngsters. Int J Obes (Lond) 2006;30:1766–1774. doi: 10.1038/sj.ijo.0803336. [DOI] [PubMed] [Google Scholar]
- 42.Odgers CL, Milne BJ, Caspi A, Crump R, Poulton R, Moffitt TE. Predicting prognosis for the conduct-problem boy: can family history help? J Am Acad Child Adolesc Psychiatry. 2007;46:1240–1249. doi: 10.1097/chi.0b013e31813c6c8d. [DOI] [PubMed] [Google Scholar]
- 43.Barnow S, Ulrich I, Grabe HJ, Freyberger HJ, Spitzer C. The influence of parental drinking behaviour and antisocial personality disorder on adolescent behavioural problems: results of the Greifswalder Family Study. Alcohol Alcohol. 2007;42:623–628. doi: 10.1093/alcalc/agm051. [DOI] [PubMed] [Google Scholar]
- 44.Janicke DM, Harman JS, Kelleher KJ, Zhang J. Psychiatric diagnosis in children and adolescents with obesity-related health conditions. J Dev Behav Pediatr. 2008;29:276–284. doi: 10.1097/DBP.0b013e31817102f8. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein RB, Dawson DA, Stinson FS, Ruan WJ, Chou SP, Pickering RP, Grant BF. Antisocial behavioral syndromes and body mass index among adults in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2008;49:225–237. doi: 10.1016/j.comppsych.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a go-nogo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardenas VA, Chao LL, Blumenfeld R, Song E, Meyerhoff DJ, Weiner MW, Studholme C. Using automated morphometry to detect associations between ERP latency and structural brain MRI in normal adults. Hum Brain Mapp. 2005;25:317–327. doi: 10.1002/hbm.20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer JR, Chan G, Dick DM, Kuperman S, Bucholz KK, Edenberg HJ, Polgreen LA, Hesselbrock VM, Schuckit MA, Nurnberger JI, Kapp ES, Porjesz B, Bierut LJ. Multiple-domain predictors of problematic alcohol use in young adulthood. J Stud Alcohol Drugs. 2008;69:649–659. doi: 10.15288/jsad.2008.69.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Kramer J, Hinrichs A, Bertelsen S, Budde JP, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate A. Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- 50.Contini V, Marques FZ, Garcia CE, Hutz MH, Bau CH. MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:305–308. doi: 10.1002/ajmg.b.30290. [DOI] [PubMed] [Google Scholar]