Abstract
Background
Studies have reported declines with age in cognitive or physical functioning, but rarely identify whether these are parallel or linked events in the same study. Furthermore, most research in this area has focused on persons in late life rather than midlife. Objective: The objective of the study was to determine (1) if cognitive functioning was related to physical functioning and whether this relationship persisted after adjustment for age, menopause status, metabolic status, depression and socioeconomic resources, and (2) if changes in physical functioning were associated with changes in cognitive functioning over a 4-year follow-up period.
Methods
Data were from the Study of Women's Health Across the Nation (SWAN), a multi-site, longitudinal study of women aged 46–56 years at follow-up examination 4. Three follow-up examinations (study years 04, 06 and 08) included measures of physical functioning perception (MOS SF-36) and cognitive functioning [Symbol Digit Modality Test (SDMT), Digit Span Backward Test (DSBT), and East Boston Memory Test (EBMT)] (n = 2,405).
Results
Women with lower cognitive functioning scores also had lower perceived physical functioning scores. While adjustment for covariates attenuated the association between perceived physical functioning and both the SDMT and EBMT cognitive measures, these associations remained statistically significant. Additionally, the 4-year change in perceived physical functioning was significantly associated with the 4-year change in the EBMT.
Conclusions
At midlife, there were associated declines in cognitive and perceived physical functioning scores, commencing at midlife in women.
Key Words: Physical functioning, Cognitive functioning, Menopause, Metabolic syndrome, MOS SF-36
Introduction
It is believed that functioning reflects the integration of cognitive, physiological and physical capacities and that the aging-related declines in any one of these may be associated with a concomitant decline in the other(s) [1]. While studies report that there are declines in cognitive or physical functioning with age, studies have not adequately addressed whether these are parallel or linked events because most studies have examined either cognitive or physical functioning. In addition, while current theories of the disablement process emphasize the importance of understanding early physical changes as a means of helping prevent or delay adverse health outcomes in older adults [2], most studies of functioning have examined populations of adults who are substantially past midlife when the implementation of prevention activities may have less impact.
Cognitive function may have a significant role in the subclinical presentation of changes in the overall functional status by impairing executive abilities that are relevant for the performance of complex activities. Alternatively, diminishing cognitive functioning may interfere with an individual's ability to accurately perceive and report his/her functional status [3]. A number of studies have shown a relationship between cognitive and physical functioning [3,4,5,6,7,8,9] using both cross-sectional [3,5,6,8] and longitudinal [4,7,9] study designs, but the studies have been limited to various elderly populations. A recent review noted that in cross-sectional studies of the elderly, executive control function and general measures of cognition are particularly strong correlates of physical functioning [10].
A number of underlying physiological and psychological processes have been proposed as mechanisms to link cognitive and physical functioning. One proposal focuses on a more compromised metabolic environment encompassing atherosclerosis, altered glucose metabolism, vascular injury and an increased acute phase inflammatory response [11]. This is supported by observations of elevated serum amyloid A in neurogenerative disease [11] and higher D-dimer levels in individuals with diminished walking ability [12]. Alternatively, it has been suggested that physical activity, acting as a surrogate for physical functioning, preserves or promotes cerebral blood flow [13] which, in turn, is associated with better performance during cognitive assessment. Several studies have shown that indicators of socioeconomic status (SES) in adulthood are related to risk of cognitive decline [14] and poor physical functioning [15]. Previous studies have also found an independent association between depressive symptoms and both physical performance declines [16,17] and cognitive decline and impairment [18,19]. Finally, few studies have addressed the role of ovarian aging and menopause status as a component of the aging process in women.
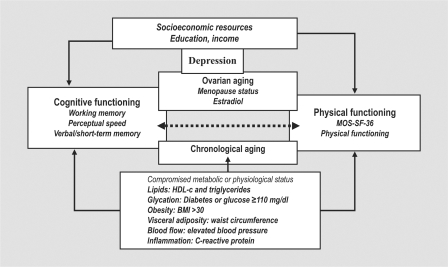
We propose that the parallel and co-occurrence of cognitive and physical functioning decline begins in the midlife and that women in the postmenopause will have poorer physical functioning than women in earlier menopause stages, apart from the expected declines associated with aging. We further propose that the decline will be conditioned by an underlying component of metabolic and physiological compromise, quantified as the metabolic syndrome, depressive symptoms and lower SES (fig. 1). We posed two related questions: (1) is there an association between measures of physical and cognitive functioning cross-sectionally and does it persist after considering SES, metabolic status, depressive symptoms and menopause status, and (2) is there an association between the longitudinal change in physical functioning and cognitive functioning over a 4-year period?
Fig. 1.
Model of cognitive and physical functioning.
Methods
Sample
The Study of Women's Health Across the Nation (SWAN) is a seven-site, longitudinal study conducted in community-based groups of women. At baseline, the 3,302 enrolled women were aged 42–52 years, premenopausal or early perimenopausal, without use of exogenous hormones, and self-identified as belonging to one of the designated race/ethnic groups, as previously described [20]. SWAN was approved by the Institutional Review Boards at each site and all participants provided written informed consent at every visit. Study sites whose data were included in these analyses were located in southeast Michigan, Chicago, Ill., Los Angeles, Calif., Alameda and Contra Costa County, Calif., Pittsburgh, Pa., and Boston, Mass. The analysis sample includes African-American, Caucasian, Chinese and Japanese women.
Cognitive and Physical Functioning Measures
Cognitive and physical functioning data were collected at the follow-up (F/U) 04, 06, and 08 examinations. The cognitive tests were chosen because: (1) they represent different aspects of cognition that are likely to change with aging; (2) they represent several aspects of cognition thought to be associated with changes in hormone status (which have largely been based on studies of hormone therapy), and (3) these measures were less likely to reflect ceiling and floor effects of tests typically administered to population whose ages exceed 70 years. Complex attention and information processing speed was assessed using the Symbol Digit Modality Test (SDMT), oral version. Participants identified as many symbol-digit matches as possible in a 90-second period [21]. Possible scores ranged from 11 to 95. Working memory was assessed using the Digit Span Backward Test (DSBT) where participants repeated backwards increasingly longer strings of digits until they made an error. Data were scored according to the Wechsler Memory Scale revised manual [22], with scores ranging from one to 12. The East Boston Memory Test (EBMT) provided information on verbal and short-term memory [23]. After being told a story, participants were asked to recall story elements immediately and 30 min later. Because results for the two time points were similar, this paper reports only data from the first recall of the story. EBMT scores ranged from zero to 12. The tests of cognitive function were independent variables in our models.
Perception of physical functioning (PF) was assessed with the 10-item perceived physical functioning scale of the Medical Outcomes Scale (SF-36) [24]. The scale reflects the self-reported difficulty in undertaking physical activities ranging from vigorous athletic activity to the ability to bathe and dress. The possible scores [0 (worst) to 100 (best)] were categorized into a three-level categorical variable as no physical limitation (score 85–100), moderate limitation (score 50–84), and severe limitation (0–49) for the analyses in table 2. The continuous SF-36 outcome was the dependent variable in our models in tables 3 and 4.
Table 2.
Means of cognitive scores by physical limitation category
| Cognitive measure | Physical limitation index |
p value∗ | |||
|---|---|---|---|---|---|
| all women | no limitation | moderate limitation | severe limitation | ||
| Symbol Digit Modality Test | |||||
| Follow-up visit 04 (n =1,914) | 56.1 | 57.3 | 54.7 | 50.5 | <0.001 |
| Follow-up visit 06 (n =1,890) | 56.8 | 58.0 | 55.7 | 50.1 | <0.001 |
| Follow-up visit 08 (n = 1,382) | 58.6 | 60.0 | 57.0 | 52.4 | <0.001 |
| Digit Span Backward Test | |||||
| Follow-up visit 04 (n = 1,915) | 6.6 | 6.8 | 6.4 | 5.9 | <0.001 |
| Follow-up visit 06 (n =1,860) | 6.8 | 6.9 | 6.5 | 6.1 | <0.001 |
| Follow-up visit 08 (n = 1,381) | 7.0 | 7.2 | 6.7 | 6.2 | <0.001 |
| East Boston Memory Test | |||||
| Follow-up visit 04 (n = 1,921) | 10.2 | 10.3 | 10.0 | 9.5 | <0.001 |
| Follow-up visit 06 (n = 1,920) | 10.3 | 10.4 | 10.1 | 9.7 | <0.001 |
| Follow-up visit 08 (n = 1,412) | 10.5 | 10.5 | 10.6 | 10.2 | <0.001 |
p value denotes significant difference in cognitive measures according to physical limitation severity category.
Table 3.
Association of measures of cognitive function with physical functioning adjusted for age, race, and study site
| Symbol Digit Modality Test |
Digit Span Backward Test |
East Boston Memory Test |
||||
|---|---|---|---|---|---|---|
| β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Basic models1 | ||||||
| Standardized cognitive measure | 1.858 (0.337) | <0.001 | 0.569 (0.309) | 0.030 | 0.744 (0.256) | 0.002 |
| Basic plus SES | ||||||
| Standardized cognitive measure | 1.285 (0.039) | <0.001 | 0.699 (0.310) | 0.411 | 0.614 (0.257) | 0.008 |
| Education | 1.519 (0.377) | <0.001 | 1.670 (0.378) | <0.001 | 1.641 (0.375) | <0.001 |
| Difficulty paying for basics | 4.983 (0.545) | <0.001 | 5.171 (0.549) | <0.001 | 5.110 (0.545) | <0.001 |
| Basic plus metabolic | ||||||
| Standardized cognitive measure | 1.800 (0.352) | <0.001 | 0.581 (0.322) | 0.035 | 0.687 (0.271) | 0.005 |
| Metabolic status | −5.532 (0.774) | <0.001 | −5.569 (0.784) | <0.001 | −5.559 (0.774) | 0.000 |
| Basic plus menopause | ||||||
| Standardized cognitive measure | 1.839 (0.336) | <0.001 | 0.561 (0.310) | 0.034 | 0.728 (0.257) | 0.002 |
| Premenopause | 5.226 (1.502) | <0.001 | 5.484 (1.521) | <0.001 | 5.221 (1.509) | <0.001 |
| Early perimenopause | 1.303 (0.718) | 0.035 | 1.583 (0.725) | 0.015 | 1.459 (0.720) | 0.021 |
| Late perimenopause | 0.761 (0.787) | 0.150 | 0.635 (0.793) | 0.211 | 0.552 (0.786) | 0.241 |
| Hormone user | 0.335 (0.950) | 0.362 | 0.493 (0.960) | 0.303 | 0.415 (0.952) | 0.332 |
| Postmenopause | reference | reference | reference | |||
| Basic plus CESD score | ||||||
| Standardized cognitive measure | 1.591 (0.331) | <0.001 | 0.530 (0.305) | 0.04 | 0.623 (0.255) | 0.004 |
| CESD sum | −0.436 (0.033) | <0.001 | −0.453 (0.033) | <0.001 | −0.444 (0.033) | <0.001 |
| Expanded models2 | ||||||
| Standardized cognitive measure | 1.039 (0.348) | 0.001 | 0.084 (0.317) | 0.39 | 0.472 (0.269) | 0.04 |
| Education | 1.002 (0.367) | 0.003 | 1.109 (0.367) | 0.001 | 1.123 (0.365) | 0.001 |
| Difficulty paying for basics | 4.406 (0.563) | <0.001 | 4.568 (0.566) | <0.001 | 4.490 (0.562) | <0.001 |
| Metabolic syndrome | −5.277 (0.755) | <0.001 | −5.383 (0.762) | <0.001 | −5.221 (0.754) | <0.001 |
| Menopause status | ||||||
| Premenopause | 4.780 (1.545) | 0.001 | 4.951 (1.561) | <0.001 | 4.820 (1.550) | 0.001 |
| Early perimenopause | 1.183 (0.731) | 0.05 | 1.359 (0.736) | 0.03 | 1.333 (0.732) | 0.03 |
| Late perimenopause | 0.462 (0.822) | 0.28 | 0.244 (0.827) | 0.38 | 0.261 (0.820) | 0.37 |
| Hormone user | −0.189 (0.969) | 0.42 | −0.100 (0.977) | 0.45 | −0.139 (0.971) | 0.45 |
| Postmenopause | reference | reference | reference | |||
| CESD sum | −0.376 (0.035) | <0.001 | −0.388 (0.035) | <0.001 | −0.385 (0.034) | <0.001 |
Basic models relate four different cognitive function assessments to physical function, adjusted for age, race, and study site.
Expanded models additionally adjusted for education, difficulty paying for basics, metabolic syndrome status, menopause status, and depression.
Table 4.
Association of 2-year change in physical functioning score and 2-year change in measures of cognitive function
| Symbol Digit Modality Test |
Digit Span Backward Test |
East Boston Memory Test |
||||
|---|---|---|---|---|---|---|
| β (SE) | p value | β (SE) | p value | β (SE) | p value | |
| Basic models1 | ||||||
| Standardized cognitive measure | 2.170 (0.829) | 0.004 | −0.185 (0.814) | 0.410 | 2.064 (0.841) | 0.007 |
| Time | 0.011 (0.192) | 0.476 | 0.162 (0.192) | 0.199 | 1.50 (0.191) | 0.216 |
| Time × standardized cognitive measure | −0.052 (0.125) | 0.388 | 0.126 (0.129) | 0.164 | −0.231 (0.139) | 0.048 |
| Expanded models2 | ||||||
| Standardized cognitive measure | 1.520 (0.878) | 0.041 | 0.127 (0.139) | 0.280 | 1.917 (0.898) | 0.016 |
| Time | 0.119 (0.194) | 0.268 | 0.209 (0.191) | 0.138 | 1.188 (0.191) | 0.162 |
| Time × standardized cognitive measure | −0.087 (0.135) | 0.256 | 0.127 (0.139) | 0.180 | −0.256 (0.150) | 0.044 |
Basic models relate physical function change with changes in four different cognitive function assessments, adjusted for age, race, and study site.
Expanded models additionally adjusted for education, difficulty paying for basics, metabolic syndrome status, depressive symptoms, and menopause status.
Physiological Environment
Menopausal status was based on menstrual bleeding predictability in the three months prior to interview with the following categories: no decreased predictability in menses timing (premenopause), decreasing predictability in the time between menses (early perimenopause), no menses for 3–11 months (late perimenopause), or no menses for 12 or more months (postmenopause). Women using estrogen and related hormones were classified as hormone users.
The Core SWAN protocol specified a blood draw following a minimum 10-hour fast with specimens analyzed for cardiovascular risk measures at baseline and follow-up visits through visit 07. Phlebotomy appointments occurred between days two through five of the follicular phase of the menstrual cycle to allow for interpretation of a standardized hormone environment among still menstruating women.
Women were defined as having metabolic syndrome by meeting at least three of the following criteria based on the National Cholesterol Education Program Adult Treatment Panel III [25] and using race-specific criteria for waist circumference [26]: (1) waist circumference >88 cm for Caucasian and African-American participants and ≥80 cm for Chinese and Japanese participants; (2) triglycerides ≥150 mg/dl; (3) HDL-c <50 mg/dl; (4) systolic blood pressure (BP) ≥130 mm Hg or diastolic BP ≥85 mm Hg or taking antihypertensive medication, and (5) fasting glucose ≥110 mg/dl or evidence of diabetes. The metabolic syndrome categorization was based on measures taken one year preceding each measure of cognitive and physical functioning status (F/U 03, 05, 07) based on our hypothesis that compromised metabolic status altered cognitive and physical function (fig. 1).
Depression
A depressive symptoms score was identified at F/U 04, 06 and 08 using the Center for Epidemiologic Studies Depression Scale (CESD) [27].
Socioeconomic Resources
Education was measured in five categories at study baseline. The categories included less than high school, high school, more than high school, college, and more than college. Women in the study were also asked if it was difficult to pay for basics such as food and housing at F/U 04, 06 and 08. The coded responses were difficult, somewhat difficult, or not difficult at all. A high value indicates that it was not hard to pay for basics. Chronological age was measured in single years at F/U 04, 06 and 08. All analyses included a dummy coded variable for study site.
Statistical Methods
In table 2, mean cognitive cores were calculated by physical function category and study year using generalized linear models. In tables 3 and 4, the statistical analyses were implemented using a repeated measures data set with 1–3 records for each woman with the records representing the cognitive and physical functioning measures for each year of study (F/U 04, 06 and 08). The independent variables representing cognition were normalized to have a 0 mean and 1 SD to facilitate comparisons in the models in tables 3 and 4. At F/U 08, there were fewer functioning data points contributing to the longitudinal analyses because, at that visit in an effort to reduce costs, sites collected data from the sample via mailed questionnaires with the postmenopausal women. Completion of the third assessment was associated with the control variables of age, ethnicity, and study site. In addition, women with lower scores on the first evaluation were less likely to complete later evaluations. This reduced the size of the decline in cognitive scores over time and may have attenuated our results.
The associations between cognitive and physical function variables were estimated from mixed models using the xtreg procedure (STATA 8 statistical package) with the GLS random effects estimator. Two sets of models were estimated with these data. In the first set, the marginal association between the cognitive measures and physical functioning was estimated (table 3). A second set of models was used to determine if change in cognitive function was related to change in physical function (table 4). Variables representing time and an interaction term between the time variable and cognitive function were added to the model. This interaction term indicates whether or not the change in the cognitive function is related to the change in physical function; the term's p value indicates the statistical significance of the relationship.
Results
Sample Characteristics
The average age of participants (at F/U visit 04) was 50 years. Half of the sample was Caucasian, 28% were African-American, 10% were Chinese, and 12% were Japanese. Approximately one-third of women reported at least some difficulty paying for basics (table 1).
Table 1.
Demographic and study variables for participants contributing to follow-up 04, 06 and 08 measures
| Total | |
|---|---|
| Number | 2,003 |
| Age in years at 04, mean ± SD | 50.0 ± 2.6 |
| Race or ethnicity, % | |
| African-American | 28 |
| Caucasian | 50 |
| Chinese | 10 |
| Japanese | 12 |
| Education at 04, % | |
| High school or less | 18.2 |
| Some college | 32.9 |
| Complete college | 22.9 |
| More than 16 years | 26.0 |
| Paying for basics 04, % | |
| Much difficulty | 6.8 |
| Some difficulty | 26.2 |
| No difficulty | 67.0 |
| Cognitive measures | |
| Cognitive-04, mean ± SD | |
| East Boston | 10.2 ± 1.8 |
| Symbol Digit | 56.0 ± 10.2 |
| Digit Backward | 6.6 ± 2.3 |
| Cognitive-06, mean ± SD | |
| East Boston | 10.3 ± 1.8 |
| Symbol Digit | 56.8 ± 10.5 |
| Digit Backward | 6.8 ± 2.4 |
| Cognitive-08, mean ± SD | |
| East Boston | 10.5 ± 1.6 |
| Symbol Digit | 58.6 ± 11.3 |
| Digit Backward | 6.9 ± 2.3 |
| Physical limitations | |
| Physical function-04 | |
| Mean ± SD | 83.6 ± 21.4 |
| Median | 90 |
| Severe limitations, % | 7.9 |
| Moderate limitations, % | 23.9 |
| No limitations, % | 68.1 |
| Physical function-06 | |
| Mean ± SD | 83.3 ± 21.8 |
| Median | 90 |
| Severe limitations, % | 8.8 |
| Moderate limitations, % | 21.4 |
| No limitations, % | 69.7 |
| Physical function-08 | |
| Mean ± SD | 82.0 ± 22.1 |
| Median | 90 |
| Severe limitations, % | 9.4 |
| Moderate limitations, % | 25.4 |
| No limitations, % | 65.2 |
| Menopause status-04, % | |
| Premenopause | 7.0 |
| Early perimenopause | 44.8 |
| Late perimenopause | 10.7 |
| Postmenopause | 25.4 |
| Hormone use | 12.1 |
| Metabolic syndrome-03, % | 19.6 |
| CESD summary score-04, mean ± SD | 9.1 ± 8.9 |
At F/U visit 04, 7% of women were premenopausal, 45% were early perimenopausal, 11% were late perimenopausal, and 25% were postmenopausal. Twelve percent of women were using exogenous hormones. Approximately 1 in 5 women was classified as having metabolic syndrome (table 1).
Physical and Cognitive Functioning
The average SF-36 physical functioning score at F/U 04 was 83.6. Sixty-eight percent of women were designated as having no physical limitations (PF score 85–100), 24% reported moderate limitations (PF score 50–84), and 8% were classified with severe limitations at the F/U 04 (PF score <50). There was a modest decline in the proportion of women reporting no limitations between F/U 04 and F/U 08.
Mean scores for the cognitive functioning measures at F/U 04 were SDMT (56, range 11–95), DSBT (6.6, range 1–12), and EBMT (10, time 1 and time 2, range 0–12). There were small overall 4-year changes in the cognitive functioning measures between F/U 04 and F/U 08.
Association of Physical and Cognitive Functioning. Women with severe physical limitations had lower scores on unadjusted cognitive measures, and this was consistently observed at each of the three visits (table 2).
Results from the cross-sectional random effects model for each cognitive measure are shown in table 3 and include three levels: (1) basic models with adjustment for age, race, education and study site; (2) basic models with one additional covariate, and (3) full models including adjustment for menopause status, depressive symptoms, metabolic status, and SES. In the basic models, all three cognitive measures positively and significantly predicted physical functioning. An increase of 1 SD in the SDMT would result in a 1.86 unit higher physical functioning score. A 1-SD increase in DSBT would result in a 0.57 points higher physical functioning score. A 1-SD increase in the EBMT would result in a 0.74 points higher physical functioning score.
The second groups of models included an additional covariate or covariate group to the basic model (table 3). First, the two variables representing SES were added to the model. Both SES variables were significant determinants of physical functioning. The coefficients of the cognitive measure were attenuated but remained statistically significant for the SDMT and the EBMT measures. Metabolic status was significantly associated with physical functioning, and all three cognitive measures remained significant. Only small changes occurred in the size of the coefficients. The addition of the menopause variable did not have a sizeable effect on the magnitude of significance of the cognitive measures. Finally, the CESD measure was significantly related to physical functioning but had only a small effect on the beta coefficients for the cognitive variables.
In the third group of models that also controlled for SES, metabolic syndrome status, menopause status and depressive symptoms (table 3), the coefficient for physical functioning remained positive and statistically significant for the SDMT and EBMT. The size of the beta coefficient dropped from 1.86 to 1.04 for the SDMT and from 0.74 to 0.47 for the EBMT.
Changes in Physical and Cognitive Functioning – Longitudinal Model (table 4). The 4-year difference in cognitive functioning was related to the 4-year difference in physical functioning as shown with a random-effects model. Models also included a variable for time and an interaction term for cognitive functioning with time to assess if the change in cognitive functioning was associated with the change in physical functioning.
In models that included both the basic and the expanded list of covariates (table 4), the interaction term between time and cognitive functioning was significant for the EBMT, suggesting that the 4-year change in verbal and short-term memory was predictive of the 4-year change in physical functioning. Interaction terms representing the other two cognitive measures were not statistically significant.
Discussion
In this longitudinal study of women at midlife: (1) lower physical functioning scores were related to lower scores on the three measures of cognitive functioning; (2) the 4-year change in physical functioning was associated with the 4-year change in a measure of verbal and short-term memory (EBMT) but not with the 4-year change in measures of complex attention and information processing speed (SDMT) or the 4-year change in a measure of working memory (DSBT), and (3) relationships between physical and cognitive functioning measures were attenuated with adjustment for socioeconomic environment, depressive symptoms and metabolic syndrome, suggesting that in part, these associations between cognitive and perception of physical functioning were influenced by measures of SES, depressive symptoms, and metabolic syndrome.
While the SDMT measure was more strongly correlated with physical functioning assessment, cross-sectionally, the change in physical functioning was more strongly associated with EBMT than with other cognitive measures. This may have occurred because memory may change more than other cognitive measures in this age group.
We used the metabolic syndrome to approximate alterations in the physiological environment that may be related to cerebral blood flow and glucose metabolism. Chronic insulin resistance appears to have a deleterious effect on memory among individuals with type II diabetes mellitus [28,29] or impaired glucose tolerance [30] and is related to impaired operant learning and classical conditioning in animals [31].
In our investigation, SES attenuated the association between physical and cognitive functioning, even after adjustment for metabolic status. This suggests that while obesity, high BP, diabetes, and reduced lung function are more prevalent among low SES groups and lower SES is a risk factor for cardiovascular disease and stroke [32], other elements in SES not linked to these metabolic measures remain operational in this sample. Notably, a longitudinal study of older adults found that those in the lowest SES group also had an increased risk of mobility limitation, even after accounting for a wide range of diseases and risk factors [33]. The magnitude and consistency of statistically significant associations with measures available suggest that the overarching impact of SES in relation to functioning is present even as modest declines are being observed among these mid-aged women [34].
Menopause status also contributed to the association of cognitive measures with physical functioning. We have previously described that progressive stages of the menopause transition were associated with increasingly diminished physical functioning among 16,065 women aged 40–55 years, after adjusting for age [35]. Studies of estrogen therapy, frequently conceived as a model of the physiological state prior to the demise of ovarian estradiol levels, have shown that estrogen users may have decreased brain white matter lesions [36], increased cerebral blood flow [37], and increased glucose metabolism in certain brain regions [38] compared to nonusers. These characteristics appear to modulate the effects of various neurotransmitter systems [39] and alter regional brain activation patterns during cognitive processes [40]. Our results incorporating a measure of depressive symptoms are also consistent with earlier research that identified that depression was associated with reduced physical functioning and attenuated the relationship between cognitive and physical functioning [16,17,18].
This study has strengths and limitations. Although this study is associated with a large number of women, the proportionately fewer Japanese and Chinese women forced us to examine overall associations, adjusting for race, rather than examine associations within each race group. While the age of this population is relatively younger compared to other populations in which physical and cognitive functioning have been examined, our assessment suggests that there is a substantial amount of perceived compromised physical functioning present. Assessment of perceived physical functioning may or may not be comparable with information from performance-based measures. More change may have been observed in physical functioning if performance-based measures were available in the study. Further, the cognitive measures were limited to the working memory, perceptual speed and verbal and short-term memory domains. Direct measures of cerebral blood flow were not possible in this large epidemiological study. Finally, while there were three assessments during the 4-year period during the menopause transition, this time period may be inadequate to confirm distinct changes in younger women who are experiencing both chronological and ovarian aging and have experienced only a modest decline in cognitive function. The small amount of change in cognitive and physical functioning may have contributed to the weaker longitudinal associations compared to the cross-sectional associations. Health studies often identify a stronger cross-sectional than longitudinal associations, with the former describing between-person variation while the longitudinal study, by design, includes within-person variation.
This study provided evidence that women at midlife have concurrently diminished physical and cognitive functioning; some decline appeared to occur even in a 4-year period of observation. Apart from chronological age, menopause status, metabolic syndrome, depression and the socioeconomic environment were clearly associated with physical functioning, and their presence attenuated the relationship between physical and cognitive functioning suggesting their potential underlying contribution to functioning.
Acknowledgements
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers
University of Michigan, Ann Arbor, MI – MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA – Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999–present; Rush University, Rush University Medical Center, Chicago, IL – Lynda Powell, PI 1994–2009; Howard Kravitz, PI 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles, CA – Gail Greendale, PI; University of Medicine and Dentistry – New Jersey Medical School, Newark-Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004–present; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office
National Institute on Aging, Bethesda, MD – Marcia Ory 1994–2001; Sherry Sherman 1994–present; National Institute of Nursing Research, Bethesda, MD – Yvonne Bryan, Program Officers.
Central Laboratory
University of Michigan, Ann Arbor, MI – Daniel McConnell, PI (CLASS-Central Ligand Assay Satellite Services).
Coordinating Center
New England Research Institutes, Watertown, MA – Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001–present.
References
- 1.Binder EF, Storandt M, Birge SJ. The relation between psychometric test performance and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M428–M432. doi: 10.1093/gerona/54.8.m428. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Herdman SJ, Kuhn KE, Rubin G, Turano K. Preclinical disability: hypotheses about the bottom of the iceberg. J Aging Health. 1991;3:285–300. [Google Scholar]
- 3.Royall RR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB. Cognitive function, gate speed decline, and comorbidities: the Health, Aging and Body Composition Study. J Gerontol. 2007;62A:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 5.Jagger C, Clark M, Cook AJ. Mental and physical health of elderly people: five year follow-up of a total population. Age Aging. 1989;18:77–82. doi: 10.1093/ageing/18.2.77. [DOI] [PubMed] [Google Scholar]
- 6.Royall RR, Palmer R, Chiodo LK, Polk MJ. Executive control mediates memory's association with change in instrumental activities of everyday living. J Am Geriatr Soc. 2005;53:11–17. doi: 10.1111/j.1532-5415.2005.53004.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Manoux A, Ferrie JE, Lynch JW, Marmot M. The role of cognitive ability (intelligence) in explaining the association between socioeconomic position and health: evidence from the Whitehall II prospective cohort study. Am J Epidemiol. 2005;161:831–839. doi: 10.1093/aje/kwi109. [DOI] [PubMed] [Google Scholar]
- 8.Skerr PA, Albert MS, Fukenstein HH, et al. Correlates of cognitive function in an elderly community population. Am J Epidemiol. 1998;128:1084–1101. doi: 10.1093/oxfordjournals.aje.a115051. [DOI] [PubMed] [Google Scholar]
- 9.Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur studies of successful aging. J Gerontol. 2002;57:M228–M235. doi: 10.1093/gerona/57.4.m228. [DOI] [PubMed] [Google Scholar]
- 10.Royall DR, Lauterbach EC, Kaufer D, Mallot P, Coburn KL, Black KJ. The cognitive correlates of functional status: a review from the Committee on Research of the Am Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19:249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 11.Chung TF, Sipe JD, McKee A, Fine RE, Schreiber BM, Liang JS, Johnson RJ. Serum amyloid A in Alzheimer's disease brain is predominantly localized to myelin sheaths and axonal membrane. Amyloid Int J Exp Clin Invest. 2000;7:105–110. doi: 10.3109/13506120009146246. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Guralnik JM, Greenland P, Green D, Liu K, Ridker PM, Chan C, Criqui MH, Ferrucci L, Taylor LM, Pearce WK, Schneider JR, Oskin SK. Inflammatory and thrombotic blood markers and walking-related disability in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2004;52:1888–1894. doi: 10.1111/j.1532-5415.2004.52514.x. [DOI] [PubMed] [Google Scholar]
- 13.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–128. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 15.Hemingway H, Nicholson A, Stafford M, Roberts R, Marmot M. The impact of socioeconomic status on health functioning as assessed by the SF-36 questionnaire: the Whitehall II Study. Am J Publ Health. 1997;87:1484–1490. doi: 10.2105/ajph.87.9.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pennix BW, Guralnik JM, Ferruci L, Simonsick EM, Deeg DG, Wallace RB. Depressive symptoms and physical decline in community dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 17.Gallo JJ, Rebok GW, Tennsted S, Wadley WD, Horgas A. Linking depressive symptoms and functional disability in late life. Aging Ment Health. 2003;7:469–480. doi: 10.1080/13607860310001594736. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Mendes de Leon CF, Bennett DA, Benias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–129. [PMC free article] [PubMed] [Google Scholar]
- 19.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Sowers MF, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 21.Smith A. Symbol Digit Modalities Test, revised manual. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- 22.Weschler D. Weschler Memory Scale, revised manual. New York: The Psychological Corporation; 1987. [Google Scholar]
- 23.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Steering Committee of the Western Pacific Region of the World Health Organization, the International Association for the Study of Obesity, and the International Obesity Task Force: The Asia-Pacific Perspective: Redefining Obesity and Its Treatment [monograph on the Internet]. Melbourne, Australia: Health Communications Australia Pty Limited; 2000 [cited 2005 Oct 25]. Available from: http://www.diabetes.com.au/pdf/obesity_report.pdf
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1997;1:385–401. [Google Scholar]
- 28.Elias PK, Elias MF, D'Agostino RB, Upples LA, Wilson PW, Silbershatz HJ, Wolfe PA. NIDDM and blood pressure as risk factors for poor cognitive performance: the Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 29.Rapp SR, Espeland MA, Schumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive functioning in postmenopausal women. The Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 30.Vanhanen M, Koivisto K, Kuusisto J, et al. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- 31.Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiol Learn Mem. 2001;75:179–189. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- 32.Marmot MG. Socio-economic factors in cardiovascular disease. J Hypertens Suppl. 1996;14:S201–S205. [PubMed] [Google Scholar]
- 33.House JS, Williams DR. Understanding and reducing socioeconomic and racial/ethnic disparities in health. In: Smedley BD, Syme SL, editors. Promoting Health: Intervention Strategies from Social and Behavioral Research. Washington: National Academy Press; 2000. pp. 81–204. [Google Scholar]
- 34.Koster A, Penninx BW, Bosma H, Kempen GI, Harris TB, Newman AB, Rooks RN, Rubin SM, Simonsick EM, van Eijk JT, Kritchevsky SB. Is there a biomedical explanation for socioeconomic differences in incident mobility limitation? J Gerontol A Biol Sci Med Sci. 2005;60:1022–1027. doi: 10.1093/gerona/60.8.1022. [DOI] [PubMed] [Google Scholar]
- 35.Sowers MF, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. J Am Geriatr Soc. 2001;49:1485–1492. doi: 10.1046/j.1532-5415.2001.4911241.x. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt R, Fazekas F, Reinhart B, Kapeller P, Fazekas G, Offenbacker H, Eber B, Schumacher M, Freidl W. Estrogen replacement therapy in older women: a neuropsychological and brain MRI study. J Am Geriatr Soc. 1996;44:1307–1313. doi: 10.1111/j.1532-5415.1996.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 37.Okura T, Teshima Y, Isse K, Matsuda H, Inoue T, Sakai Y, Iwasaki W, Yaoi Y. Estrogen increases cerebral and cerebellar blood flows in postmenopausal women. Menopause. 1995;2:13–18. [Google Scholar]
- 38.Rasgon NL, Silverman D, Siddarth P, Miller K, Ercoli LM, Elman S, Lavretsky H, Huang SC, Phelps ME, Small GW. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Smith YR, Zubieta JK. Neuroimaging of aging and estrogen effects on central nervous system physiology. Fertil Steril. 2001;76:651–659. doi: 10.1016/s0015-0282(01)01985-9. [DOI] [PubMed] [Google Scholar]
- 40.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]