Abstract
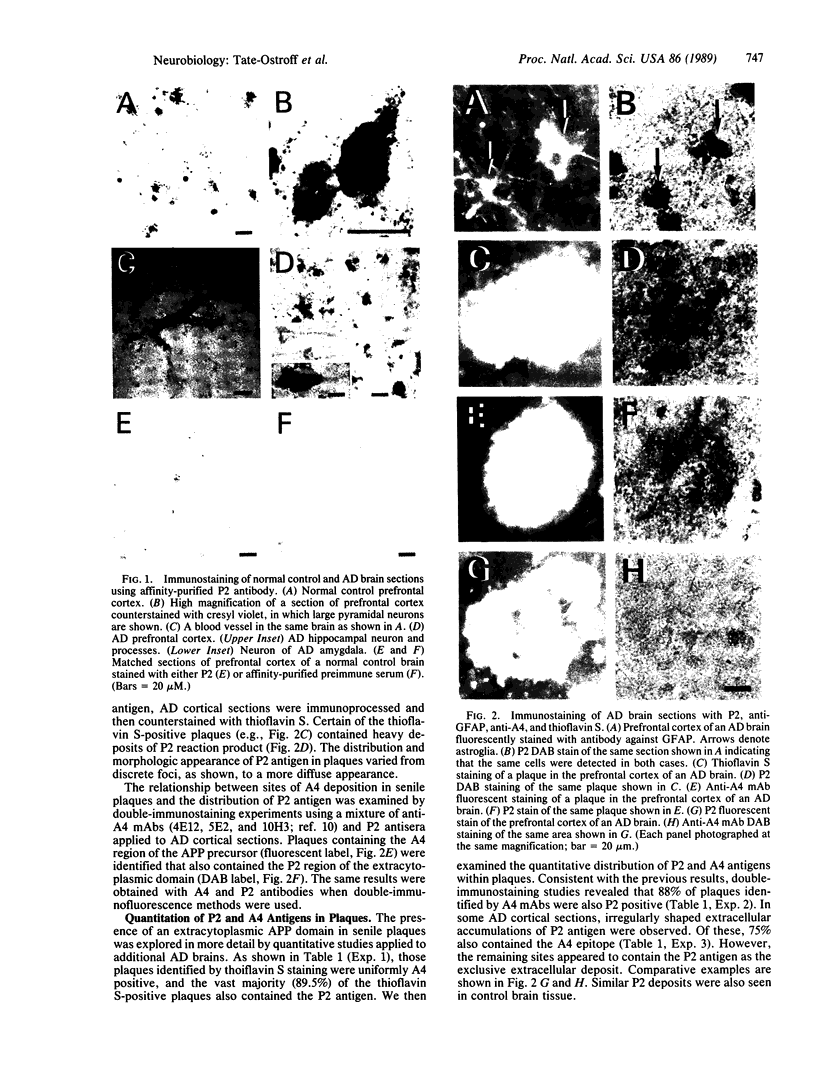
Information concerning the distribution of various subdomains of the amyloid precursor protein (APP) in brain may illuminate aspects of the normal metabolism of this membrane-associated protein, as well as putative abnormal processing that may occur in Alzheimer disease (AD). We prepared affinity-purified antibody, P2, against an extracytoplasmic APP site and applied it, along with monoclonal antibodies to the beta-peptide, or A4 region, in conjunction with selective cytochemical staining methods, to control and AD tissues. The following was noted: (i) in contrast to A4 epitopes, which are easily demonstrable primarily in extracellular senile plaques of AD patients, the extracytoplasmic P2 antigen was found in association with neurons, glia, and blood vessels in both normal and AD prefrontal cortex; (ii) a subset of senile plaques contained both A4 and P2 antigens; (iii) in some instances, P2 antigen occurred as an extracellular deposit in the absence of A4; (iv) the P2 antigen, but not A4, was also associated with corpora amylacea. In addition to identifying the unique cellular distribution of the APP extracytoplasmic antigen, the results support the view that a segment of this domain undergoes processing and deposition at extracellular sites, including a subset of senile plaques.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzil A. P., Herrlinger H., Blinzinger K., Kronski D. Intraneuritic corpora amylacea. Demonstration in orbital cortex of elderly subjects by means of early postmortem brain sampling and electron microscopy. Virchows Arch A Pathol Anat Histol. 1974;364(4):297–301. doi: 10.1007/BF00432727. [DOI] [PubMed] [Google Scholar]
- Averback P. Parasynaptic corpora amylacea in the striatum. Arch Pathol Lab Med. 1981 Jun;105(6):334–335. [PubMed] [Google Scholar]
- Brown B. A., Majocha R. E., Staton D. M., Marotta C. A. Axonal polypeptides cross-reactive with antibodies to neurofilament proteins. J Neurochem. 1983 Feb;40(2):299–308. doi: 10.1111/j.1471-4159.1983.tb11283.x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Castano E. M., Sarma R., Frangione B. Ten to fourteen residue peptides of Alzheimer's disease protein are sufficient for amyloid fibril formation and its characteristic x-ray diffraction pattern. Biochem Biophys Res Commun. 1987 Sep 15;147(2):854–862. doi: 10.1016/0006-291x(87)91008-4. [DOI] [PubMed] [Google Scholar]
- Ikeda S., Wong C. W., Allsop D., Landon M., Kidd M., Glenner G. G. Immunogold labeling of cerebrovascular and neuritic plaque amyloid fibrils in Alzheimer's disease with an anti-beta protein monoclonal antibody. Lab Invest. 1987 Oct;57(4):446–449. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Liu H. M., Anderson K., Caterson B. Demonstration of a keratan sulfate proteoglycan and a mannose-rich glycoconjugate in corpora amylacea of the brain by immunocytochemical and lectin-binding methods. J Neuroimmunol. 1987 Feb;14(1):49–60. doi: 10.1016/0165-5728(87)90100-7. [DOI] [PubMed] [Google Scholar]
- Majocha R. E., Benes F. M., Reifel J. L., Rodenrys A. M., Marotta C. A. Laminar-specific distribution and infrastructural detail of amyloid in the Alzheimer disease cortex visualized by computer-enhanced imaging of epitopes recognized by monoclonal antibodies. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6182–6186. doi: 10.1073/pnas.85.16.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majocha R. E., Marotta C. A., Benes F. M. Immunostaining of neurofilament protein in human postmortem cortex: a sensitive and specific approach to the pattern analysis of human cortical cytoarchitecture. Can J Biochem Cell Biol. 1985 Jun;63(6):577–584. doi: 10.1139/o85-076. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Olszewski W. A. Widening of inter-Purkinje cell distances in association with corpora amylacea. J Gerontol. 1985 Nov;40(6):700–702. doi: 10.1093/geronj/40.6.700. [DOI] [PubMed] [Google Scholar]
- RAMSEY H. J. ULTRASTRUCTURE OF CORPORA AMYLACEA. J Neuropathol Exp Neurol. 1965 Jan;24:25–39. doi: 10.1097/00005072-196501000-00003. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Stam F. C., Roukema P. A. Histochemical and biochemical aspects of corpora amylacea. Acta Neuropathol. 1973 Jul 11;25(2):95–102. doi: 10.1007/BF00687554. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Agari M., Nakamura H. Intra-axonal Corpora amylacea in ventral and lateral horns of the spinal cord. Acta Neuropathol. 1975;31(2):151–158. doi: 10.1007/BF00688149. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S. B., Salim M., Chou W. G., Sajdel-Sulkowska E. M., Majocha R. E., Marotta C. A. Molecular cloning of amyloid cDNA derived from mRNA of the Alzheimer disease brain: coding and noncoding regions of the fetal precursor mRNA are expressed in the cortex. Proc Natl Acad Sci U S A. 1988 Feb;85(3):929–933. doi: 10.1073/pnas.85.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Herget T., Salbaum J. M., Schubert W., Hilbich C., Cramer M., Masters C. L., Multhaup G., Kang J., Lemaire H. G. Localization of the putative precursor of Alzheimer's disease-specific amyloid at nuclear envelopes of adult human muscle. EMBO J. 1988 Feb;7(2):367–372. doi: 10.1002/j.1460-2075.1988.tb02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]