Abstract
Objective
Diet-induced obesity (DIO) in mice causes vascular inflammation and insulin resistance that are accompanied by decreased endothelial-derived nitric oxide (NO) production. We sought to determine whether reduced NO-cGMP signaling contributes to the deleterious effects of DIO on the vasculature and, if so, whether these effects can be blocked by increased vascular NO-cGMP signaling.
Method/Results
Using an established endothelial cell culture model of insulin resistance, exposure to palmitate (100 µM) for 3 h induced both cellular inflammation (activation of IKKβ-NF-κB) and impaired insulin signaling via the IRS-PI3K pathway. Sensitivity to palmitate-induced endothelial inflammation and insulin resistance was increased when NO signaling was reduced using an eNOS inhibitor, whereas endothelial responses to palmitate were blocked by pretreatment with either a NO donor or a cGMP analog. To investigate whether endogenous NO/cGMP signaling protects against vascular responses to nutrient excess in vivo, adult male mice lacking eNOS were studied. As predicted, both vascular inflammation (phospho-IκBα, ICAM) and insulin resistance (pAkt, peNOS) were increased in eNOS−/− mice, reminiscent of the effect of DIO in wild-type (WT) controls. We next asked whether the vascular response to DIO in WT mice can be reversed by a pharmacological increase of cGMP signaling. C57Bl6 mice were fed either a high fat (HF) diet or remained on a low-fat (LF) diet for 8 wk. During the final 2 wk of the study, mice on each diet received either placebo or the phosphodiesterase-5 (PDE-5) inhibitor sildendafil (0.1 mg/kg/day orally). In HF-fed mice, vascular inflammation and insulin resistance were completely prevented by sildenafil administration at a dose that had no effect in mice fed the LF diet.
Conclusion
We conclude that reduced signaling via the NO/cGMP pathway is a mediator of vascular inflammation and insulin resistance during over-nutrition induced by HF feeding. PDE-5, soluble guanylyl cyclase and other molecules in the NO/cGMP pathway (e.g., protein kinase G) therefore constitute potential targets for the treatment of vascular dysfunction in the setting of obesity.
Keywords: Nitric Oxide, eNOS, vascular inflammation
Introduction
In conditions of nutrient excess such as obesity and diabetes, elevated free fatty acid (FFA) levels are implicated in the pathogenesis of both inflammation and insulin resistance in a variety of tissues, including endothelial cells.1 2–4 At the cellular level, nutrient excess is linked to vascular insulin resistance via activation of IKKβ and, subsequently, NF-κB, a key transcriptional mediator of inflammation.1 Among the responses observed in association with obesity-induced vascular inflammation and insulin resistance is a reduction in vascular levels of nitric oxide (NO), which in other models has been linked to endothelial inflammation, thrombosis, and vasoconstriction.5, 6 These observations raise the possibility that reduced levels of vascular NO, which occurs relatively early during time course of diet-induced obesity (DIO),7 underlies vascular inflammation triggered by nutritional excess. In the current studies we sought to investigate whether interventions that reduce NO signaling predispose vascular tissues to inflammation and insulin resistance during nutrient excess and, conversely, whether an increase of endothelial-derived NO signaling might block these deleterious vascular responses.
The relationship between NO and cellular inflammation is complex since, at high concentrations, NO is cytotoxic and pro-inflammatory, effects that are opposite to those induced by much lower concentrations of NO that involve the cGMP-PKG pathway. Inducible NOS (iNOS) is an enzyme found in activated macrophages and other immune cells that produces NO in the micromolar range. At these concentrations, NO induces oxidative DNA damage and modifications of protein structure and function that can lead to cell death. By comparison, NO produced by eNOS is typically present at nanomolar concentrations and, via increased cGMP signaling and perhaps other mechanisms, has well documented anti-inflammatory effects. For example, NO can inhibit vascular inflammatory NF-κB activity by induction of IκBα,8 by inhibition of NF-κB-DNA binding9, or by indirect inhibitory effects on NF-κB by activation of PKA.10 In addition, eNOS has been shown to regulate expression of NF-κB,11, 12 and NO-mediated S-nitrosylation of p50 subunit of NF-κB12 (resulting in inhibition of NF-κB) is suggested to limit endothelial inflammation.
Endothelial-derived NO activates guanylyl cyclase in vascular smooth muscle and other cell types, thereby increasing cyclic guanosine monophosphate (cGMP) production. Intracellular levels of cGMP levels are not only governed by guanylyl cyclase activity but also by phosphodiesterase conversion of cGMP back to GMP. Sildendafil (marketed as Viagra) is a phosphodiesterase type 5 (PDE-5) inhibitor that attenuates intracellular catabolism of cGMP. In addition to its use in the management of erectile dysfunction, sildenafil’s effect to enhance signaling via cGMP is also effective in the treatment of pulmonary hypertension and congestive heart failure and it may also promote ischemia-induced angiogenesis13 and immune regulation.14 A link between cGMP signaling and insulin action was identified in a recent study in which chronic treatment with sildenafil improved energy balance and insulin sensitivity in mice fed a high-fat (HF) diet,15 and sildenafil also ameliorated diabetes-induced endothelial dysfunction in both a rat model and in humans.16, 17 Combined with evidence that vascular NO content falls early in the course of DIO in mice, these observations raise the possibility that reduced NO/cGMP signaling is a trigger for the development of vascular insulin resistance and inflammation during HF-feeding and hence that increased signaling via this pathway may have therapeutic effects in such conditions.
In this study we demonstrate that genetic and pharmacological interventions that reduce NO/cGMP signaling predispose to vascular inflammation and insulin resistance during nutrient excess whereas interventions that increase signaling via this pathway exert a protective effect, both in vivo and in an endothelial cell culture model. These findings collectively suggest that NO/cGMP levels play a critical role in to maintain vascular insulin sensitivity, and that reduced signaling via this pathway is a key mediator of vascular inflammation and insulin resistance that occurs during HF feeding.
Methods
Materials
Anti-phospho-eNOS (Ser1177), phospho-Akt (Ser473), anti-Akt, anti-phospho-IκBα, anti-IRS-1 rabbit polyclonal antibodies and monoclonal anti-phosphotyrosine antibody were obtained from Cell Signaling (Beverly, MA), anti-eNOS antibody was obtained from Transduction Labs, BD Biosciences (Lexington, Kentucky), and anti-ICAM antibody was purchased from R and D Systems (Minneapolis, MN). Total Akt and pAkt (serine 473) ELISA kits were obtained from Biosource (Camarillo, CA). (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NO), 8-Bromoguanosine-3’,5’-cyclic Monophosphate (8 Br cGMP) were purchased from Alexis Biochemical and the soluble guanylyl cyclase inhibitor- 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and PKG inhibitor-(KT 5823) was purchased from Cayman Chemical.
Cell Culture
Human microvascular endothelial cells (HMEC) were purchased from Invitrogen-Cascade Biological and were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and 12 µg/ml of bovine brain extract (Clonetics, Walkersville, MD), L-glutamine (2 mM), sodium pyruvate (1 mM) and nonessential amino acids in the presence of penicillin (100 units/ml) and maintained at 37°C in 5% CO2. All Western bots were performed as described18, using equal amounts of total protein for each condition and experiment. SDS gel electrophoresis was performed using a 4% by 20% gradient gel.
Study protocol
Adult male C57BL6 (WT) mice and eNos −/− mice were purchased from Jackson Laboratories. Age-matched groups (6–12 weeks old) (n=10 per group) were maintained in a temperature-controlled facility with a 12-hour light-dark cycle and were fed an equicaloric diet that was either low (10% saturated fat) or high in fat content (60% saturated fat) (Research Diets, number D12492, D12450B). Body weight and food intake were measured weekly. In one study involving WT mice only, sildenafil tablets (100 mg) were ground into a powder and mixed into a highly palatable ‘treat’ (300 mg) containing peanut butter and a high fat food pellet (Research Diets) so as to deliver sildenafil orally at a dose of 0.1 mg/kg/day. Vehicle-treated controls received the same daily treat without sildenafil, which was reliably and rapidly consumed. Sildenafil or vehicle was given once daily for the final 2 wk of an 8 w study protocol in which mice were fed either the HF or LF diet.
At the conclusion of the protocol, each animal received an IP injection of either vehicle (normal saline) or regular insulin (2 U in 300 µl of normal saline) after an overnight fast. Fifteen minutes later, mice were euthanized with an overdose of CO2 followed by cervical dislocation. Thoracic aorta and surrounding connective tissue was quickly removed and snap-frozen on dry ice. Protein was subsequently extracted from tissue samples and, after protein levels were quantified using Micro BCA Protein Assay Kit (Pierce, Rockford Il), equal amounts of protein were used for each condition in each assay. Total Akt and phospho-Akt (serine 473) levels, a measure of PI3 kinase signal transduction, were determined using ELISA assay kits (Biosource). Total eNOS, peNOS, ICAM, and phospho-IκBα were assessed using Western blot analysis and were quantified using Image J software (NIH). All procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Statistical analysis
In all experiments, densitometry measurements were normalized to controls incubated with vehicle and fold increase above the control condition was calculated. Analysis of the results was performed using the STATA8 statistical package. Data are expressed as mean ± SEM, and values of p<0.05 were considered statistically significant. A two-tailed t test was used to compare mean values in two-group comparisons. To compare responses between sildenafil and vehicle-treated mice that also received either vehicle or insulin, data were analyzed by two-way analysis of variance, and the Bonferoni-post-hoc comparison test was used to compare mean values between groups.
Results
Effect of reduced NO bioavailability on vascular inflammation and insulin signaling
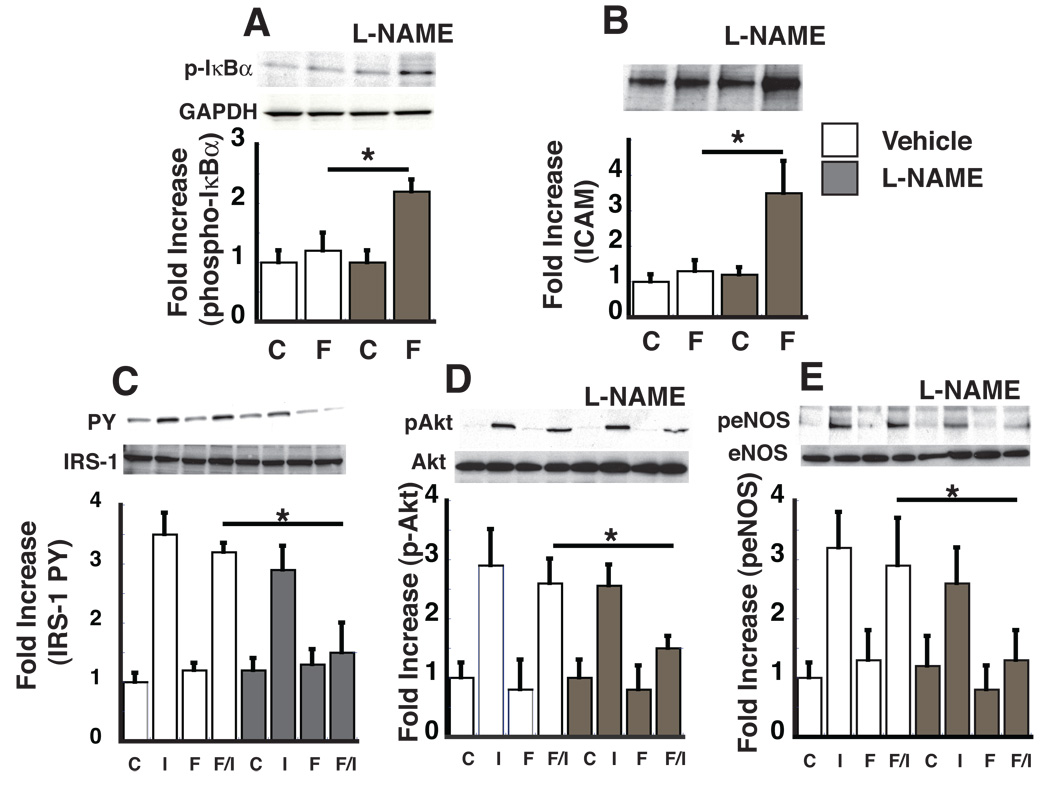
To determine if basal NO signaling is required to prevent endothelial inflammation and insulin resistance, we used the eNOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), to reduce basal endothelial NO levels, and asked whether this intervention increases susceptibility to palmitate-induced endothelial dysfunction. This was accomplished by exposing HMEC to palmitate complexed with BSA (10 µM) at a concentration below that needed to activate endothelial inflammation, in both the presence and absence of L-NAME. By design, exposure of HMEC to this low dose of palmitate-BSA failed to increase phospho-IκBα or ICAM protein levels compared to BSA controls (Figure 1A, B). In HMEC pretreated with L-NAME (50 µM), however, incubation with this same low dose of palmitate-BSA robustly induced phospho-IκBα and ICAM expression (Figure 1 A,B). Similarly, exposure of HMEC to 10 µM palmitate did not induce insulin resistance at the level of IRS-1 tyrosine phosphorylation, pAkt and peNOS in the absence of L-NAME, but clearly did so when pretreated with L-NAME (Fig 1 C,D, E). Importantly, L-NAME did not affect any of these parameters in the absence of palmitate. These results demonstrate that although reduced NO bioavailability does not in and of itself cause endothelial inflammation, it increases the susceptibility of endothelial cells to the inflammatory effects of palmitate and subsequent endothelial insulin resistance.
Figure 1. The effect of eNOS inhibitor, L-NAME, on palmitate-mediated endothelial inflammation and insulin signaling.
HMEC were treated with vehicle or the eNOS inhibitor (L-NAME) 50 µM 3 h and then treated with BSA (C) or BSA-palmitate (F) (10 µM) for 3 h, followed by insulin stimulation (I) (100 nM, 15 min). Cell lysates were analyzed by Western blot and fold increase over vehicle, BSA control group was calculated for each experiment (n=3) A. Fold increase of phospho-IκBα protein normalized to GAPDH levels. B. Fold increase of ICAM protein levels normalized to GAPDH levels. C. Fold increase in IRS-1 tyrosine phosphorylation normalized to total IRS-1. D. Fold increase in pAkt normalized to total Akt E. Fold increase in peNOS normalized to total eNOS levels. *p<0.05
Effect of increased NO bioavailability on palmitate-mediated vascular inflammation and insulin signaling in endothelial cells
Since eNOS inhibition predisposes to the deleterious effects of palmitate, whereas increased NO bioavailability decreases expression of NF-κB-dependent adhesion molecule expression in endothelial cells,10, 19 we next determined whether interventions that increase NO bioavailability would attenuate palmitate-mediated activation of endothelial NF-κB signaling and endothelial insulin resistance. As previously shown1, palmitate (100 µM for 3 h) inhibited insulin-mediated increases of IRS-1 tyrosine phosphorylation, pAkt and peNOS protein levels in HMEC, and pretreatment with DETANO (50 µM for 12 h) blocked each of these responses (Figure 2A–C). In HMEC, phospho-Iκbα and IL-6 protein levels, markers of endothelial inflammation, were increased >2-fold over BSA-treated controls in response to palmitate-BSA (100 µM), consistent with previous findings.1 However, pre-treatment with the NO donor DETANO (50 µM) attenuated each of these responses to palmitate (Figure 2D, E). In the presence of soluble guanylyl cyclase (sGC) inhibitor ODQ (1 µM) or the PKG inhibitor KT 5823 (1 µM), palmitate increased IL-6 levels, suggesting that the anti-inflammatory effect of DETANO requires sGC and PKG signaling elements (Figure 2E). These results demonstrate that increased NO bioavailability attenuates palmitate-mediated inflammation and its deleterious effects on endothelial insulin signaling.
Figure 2. The effect of the NO donor, DETANO on palmitate-mediated endothelial inflammation and insulin resistance.
HMEC were treated with vehicle or the NO donor-DETANO (50 µM) overnight and then treated with BSA (C) or BSA-palmitate (F) (100 µM) for 3 h, followed by insulin stimulation (I)(100 nM, 15 min). Cell lysates were analyzed by Western blot and fold increase over the vehicle, BSA control group was calculated for each experiment (n=3) A–C. Representative IRS-1 tyrosine, pAkt and peNOS Western blots are shown. IRS-1 tyrosine phosphorylation levels were normalized to total IRS-1 levels and pAkt and peNOS were normalized to total Akt and eNOS levels and the fold increase was calculated. * p<0.05. D. phospho-IκBα protein levels normalized to GAPDH E. Fold increase in IL-6 concentration in response to palmitate in the presence of DETANO (50 µM), sGC inhibitor ODQ (1 µM), or PKG inhibitor KT-5823 (1 µM) from HMEC supernatant as determined by ELISA. * p<0.05.
Effect of increased cGMP levels on inflammatory signaling in endothelial cells
The inhibitory effects of NO on NF-κB-dependent adhesion protein expression reportedly involve mechanisms that are both dependent on and independent of guanylyl cyclase/cGMP activity.8,20 To investigate the role of the guanyly cyclase pathway in palmitate-induced endothelial inflammation, we used the cell permeable cGMP analogue, 8-Br-cGMP. Pretreatment of HMEC with 8 Br-cGMP (50 µM) for 12 h attenuated increases of phospho-IκBα induced by either palmitate or lipopolysaccaride (LPS) which, like palmitate, induces cellular inflammation via activation of TLR4 (Figure 3A,B). In the presence of sGC inhibitor ODQ increased cGMP levels are still associated with decreased inflammatory responses in the presence of palmitate (Figure 3B). Thus, increased guanylyl cyclase/cGMP signaling mimics the anti-inflammatory effects of NO in endothelial cells exposed to palmitate. Similarly, treatment with 8-Br-cGMP attenuated palmitate-mediated impairment of insulin-stimulated pAkt (Figure 3C) suggesting that the anti-inflammatory effect of increased cGMP signaling can preserve endothelial insulin signaling in the presence of palmitate.
Figure 3. The effect of 8 Br-cGMP on endothelial inflammation and insulin signaling.
HMEC were treated with 50 µM 8-Br-cGMP for 12 h in the presence or absence of ODQ (1 µM) and then stimulated with LPS (5 ng/ml) for 1 h or palmitate (100 µM) for 3 h. A.B. Phospho-IκBα levels were assessed by Western blot and normalized to GAPDH and fold increased over the control condition was calculated following densitometry. C. Following treatment with vehicle or 8-Br-cGMP, HMEC were stimulated with 100 nM insulin for 15 min and pAkt levels were assessed by Western blot. * p<0.05.
Vascular inflammation and insulin resistance in eNos−/− mice
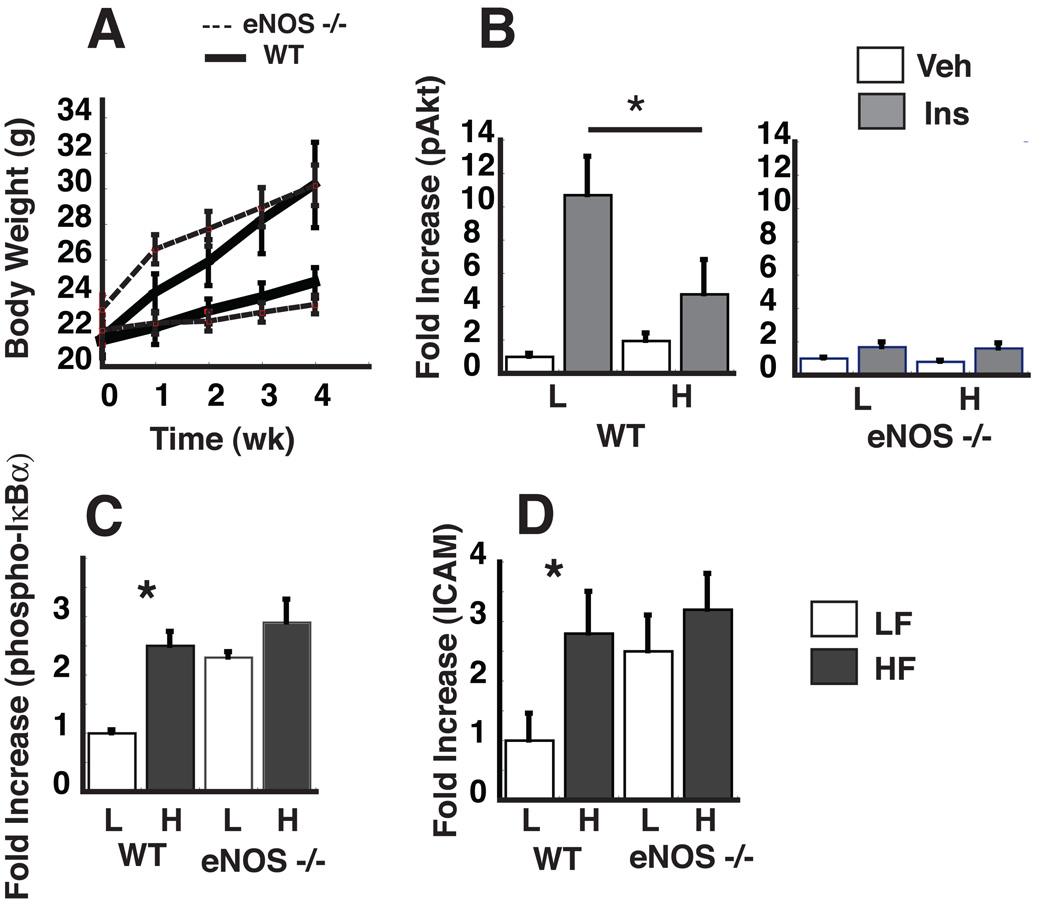
To test the hypothesis that the absence of vascular eNOS predisposes the vasculature to the development of inflammation and insulin resistance in vivo, we studied both eNos−/− and WT mice fed either a LF or a HF diet for 4 wk. As expected, HF feeding in WT mice was associated with rapid weight gain compared to LF-fed controls, and this effect did not differ by genotype, suggesting that eNOS is not required for weight gain in this setting (Figure 4A). Compared to WT controls, however, eNos−/− mice exhibited significant reductions in insulin-mediated activation of pAkt in thoracic aortic lysates (Figure 4B) even on the LF diet. Similarly. eNos−/− mice exhibited increased aortic phospho-IκBα and ICAM protein levels when fed a LF diet whereas WT mice exhibited increased aortic phospho-IκBα protein levels and reduced insulin-mediated activation of pAkt only when fed the HF diet (Figure 4C,D). These results demonstrate that in vascular tissue, the absence of eNOS (and, consequently, of NO) mimics the effect of HF feeding to induce vascular inflammation and insulin resistance.
Figure 4. The effect of HF-feeding in eNos −/− mice on body weight, vascular insulin signaling and inflammation.
WT and eNos −/− mice were fed a HF or LF diet for 4 wk. A. Weight gain for both WT and eNos −/− mice on the LF or HF diet. B. Fold increase in p-Akt/total Akt as measured by ELISA. C. Fold increase in phospho-IκBα normalized to GAPDH levels as measured by Western blot. D. ICAM protein levels normalized to total GAPDH levels. *p<0.05.
Effect of sildenafil on vascular insulin signaling and inflammation during HF feeding
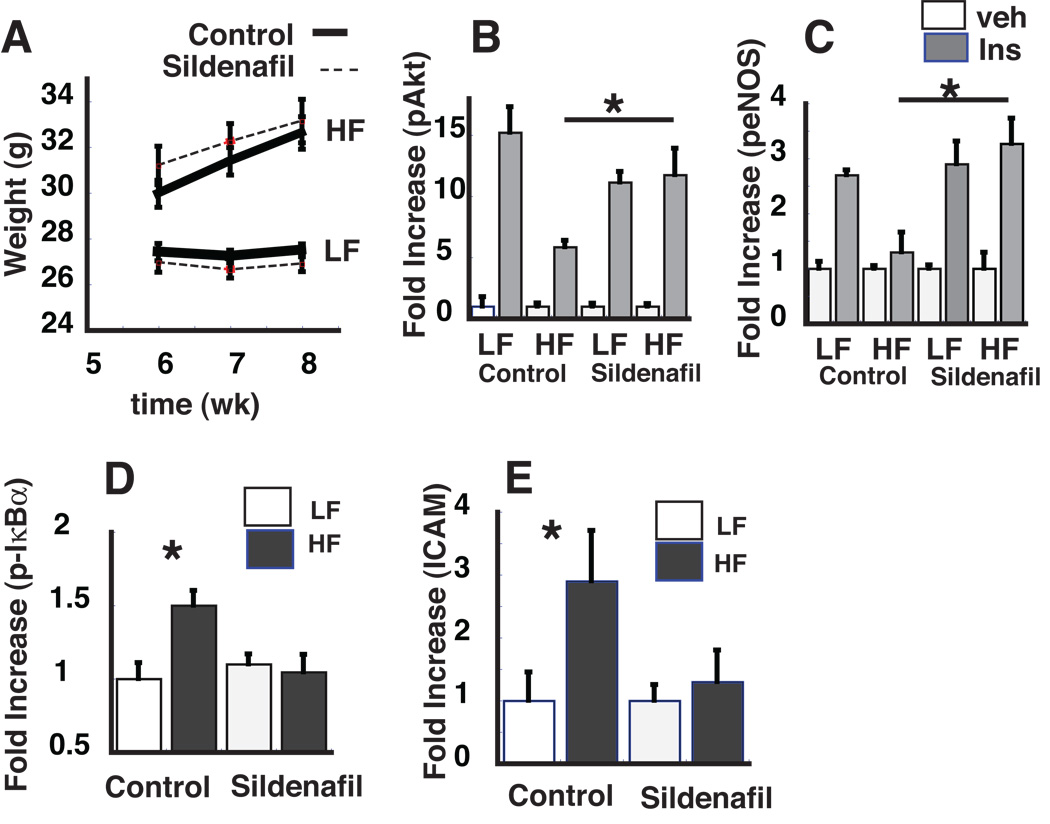
Since reduced NO predisposes to the effect of nutrient excess to cause vascular inflammation and insulin resistance, we hypothesized that increased NO-cGMP signaling would exert a protective effect. To test this hypothesis in an in vivo model, adult male C57BL6 (WT) mice were fed either a low fat (LF) (10% saturated fat) or high fat (HF) (60% saturated fat) diet for 8 wk to cause obesity, vascular inflammation and insulin resistance.21 During the final 2 wk of the HF feeding protocol, mice on each diet received daily oral administration of either vehicle or the phosphodiesterase-5 (PDE-5) inhibitor, sildendafil (0.1 mg/kg/day). As shown in Figure 5A, daily sildenafil treatment at this dose did not affect weight gain on either diet compared to the vehicle control group.
Figure 5. Effect of daily sildenafil during HF feeding on weight gain, vascular insulin signaling and inflammation.
Male C57BL6 mice were maintained on either a low fat (LF; 10% saturated fat) or high fat (HF; 60% saturated fat) diet for 8 wk and for the last 2 wks of the diet study, mice received 0.1 mg/kg/day of sildenafil or placebo (control). A. Mean weekly weight during wk 6–8. B,C. Fold increase in pAkt and peNOS in response to insulin (0.06 U/g body weight IP) *P<0.05 vs. LF controls. D,E Fold increase in phospho-IκBα and ICAM in response to 8 wk of HF or LF feeding with sildenafil or placebo (control). *, p<0.05.
In thoracic aortic lysates from vehicle-treated mice, insulin-mediated activation of pAkt and peNOS were reduced in HF- compared to LF-fed mice, consistent with our previous findings.7 By comparison, these defective vascular responses to HF-feeding were not detected in mice treated with sildenafil (Figure 5B,C). Similar results were obtained when vascular inflammation was assessed. Thus, protein levels of phospho-IκBα and ICAM were increased in thoracic aorta of vehicle-treated mice in response to HF feeding (but were not observed in LF-fed controls), whereas in sildenafil-treated mice, these adverse responses to HF-feeding were not observed (Figure 5 D,E). In mice, therefore, vascular inflammation and insulin resistance induced by HF-feeding can be fully blocked by pharmacological inhibition of PDE5, which increases intracellular signaling via cGMP.
Discussion
During nutritional excess, inflammatory pathways are activated rapidly in both cultured endothelial cells and in vascular tissue in vivo, and growing evidence implicates these inflammatory responses in the mechanism linking cardiovascular disease to obesity and related metabolic disorders.22–24 Since endothelial nitric oxide signaling has anti-inflammatory properties, and since vascular nitric oxide levels decline early in the course of DIO,7 we hypothesized an etiological role for reduced NO/cGMP signaling in the pathogenesis of vascular inflammation and insulin resistance in this setting. Using an endothelial cell culture model, we found that susceptibility to the inflammatory effects of palmitate increases when eNOS is inhibited, whereas interventions that increase bioavailability of either NO or cGMP reverse palmitate-mediated NF-κB activation and insulin resistance. Similar results were obtained using a mouse model of obesity and insulin resistance induced by HF feeding. Here, we found that in eNos−/− mice, genetic deficiency of vascular NO is associated with vascular inflammation and insulin resistance even on a LF diet, suggesting that endothelial NO signaling is required to restrain these responses. Conversely, the deleterious vascular effects of HF feeding were prevented in normal mice by daily oral dosing of sildenafil, a PDE5 inhibitor that increases signaling downstream of NO. Together, these results provide direct evidence in support of the hypothesis that vascular NO/cGMP signaling plays a physiological role to protect against vascular inflammation and insulin resistance and suggest that the effect of DIO to inhibit eNOS is a mediator of these deleterious vascular responses.
Clinical studies have demonstrated a profound effect of FFA on NO production. In normal patient volunteers, the ingestion of a single high-fat meal transiently impairs endothelial function as measured by flow-mediated brachial artery vasodilation.25 Infusion of high doses of intralipid plus heparin into normal volunteers raises circulating FFA concentrations from a starting concentration of 350 µM to a peak of 3800 µM and Methacholine-induced vasodilation was reduced by as much as 20%, indicating that elevated FFA levels induce endothelial dysfunction.26 In a separate study, raising FFA levels resulted in impairment of basal and insulin-mediated NO production.27 These human studies suggest that the production of NO is impaired in the presence of high circulating levels of FFA and we have previously demonstrated that cellular mechanism of FFA-mediated impairment of NO is dependent on NF-κB signaling.
Cellular inflammation has emerged as an important mechanism underlying insulin resistance in muscle, liver, adipose tissue and vascular tissue,21, 28 and interventions that block inflammatory responses have been shown to improve insulin sensitivity in vivo.28, 29 Consistent with these observations, we report that whereas reduced NO signaling (induced by pharmacological inhibition of eNOS) enhances endothelial inflammation induced by palmitate, increased NO/cGMP signaling has the opposite effect in both cultured endothelial cells and in a mouse model of DIO. Specifically, we found that in mice, daily oral administration of sildenafil for 2 wk fully reversed the vascular inflammation and insulin resistance induced by HF feeding at a dose that had no effect on body weight gain or fat mass. This observation identifies vascular NO-cGMP signaling as a potential target in the treatment of vascular inflammation associated with human obesity and related metabolic disorders.
That IKKβ-NF-κB activation mediates palmitate-induced insulin resistance in endothelial cells was established in previous work showing that IKKβ inhibition blocks this effect, whereas IKKβ activation recapitulates it.1 Combined with evidence that signaling via the innate immune receptor TLR4 is required for IKKβ-NF-κB activation by palmitate in endothelial cells,21 these findings suggest that NO antagonizes the ability of palmitate to increase endothelial signaling via TLR4 and subsequently activate IKKβ-NF-κB. Whether the mechanism underlying this NO effect involves an interaction directly with NF-κB or with upstream signaling molecules is unknown, and several possibilities exist. For one, NO is reported to scavenge superoxide, thereby reducing generation of H2O2 and impeding the activation of NF-κB and subsequent expression of inflammatory mediators that promote leukocyte adhesion30, 31 and macrophage recruitment.32 Nitric oxide also upregulates and stabilizes IκBα, the inhibitor of NF-κB, thereby suppressing NF-κB activity,33 and it may also inhibit DNA binding by NF-κB, thereby decreasing the transcription of genes involved in cellular inflammation.9 Additional studies are warranted to identify mechanisms underlying NO-mediated inhibition of the endothelial response to palmitate, and to determine whether NO exerts similar protective effects in other cell types, as suggested by evidence that reduced eNOS activity exacerbates liver injury in response to the bacterial endotoxin, lipopolysaccharde (LPS which, like palmitate induces inflammation through TLR4 activation).34 Conversely, increased NO signaling by either NO donor administration or eNOS overexpression can prevent liver injury in animal models of hepatoxicity.35, 36
The findings of the present study are also consistent with recent work on AMP-activated kinase (AMPK), inflammation, and NO. Although, the AMPK pathway is traditionally thought of as an intracellular fuel gauge and regulator of metabolism it is also important in the regulation and maintenance of endothelial function.37 AMPK and NO are integrally related since AMPK has been shown to directly activate eNOS activity38, 39 and recently, NO has been shown to be an endogenous activator of AMPK,40 suggesting a reciprocal relationship between AMPK and eNOS activity. Furthermore, palmitate-mediated activation of NF-κB and insulin resistance can be inhibited by AMPK.41 Therefore, a reduction in NO bioavailability could result in reduced activation of AMPK, leading in turn to increased palmitate-mediated activation of vascular inflammation or increased susceptibility to inflammatory effects of palmitate. In addition, increased NO levels could activate AMPK activity and through its “anti-inflammatory” effects, AMPK could attenuate palmitate-mediated activation of NF-κB. Thus, it is possible that the protective effects of NO/cGMP could be mediated by AMPK and further studies to address this question are warranted.
While our findings suggest a key role for NO to prevent or limit vascular inflammation induced by overnutrition, it is important to recognize that at the same time, NO biosynthesis is inhibited by activation of the IKKβ-NFκB pathway in endothelial cells.7 The mechanism underlying this observation involves the effect of NFκB activation to inhibit signal transduction via the IRS-PI3K pathway, a key positive regulator of eNOS activity. Since the time course of reduced NO levels during HF feeding coincides with the onset of vascular inflammation and insulin resistance7, reduced NO signaling may both mediate and be a consequence of vascular inflammation in the setting of DIO. These considerations support a bidirectional model in which the effect of nutrient excess to inhibit endothelial NO production predisposes to inflammation that, in turn, inhibits eNOS and further reduces NO synthesis and release. Thus, we propose that reduced vascular NO levels are an integral component of a vicious cycle that operates in states of nutrient excess. Consistent with this hypothesis, studies in mice have shown that although insulin-stimulated induction of vascular phospho-eNOS is measurably reduced (by ~40%) within the first week of HF-feeding, this response declines progressively over time such that it is entirely absent after 8 wk on this diet.7 This hypothesis is also in agreement with the negative feedback loop proposed Grumbach, et. al,12 to explain the observation that in endothelial cells, shear stress activates eNOS and increases NO signaling on the one hand, while also activating NF-κB on the other. To explain these seemingly paradoxical findings, the authors hypothesized that eNOS activation by shear stress serves to prevent sustained activation of NF-κB by shear stress. Accordingly, we propose that the early decline of NO signaling in DIO7 predisposes endothelial cells to inflammation; this in turn causes insulin resistance leading to eNOS inhibition that further reduces NO bioavailability and thereby exacerbates endothelial inflammation.
Direct evidence in support of this hypothesis stems from our observation that aortic samples from eNos−/− mice fed a LF diet display the same pattern of inflammation and insulin resistance that occurs in the vasculature of WT mice rendered obese by HF feeding. This vascular response is characterized by activation of the IKKβ-NFκB pathway and induction of the adhesion molecule ICAM, and by resistance to the ability of insulin to activate either PI3K or eNOS in aortic tissue. This change in vascular inflammation does not appear to be a result of changes in blood pressure between eNos−/− and WT mice since no differences in ICAM expression was detected when both mouse strains were fed a chow diet (See Supplementary Figure III). Complementary evidence is provided by our observation that a two-week course of the PDE5 inhibitor sildenafil fully reversed the deleterious vascular consequences of DIO in WT mice and thereby recapitulates the protective effect exerted by deficiency of TLR4. Similarly, both TLR4 deficiency21 and sildenafil15 administration can ameliorate systemic insulin resistance in mice with DIO.
One potential advantage of a therapeutic approach based on PDE5 inhibition is that, unlike approaches that increase NO levels, this strategy averts the risks associated with excessive NO signaling. At high concentrations, NO is cytotoxic and its release from activated macrophages and other immune cells (via activation of iNOS) plays a key role in the host defense against pathogens. Yet excessive NO can have detrimental effects even when derived from eNOS. For example, transgenic eNOS expression paradoxically increased vascular lesion formation in atherosclerosis-prone apoE−/− mice.42 One mechanism forwarded to explain this finding is that excessive NO production can act via a mechanism independent of soluble guanylyl cyclase to increase oxidative stress and favor the development of atherosclerosis. 43 This sequence of events would not be expected to arise from PDE-5 inhibition or alternatively, from direct activators of soluble guanylyl cyclase or the cGMP target, protein kinase G. Each of these molecules therefore constitutes a potential target for the treatment of vascular dysfunction in the setting of obesity.
Thoracic aortic tissue lysates used in figure 4 and figure 5 contain endothelial cells, vascular smooth muscle cells, and adventitial tissues and changes in Akt and inflammatory signaling could reflect changes in non-endothelial tissues. To verify the inflammatory effects of palmitate on smooth muscle cells, we utilized a mouse smooth muscle culture model (supplementary figure I). As expected, palmitate increased inflammatory markers (IL-6, TNF-α) and attenuated insulin-mediated Akt phosphorylation, which suggest that changes observed in the in vivo experiments are consistent from changes in both endothelial and smooth muscle cell population. We are unable to determine the relative contribution from the different tissue types, however, data on the changes in eNOS phosphorylation are relatively specific to endothelial tissues. The overall significance and contribution of SMC inflammation during HF-feeding await further study.
In conclusion, our findings suggest that NO/cGMP signaling plays a physiological role to attenuate vascular inflammation induced by nutrient excess both in vivo and in an endothelial cell culture model. Consequently, the effect of DIO to reduce vascular NO/cGMP signaling is implicated in the mechanism underlying vascular inflammation and insulin resistance in this setting. Therapeutic strategies designed to increase vascular NO/cGMP signaling may therefore be of value in the prevention and treatment of obesity-associated cardiovascular disease.
Supplementary Material
Acknowledgments
Source of Funding: This study was supported by NIH grants DK073878 (FK), DK52989 and DK68384 (MWS) and a grant from the John L. Locke Jr. Charitable Trust and from the Kenneth H. Cooper Endowed Professorship in Preventive Cardiology (FK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 2.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 6.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- 9.Matthews JR, Botting CH, Panico M, Morris HR, Hay RT. Inhibition of NF-kappaB DNA binding by nitric oxide. Nucleic Acids Res. 1996;24:2236–2242. doi: 10.1093/nar/24.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res. 2003;93:406–413. doi: 10.1161/01.RES.0000091074.33584.F0. [DOI] [PubMed] [Google Scholar]
- 11.Connelly L, Jacobs AT, Palacios-Callender M, Moncada S, Hobbs AJ. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J Biol Chem. 2003;278:26480–26487. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- 12.Grumbach IM, Chen W, Mertens SA, Harrison DG. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol. 2005;39:595–603. doi: 10.1016/j.yjmcc.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Senthilkumar A, Smith RD, Khitha J, Arora N, Veerareddy S, Langston W, Chidlow JH, Jr, Barlow SC, Teng X, Patel RP. Lefer DJ, Kevil CG. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27:1947–1954. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 14.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes. 2007;56:1025–1033. doi: 10.2337/db06-0883. [DOI] [PubMed] [Google Scholar]
- 16.Aversa A, Vitale C, Volterrani M, Fabbri A, Spera G, Fini M, Rosano GM. Chronic administration of Sildenafil improves markers of endothelial function in men with Type 2 diabetes. Diabet Med. 2008;25:37–44. doi: 10.1111/j.1464-5491.2007.02298.x. [DOI] [PubMed] [Google Scholar]
- 17.Schafer A, Fraccarollo D, Pfortsch S, Flierl U, Vogt C, Pfrang J, Kobsar A, Renne T, Eigenthaler M, Ertl G, Bauersachs J. Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br J Pharmacol. 2008;153:886–893. doi: 10.1038/sj.bjp.0707459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 19.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong D, Prameya R, Dorovini-Zis K, Vincent SR. Nitric oxide regulates interactions of PMN with human brain microvessel endothelial cells. Biochem Biophys Res Commun. 2004;323:142–148. doi: 10.1016/j.bbrc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 22.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 23.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 24.Yki-Jarvinen H. Insulin resistance and endothelial dysfunction. Best Pract Res Clin Endocrinol Metab. 2003;17:411–430. doi: 10.1016/s1521-690x(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 25.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 28.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 31.Chen XL, Zhang Q, Zhao R, Ding X, Tummala PE, Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J Pharmacol Exp Ther. 2003;305:573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 32.Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol. 2004;286:H1001–H1007. doi: 10.1152/ajpheart.00716.2003. [DOI] [PubMed] [Google Scholar]
- 33.Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–14219. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 34.Vos TA, Gouw AS, Klok PA, Havinga R, van Goor H, Huitema S, Roelofsen H, Kuipers F, Jansen PL, Moshage H. Differential effects of nitric oxide synthase inhibitors on endotoxin-induced liver damage in rats. Gastroenterology. 1997;113:1323–1333. doi: 10.1053/gast.1997.v113.pm9322528. [DOI] [PubMed] [Google Scholar]
- 35.Rivera-Chavez FA, Toledo-Pereyra LH, Dean RE, Crouch L, Ward PA. Exogenous and endogenous nitric oxide but not iNOS inhibition improves function and survival of ischemically injured livers. J Invest Surg. 2001;14:267–273. doi: 10.1080/089419301753170048. [DOI] [PubMed] [Google Scholar]
- 36.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980–H2986. doi: 10.1152/ajpheart.01173.2005. [DOI] [PubMed] [Google Scholar]
- 37.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 38.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Xie Z, Dong Y, Wang S, Liu C, Zou MH. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, Masada M, Yokoyama M. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest. 2002;110:331–340. doi: 10.1172/JCI15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaya T, Hirata K, Yamashita T, Shinohara M, Sasaki N, Inoue N, Yada T, Goto M, Fukatsu A, Hayashi T, Alp NJ, Channon KM, Yokoyama M, Kawashima S. A specific role for eNOS-derived reactive oxygen species in atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2007;27:1632–1637. doi: 10.1161/ATVBAHA.107.142182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.