Abstract
Biglycan (bgn)-deficient mice (KO) have defective osteoblasts which lead to changes in the amount and quality of bone. Altered tissue strength in C57BL6/129 (B6;129) KO mice, a property which is independent of tissue quantity, suggests that deficiencies in tissue quality are responsible. However, the response to bgn-deficiency is inbred strain-specific. Mechanical loading influences bone matrix quality in addition to any increase in bone mass or change in bone formation activity. Since many diseases influence the mechanical integrity of bone through altered tissue quality, loading may be a way to prevent and treat extracellular matrix deficiencies. C3H/He (C3H) mice consistently have a less vigorous response to mechanical loading vs. other inbred strains. It was therefore hypothesized that the bones from both wild type (WT) and KO B6;129 mice would be more responsive to exercise than the bones from C3H mice. To test these hypotheses at 11 weeks of age, following 21 consecutive days of exercise, we investigated cross-sectional geometry, mechanical properties, and tissue composition in the tibiae of male mice bred on B6;129 and C3H backgrounds. This study demonstrated inbred strain-specific compositional and mechanical changes following exercise in WT and KO mice, and showed evidence of genotype-specific changes in bone in response to loading in a gene disruption model. This study further shows that exercise can influence bone tissue composition and/or mechanical integrity without changes in bone geometry. Together, these data suggest that exercise may represent a possible means to alter tissue quality and mechanical deficiencies caused by many diseases of bone.
Keywords: Transgenic, Mechanical properties, Exercise, Micro CT, Raman
INTRODUCTION
Biglycan (bgn) is a small leucine-rich proteoglycan (SLRP) that is enriched in the extracellular matrix (ECM) of bone and other connective tissues.7 Much of what is known about biglycan's roles in regulating bone structure and mechanical function in vivo has been determined using bgn-deficient mice, which exhibit a defect in the growth and differentiation of osteoblast precursor cells12–14 resulting in a decrease in the amount of bone and changes in the composition of the tissue.36,38,46 Alterations in the mineral phase of bgn-deficient bone include increased volumetric bone mineral density (vBMD), mineral/matrix ratio and mineral crystallinity vs. wild type (WT) mice.36,38 The diameter of collagen fibrils in bgn-deficient mice is larger and more variable than in WT mice and fibrils often exhibit notches, protuberances and irregular spacing.4,15 Changes in collagen architecture are accompanied by increased collagen gene expression and decreased levels of mature collagen cross-links.36 These changes in tissue composition and quality are accompanied by decreased tissue-level strength and structural-level stiffness and strength but increased structural-level post-yield and failure deformation in bgn-deficient male mice bred on the C57BL6/129 (B6;129) inbred strain at 11 weeks of age. The response to bgn-deficiency is inbred strain-specific; the gene deletion in C3H/He (C3H) mice fails to impact any mechanical properties, but leads to significantly increased collagen gene expression, vBMD, mineral/matrix ratio and carbonate to phosphate ratio and a decreased collagen cross-linking ratio at 11 weeks of age.36 It is now recognized that the phenotype determined from gene disruption studies needs to be qualified with respect to the background strain of mice,36 age,36 gender and bone38 under investigation.
For at least 100 years, it has been known that mechanical stimulation can influence bone through increased mass and changes in structural architecture.45 Mechanical loading also has effects on bone that influence the quality of the bone matrix in addition to or in lieu of any increase in bone mass or change in bone formation activity, and these changes may increase the bone's resistance to fragility.22,39 Mechanical stimulation may therefore be a way to prevent and treat ECM deficiencies in bone, such as those noted in bgn-deficient mice. However, the response of mice with genetic deletions to mechanical stimulation is largely unknown. Biglycan's roles in mechanotransduction are unclear, but it does play a direct role in the growth and differentiation of osteoblast precursors,13,31 cells which are well known to be mechanically sensitive. Biglycan is also expressed at high levels in osteoblasts and on bone surfaces7 and, unlike many matrix proteins, in osteocytes,7 which are believed to be the primary cells involved in mechanotransduction in bone. This evidence suggests that biglycan could be important in a bone's response to mechanical loading.
Inbred strain-specific changes in mice have been demonstrated in response to mechanical loading and unloading,2,3,21 in the amount of bone regeneration following injury25 and in new bone induction at an extraskeletal site.26 Consistently, C3H mice demonstrate less intense changes in bone formation and mechanical integrity vs. control mice following these stimuli compared with other inbred strains. These differences may stem from differences in the basal level of mineral and/or osteoblast function. Therefore, it is hypothesized that the bones from wild type B6;129 mice will be more responsive to running than the bones from C3H mice (hypothesis 1). Despite the more compelling phenotype of bgn-deficient mice bred on the B6;129 background strain and the fact that bgn-deficiency altered mineral and collagen in both inbred strains,36 it is unclear what precise role, if any, biglycan plays in mechanosensitivity. Therefore, it is further hypothesized that the bones from bgn-deficient B6;129 mice will also be more responsive to exercise than the bones from C3H mice (hypothesis 2).
These two hypotheses were tested in wild type and bgn-deficient mice bred on B6;129 and C3H backgrounds. At 11 weeks of age, following 21 consecutive days of running on a treadmill,37,39 we investigated changes in cross-sectional geometry (via microCT), mechanical properties (tissue and structural-level via four-point bending) and tissue composition (via Raman microspectroscopy) to test for inbred strain-specific effects of exercise in wild type (hypothesis 1) and bgn-deficient mice (hypothesis 2). Exercise-related changes in these properties relative to non-exercise control mice were assessed in the cortical bone of male tibiae from both inbred strains and genotypes. This bone and gender were chosen because the bgn-deficient phenotype was strongest in the male tibiae,38 as was the response to exercise.37
MATERIALS AND METHODS
Animals
All animal procedures were performed at the University of Michigan with University Committee on Use and Care of Animals (UCUCA) approval (UCUCA animal approval protocol #8518). Biglycan-deficient (KO) and wild type (WT) breeder mice were the generous gift of Dr. Marian F. Young from the National Institute of Dental and Craniofacial Research (NIDCR). Mice from the B6;129 background strain were originally generated by homologous recombination in embryonic stem cells.46 Mice from this background strain were then backcrossed to the C3H/HeNHsd (C3H) strain to a purity of greater than 95%. Upon arrival at the University of Michigan, the genotype of all breeder pairs was verified via polymerase chain reaction (PCR) using DNA extracted from a tail biopsy of each mouse. This process was repeated for the first F1 generation of mice from each breeder pair as verification.
To determine proper sample sizes for detecting the inbred strain-specific effects of exercise within each genotype, power calculations were performed based on previously published values for tissue composition, cross-sectional geometry, and mechanical properties2,37,38 using a value of α = 0.05 and a power (1 – β) of 0.80. To be able to detect differences in cross-sectional geometric properties and mechanical properties, a sample size of n = 15 was needed. For measures of tissue composition, which have much smaller standard deviations than other properties, a sample size of n = 6 was needed.
At 3 weeks of age, mice were weaned and maintained in standard cages with access to food, water, and cage activity ad libitum. At 8 weeks of age (Day 0), mice from each background strain/genotype were randomly assigned to 1 of 2 weight-matched groups (exercise or control). In total, 2 inbred strains × 2 genotypes × 2 exercise groups × n = 15 per group meant 120 mice were used. Control mice remained in cages for the duration of the study with normal in-cage activity. Exercise consisted of running on a treadmill (12 m/min at a 5° incline) for 30 min/day, 7 days/week for 21 consecutive days (Columbus Instruments, Model 1055 M, Columbus, OH), a protocol previously shown to be sufficient to induce mechanical changes in the tibiae of mice.22,37,39 Exercise and control mice were sacrificed by CO2 inhalation following the last bout of exercise on Day 20, at which time final body mass was measured and both tibiae were harvested, stripped of soft tissue, wrapped in gauze soaked in a calcium-buffered saline solution and stored at –20°C.
Micro Computed Tomography (μCT) Evaluation
Left tibiae were analyzed by micro computed tomography (μCT) to assess cross-sectional geometric properties. Bones were scanned at 18 μm/voxel resolution (GE/EVS MS-8 specimen scanner, GE Healthcare, London, Ontario, Canada) and three-dimensional images were reconstructed. Each three-dimensional data set was arranged as a series of 18 μm-thick slices oriented along the long axis of the tibia. Cross-sectional geometry from the fracture site (to normalize whole bone mechanical properties, see methods section on mechanical testing) and from a standard site in the diaphysis of each bone were determined. The standard site was located at a position 792 μm proximal to the tibia–fibula junction (TFJ), and was chosen to lie just distal to the mechanical loading region (which began 800 μm proximal to the TFJ). Properties were determined from 6 μCT slices (108 μm) centered at each location. For the measurement of geometric properties, each section was thresholded into bone and non-bone voxels using a previously defined method.23,36 Geometric properties for each region of interest were then determined using a custom analysis program. Properties of interest included cross-sectional area, cortical area, marrow area, average cortical thickness, anterior–posterior (AP) width, medial–lateral (ML) width, and bending moment of inertia about the AP and ML axes (IAP, IML).
Mechanical Testing
Left tibiae were brought to room temperature before testing and were kept hydrated in calcium-buffered saline until the test was complete. Each bone was tested in the ML direction (medial surface in tension) in a four-point bending configuration (Admet eXpert 450 Universal Testing Machine; Norwood, MA). The fibula was carefully removed from each bone using a scalpel and the bones were positioned such that the TFJ was lined up with the outside edge of one loading roller. The bones were preloaded to 0.5 N, preconditioned for 8 cycles (2 Hz, mean load of 2N ± 2 N) and then monotonically tested to failure in displacement control at a rate of 0.025 mm/s. During each test, load and deflection were recorded, from which structural strength (yield force and ultimate force), stiffness (the slope of the linear portion of the force vs. displacement curve) and deformation (yield deformation, failure deformation and post-yield deformation) were derived at the whole bone level.36–38 After testing, the distal end of each bone was placed in 70% ethanol.
During testing, the bone was visually monitored and the point of fracture initiation was noted and measured relative to the proximal end of the bone. In order to normalize structural-level mechanical properties, a subset of geometric properties at the fracture site was obtained from μCT data (IAP and the distance from the centroid to the tensile surface of the bone, c). Together with the load and deflection data, IAP and c were used to map force and displacement (structural level properties dependent on bone structural organization) into stress and strain (predicted tissue level properties) from standard beam-bending equations for four-point bending:
In these equations, F is the force, d is the displacement, a is the distance from the support to the inner loading point (3 mm), and L is the span between the outer supports (9 mm). The yield point was calculated using the 0.2% offset method based on the stress–strain curve.34 The modulus of elasticity was calculated as the slope of the linear portion of the stress–strain curve. As these equations are only valid in the pre-yield regime, tissue-level post-yield, and failure properties are not reported.
Raman Microspectroscopy
Following mechanical testing, the distal half of all bones was dehydrated in graded ethanol (70, 80, 95, 100%), defatted in Clear-Rite 3 (Richard-Allen Scientific; Kalamazoo, MI) and infiltrated in a liquid methylmethacrylate monomer (Koldmount™ Cold Mounting Liquid, Mager Scientific). The bones were then embedded in poly(methyl methacrylate) (Koldmount™ Cold Mounting Kit, Mager Scientific). Not all of the 15 bones in each group were acceptable for Raman analysis (i.e., they did not meet the requirements of having a sampling region outside of the mechanical testing region and at a standard axial location). Six bones in each group that were acceptable were chosen at random for Raman analysis. Using a low-speed sectioning saw (South Bay Technology, Model 650; San Clemente, CA) with a diamond wafering blade (Mager Scientific), thick sections (≥3 mm in thickness) were made and hand polished using wet silicon carbide abrasive discs (the average distance was 488 ± 290 μm distal to the TFJ).
The Raman imaging system has been described.11,33 Briefly, Raman scatter was excited using a 785 nm laser with a rectangular beam profile (Kaiser Optical Systems, Ann Arbor, MI). The beam was passed through a 20× objective onto the sample which focuses the line-shaped beam (~100 μm in length). Raman scattered light from every point on the line was simultaneously passed back through the objective and through a dichroic mirror to a charge coupled device (CCD) detector.
Three band areas were determined: a phosphate band ( ν1 symmetric stretch at 957 cm–1), a carbonate band ( ν1 symmetric stretch at 1070 cm–1) and the Amide I envelope (C=O stretch at 1595–1720 cm–1). The mineral/matrix ratio (indicative of the relative amount of mineral) was determined by dividing the phosphate band area by the Amide I band area. The carbonate/phosphate ratio (indicative of carbonate substituting in the crystal lattice for phosphate ions), was determined by dividing the carbonate band area by the phosphate band area. Mineral crystallinity (indicative of the size, shape, and perfection of mineral crystals) was obtained from the inverse of the full bandwidth at half peak intensity of the phosphate band. The Amide I band was decomposed into two smaller bands at 1660 and 1690 cm–1. The collagen cross-linking ratio (indicative of the amount of non-reducible/reducible cross-linking) was determined by dividing the 1660 cm–1 band area by the 1690 cm–1 band area.29 Twelve spectral line scans were collected from each bone sample. In each anatomic quadrant (A, P, M, L), scans were performed in periosteal, intracortical, and endocortical radial locations. Repeated measures ANOVA were performed to determine if there were statistical differences between quadrants or between radial locations in the cross sections. Because there were no statistical differences noted, the 12 measurements made in each bone were averaged to yield one number for each bone or n = 6 for each experimental condition.
Statistical Analysis
All statistical analyses were performed using Sigma Stat (Version 3.1, Systat Software Inc.) or SPSS (Version 16.0, SPSS Inc.). We sought to determine if an inbred strain-specific response to exercise existed in WT mice (hypothesis 1) and KO mice (hypothesis 2). Two-way ANOVA with post hoc Student–Newman–Keuls tests were employed within each genotype to investigate the effects of background strain and exercise, testing hypotheses 1 (in WT mice) and 2 (in KO mice). For all investigations, a value of p < 0.05 was considered significant.
RESULTS
Inbred Strain-Specific Effects of Exercise in Wild Type Mice
In B6;129 WT mice, exercise did not impact body mass compared with control levels (WT control, 27.07 ± 0.81 g; WT exercise, 26.19 ± 0.61 g; p = 0.29). There was similarly no impact of exercise on body mass in C3H WT mice (WT control, 25.47 ± 0.45 g; WT exercise, 25.31 ± 0.41 g; p = 0.793).
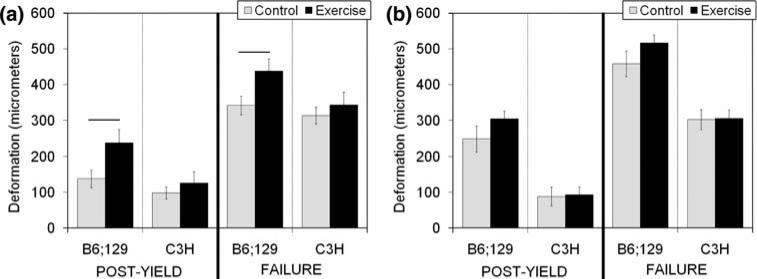
Exercise significantly increased post-yield deformation (p = 0.017; Fig. 1a) and failure deformation (p = 0.031; Fig. 1a) in B6;129 WT mice vs. control mice, but structural-level mechanical behavior was not significantly impacted by exercise in C3H WT mice (Fig. 1a, Table 1). There were no significant tissue-level mechanical changes with exercise in either B6;129 or C3H WT mice (Fig. 2, Table 2).
FIGURE 1.
Structural-level deformation from wild type (WT, a) and bgn-deficient (KO, b) tibiae. In B6;129 WT mice, post-yield deformation (p = 0.017) and failure deformation (p = 0.031) were significantly increased vs. control mice. These changes were inbred strain-specific. Data are presented as mean ± SEM.
TABLE 1.
Structural-level mechanical properties.
| Group | Yield force (N) | Ultimate force (N) | Yield deformation (μm) | Stiffness (N/mm) |
|---|---|---|---|---|
| B6;129 WT Control | 13.65 ± 0.81 | 15.44 ± 0.74 | 203.99 ± 6.46 | 76.61 ± 4.49 |
| B6;129 WT Exercise | 13.03 ± 0.64 | 14.83 ± 0.62 | 200.25 ± 6.38 | 75.07 ± 3.51 |
| C3H WT Control | 16.43 ± 0.58 | 17.06 ± 0.60 | 215.50 ± 9.47 | 93.24 ± 8.30 |
| C3H WT Exercise | 17.38 ± 0.69 | 17.77 ± 0.72 | 220.01 ± 9.14 | 96.53 ± 7.86 |
| B6;129 KO Control | 11.74 ± 0.55 | 13.46 ± 0.64 | 210.34 ± 4.18 | 65.15 ± 2.48 |
| B6;129 KO Exercise | 11.23 ± 0.30 | 12.47 ± 0.39 | 211.74 ± 4.57 | 61.87 ± 2.01 |
| C3H KO Control | 17.41 ± 0.56 | 18.09 ± 0.70 | 213.59 ± 9.64 | 96.14 ± 5.35 |
| C3H KO Exercise | 16.72 ± 0.54 | 17.16 ± 0.52 | 211.23 ± 7.16 | 93.21 ± 5.61 |
Data are presented as mean ± SEM. There were no significant effects of exercise in any groups in these structural-level mechanical properties.
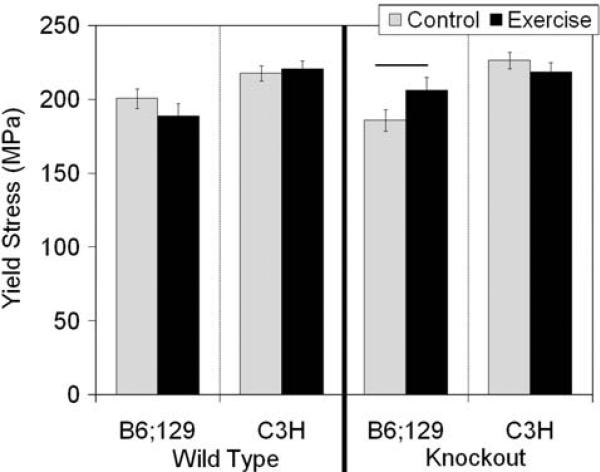
FIGURE 2.
Yield stress measured in wild type and bgn-deficient tibiae. In B6;129 KO mice, exercise led to a significant increase in yield stress compared with control mice (p = 0.050), a change which was inbred strain-specific. Data are presented as mean ± SEM.
TABLE 2.
Estimated tissue-level mechanical properties.
| Group | Ultimate stress (MPa) | Yield strain (με) | Modulus (GPa) |
|---|---|---|---|
| B6;129 WT Control | 228.09 ± 6.43 | 14,542 ± 561 | 15.99 ± 0.73 |
| B6;129 WT Exercise | 215.03 ± 8.99 | 13,832 ± 373 | 15.70 ± 0.69 |
| C3H WT Control | 226.01 ± 4.98 | 15,696 ± 690 | 16.75 ± 1.16 |
| C3H WT Exercise | 228.56 ± 5.42 | 15,889 ± 621 | 17.10 ± 1.10 |
| B6;129 KO Control | 212.82 ± 8.75 | 13,382 ± 381 | 16.29 ± 0.55 |
| B6;129 KO Exercise | 228.47 ± 9.91 | 13,477 ± 231 | 17.66 ± 0.60 |
| C3H KO Control | 223.73 ± 7.22 | 15,115 ± 764 | 17.44 ± 1.09 |
| C3H KO Exercise | 223.99 ± 6.27 | 15,281 ± 565 | 16.83 ± 0.94 |
Data are presented as mean ± SEM. There were no significant effects of exercise in any group in these tissue-level mechanical properties.
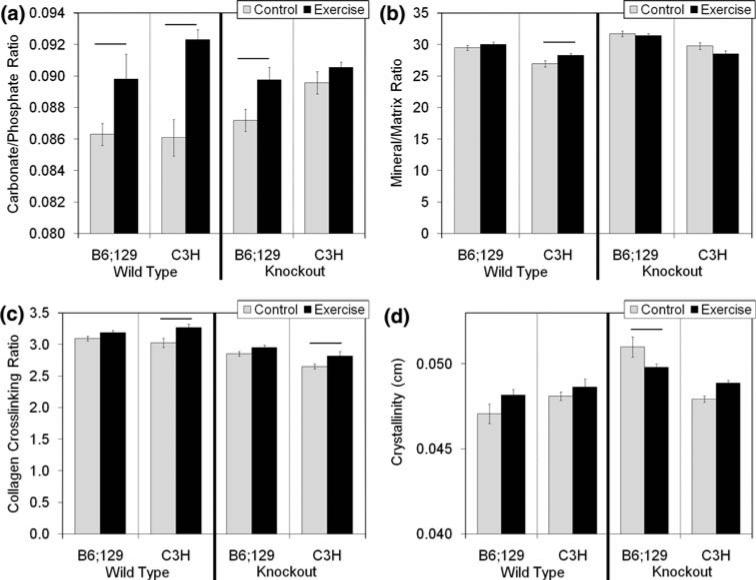
The carbonate/phosphate ratio was significantly increased with exercise in both B6;129 (p = 0.034) and C3H mice (p < 0.001; Fig. 3a). Additionally, exercise significantly increased the mineral/matrix ratio (p = 0.030; Fig. 3b) and the collagen cross-linking ratio (p = 0.003; Fig. 3c) in C3H WT mice.
FIGURE 3.
Carbonate/phosphate ratio (a, indication of carbonate substituting for phosphate in the crystal lattice), mineral/matrix ratio (b, indicative of the relative amount of mineral), collagen crosslinking ratio (c, indicative of the ratio of non-reducible to reducible crosslinking), and crystallinity (d, indicative of the size, shape, and perfection of mineral crystals) as measured by Raman spectroscopy. There was a significant increase in the carbonate/phosphate ratio with exercise in B6;129 (p = 0.034) and C3H (p < 0.001) wild type mice. In B6;129 bgn-deficient mice, there was a significant increase in carbonate/phosphate ratio with exercise (p = 0.012) which was inbred strain-specific. In C3H WT mice, there was a significant increase in mineral/matrix ratio vs. control mice (p = 0.030) which was inbred strain-specific. Inbred strain-specific increases in the collagen cross-linking ratio were present in both WT (p = 0.003) and KO (p = 0.024) C3H mice compared with control mice. Finally, there was an inbred strain specific decrease in crystallinity with exercise in B6;129 KO mice (p = 0.020). Data are presented as mean ± SEM.
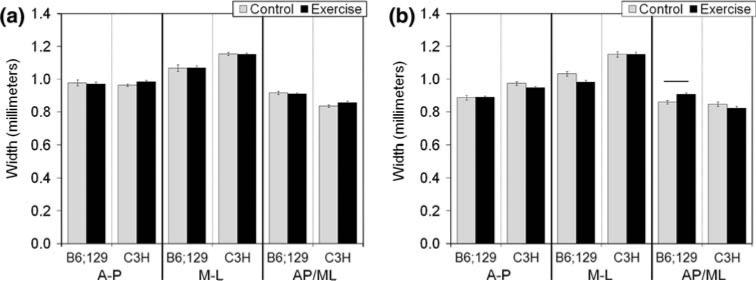
Cross-sectional geometric properties were not impacted by exercise in B6;129 or C3H WT mice (Fig. 4, Table 3).
FIGURE 4.
Cross-sectional width of wild type (WT, a) and bgn-deficient (KO, b) tibiae. In B6;129 KO mice, exercise led to a significant increase in the AP/ML ratio of the bone compared with control mice (p = 0.003), a change which was inbred strain-specific. Data are presented as mean ± SEM.
TABLE 3.
Standard site cross-sectional geometric properties.
| Group | Total area (mm2) | Cortical area (mm2) | Marrow area (mm2) | A-P moment of inertia (mm4) | M-L moment of inertia (mm4) | Average cortical thickness (mm) |
|---|---|---|---|---|---|---|
| WT | ||||||
| B6;129 Control | 0.752 ± 0.024 | 0.515 ± 0.017 | 0.237 ± 0.009 | 0.050 ± 0.003 | 0.045 ± 0.003 | 0.214 ± 0.004 |
| B6;129 Exercise | 0.742 ± 0.017 | 0.509 ± 0.011 | 0.233 ± 0.008 | 0.048 ± 0.002 | 0.044 ± 0.002 | 0.214 ± 0.003 |
| C3H Control | 0.802 ± 0.011 | 0.618 ± 0.008 | 0.184 ± 0.004 | 0.065 ± 0.002 | 0.047 ± 0.001 | 0.263 ± 0.002 |
| C3H Exercise | 0.818 ± 0.012 | 0.634 ± 0.010 | 0.184 ± 0.004 | 0.066 ± 0.002 | 0.050 ± 0.002 | 0.268 ± 0.003 |
| KO | ||||||
| B6;129 Control | 0.657 ± 0.018 | 0.483 ± 0.013 | 0.174 ± 0.006 | 0.041 ± 0.002 | 0.033 ± 0.002 | 0.223 ± 0.003 |
| B6;129 Exercise | 0.634 ± 0.011 | 0.462 ± 0.010 | 0.172 ± 0.005 | 0.036 ± 0.001 | 0.031 ± 0.001 | 0.216 ± 0.004 |
| C3H Control | 0.805 ± 0.017 | 0.634 ± 0.011 | 0.171 ± 0.006 | 0.066 ± 0.003 | 0.047 ± 0.002 | 0.273 ± 0.002 |
| C3H Exercise | 0.786 ± 0.016 | 0.622 ± 0.009 | 0.164 ± 0.008 | 0.064 ± 0.003 | 0.044 ± 0.001 | 0.272 ± 0.002 |
Data as presented as mean ± SEM. There were no significant effects of exercise in any groups in these cross-sectional geometric properties.
In summary for WT mice, there were inbred strain-specific increases in structural mechanical properties (post-yield and failure deformation) in B6;129 WT mice with exercise, supporting hypothesis 1 (Table 4). There were inbred strain-specific increases in compositional measures (mineral/matrix ratio and collagen crosslinking ratio) in C3H WT mice with exercise. However, these compositional changes in C3H WT mice were not associated with changes in any mechanical properties.
TABLE 4.
Summary of significant changes with exercise.
| Wild type |
Bgn-deficient |
|||
|---|---|---|---|---|
| Properties | B6;129 | C3H | B6;129 | C3H |
| Body mass |
↔
|
↔
|
↔
|
↔
|
| Structural-level mechanical |
↑Post-yield deformation ↑Failure deformation |
↔
|
↔
|
↔
|
| Tissue-level mechanical |
↔
|
↔
|
↑ Yield stress |
↔
|
| Tissue composition |
↑ Carbonate/phosphate ratio |
↑Carbonate/phosphate ratio ↑mineral/matrix ratio ↑collagen cross-linking ratio |
↑ Carbonate/phosphate ratio ↓ crystallinity |
↑ Collagen crosslinking ratio |
| Cross-sectional geometry | ↔ | ↔ | ↑ AP/ML ratio | ↔ |
This table summarizes the significant effects of exercise in each group. An ↔ indicates that no properties were changed with exercise.
Inbred Strain-Specific Effects of Exercise in Bgn-Deficient Mice
In B6;129 KO mice, exercise did not impact body mass compared with control levels (KO control, 25.39 ± 0.61 g; KO exercise, 24.11 ± 0.42 g; p = 0.10). Similarly, there was no impact of exercise on body mass in C3H KO mice (KO control, 26.79 ± 0.46 g; KO exercise, 26.88 ± 0.42 g; p = 0.882).
There were no significant structural-level mechanical changes in B6;129 or C3H KO mice (Fig. 1b, Table 1). Exercise significantly increased yield stress in B6;129 KO mice compared with control mice (p = 0.050; Fig. 2), but there were no significant tissue-level mechanical changes with exercise in C3H KO mice (Fig. 2, Table 2).
In B6;129 KO mice, exercise significantly increased the carbonate/phosphate ratio (p = 0.012; Fig. 3a) and significantly decreased crystallinity (p = 0.02; Fig. 3d). In C3H KO mice, the collagen cross-linking ratio was significantly elevated with exercise (p = 0.024; Fig. 3c).
In B6;129 KO mice, exercise led to a significant increase in the AP/ML ratio of the bone cross section (p = 0.003, Fig. 4b). No other cross-sectional geometric properties were altered with exercise in KO mice from either inbred strain (Table 3).
In summary, there were inbred strain-specific changes in tissue strength, composition (carbonate to phosphate ratio and crystallinity) and cross-sectional shape in B6;129 KO mice with exercise, supporting hypothesis 2 (Table 4). There was also an inbred strain-specific increase in the collagen crosslinking ratio in C3H KO mice with exercise. However, this compositional change in C3H KO mice was not associated with changes in any mechanical properties.
DISCUSSION
It was hypothesized that bones from WT B6;129 mice would be more responsive to running than the bones from C3H mice. Tibiae from WT mice of both inbred strains had significantly increased carbonate/phosphate ratio with exercise (Fig. 3a). In addition, WT C3H mice had significantly increased mineral/matrix ratio (Fig. 3b) and collagen cross-linking ratio (Fig. 3c), inbred strain-specific changes which were not present in B6;129 WT mice. These compositional changes indicate that the C3H bones were responsive to exercise. However, exercise failed to alter the size and shape of the C3H WT bones or induce changes in mechanical integrity over the time scale of the study. In B6;129 WT mice, fewer compositional changes occurred with exercise compared with the C3H mice, but these changes were accompanied by an inbred strain-specific increase in structural-level post-yield behavior (Fig. 1). Since the ultimate goal of this exercise regimen is to alter mechanical function of the bones, this combination of altered properties across multiple levels of the bone hierarchy in B6;129 WT mice but not C3H mice is supportive of hypothesis 1 (Table 4).
In WT B6;129 mice, exercise increased structural-level post-yield mechanical properties, a finding which is consistent with previous exercise studies in this background strain of mouse.37,39 Many in the literature suggest that the post-yield properties of bone are primarily dictated by the organic portion of the ECM,8,40,41 although the mineral may contribute to the plastic behavior of bone.10 The only measure of the state of the organic matrix in the current study, the collagen crosslinking ratio, was not altered with exercise in the B6;129 WT bones. However, this property only provides information about one aspect of the collagen matrix at the nanoscale (i.e., the ratio of mature/immature crosslinks). Other properties such as the quality or orientation of the collagen fibrils, properties which may be altered when bone is loaded,20 are not captured by this measure. Regardless, post-yield behavior is important to the overall toughness of a structure and suggests an increased ability of the structure to absorb energy before a catastrophic failure. In the absence of other mechanical changes, increased post-yield and failure deformation can be considered beneficial results of exercise.
The disparity in response to exercise between the two inbred strains was also present in KO mice and supportive of hypothesis 2 which proposed that the bones from KO B6;129 mice would be more responsive to exercise than the bones from KO C3H mice. Inbred strain-specific changes in composition (increased carbonate/phosphate ratio and decreased crystallinity, Fig. 3) and cross-sectional shape (increased AP/ML ratio, Fig. 4) in B6;129 KO mice were accompanied by significantly increased tissue-level yield stress (Fig. 2) compared with control mice. In C3H KO mice, only the collagen crosslinking ratio was altered with exercise (Fig. 3), a property that also had an increasing trend in B6;129 KO mice. There were no corresponding mechanical changes with exercise in C3H KO mice. The combination of inbred strain-specific changes across the bone hierarchy in the B6;129 KO mice (from composition to mechanical integrity and cross-sectional shape, Table 4) supports hypothesis 2.
In the B6;129 KO mice, exercise resulted in an increase in tissue-level yield stress, consistent with other studies on the mechanical loading of bone.19,28 Many studies have revealed positive correlations between the level of mineral in a bone and the stiffness and strength of the material.17,27 Relating carbonate content with mechanical integrity is less clear. Carbonate substituting for phosphate in the mineral lattice could lead to an atomistic-level increase in rigidity, as the presence of carbonate increases the c-axis dimension of the unit cell.5 Changes in carbonate content can also impact crystallinity due to alterations in the size and shape of the mineral crystals. The increase in carbonate/phosphate ratio and decrease in crystallinity in B6;129 KO bone may therefore have driven the increase in yield stress noted. The B6;129 KO mice also had an increase in the AP/ML shape factor of the bone indicating a shift in the distribution of tissue away from the ML direction. Bones in the current study were mechanically tested in the ML direction, so a decrease in the AP/ML ratio could explain why the increase in tissue-level strength was not accompanied by an increase in structural-level strength.
Changes in tissue composition resulted in inconsistent alterations in mechanical properties. The relationships between compositional measures at the microscale and mechanical properties at the tissue-level and structural-level are not clearly established. In an attempt to explain the relationship between properties measured in the current study, regressional analyses were performed between the compositional and mechanical properties (data not shown). Linear and multiple regressions failed to provide significant correlative relationships. One reason for the lack of correlation may be the different size scales of these measurements. Compositional measurements were made at the microscale, but are measuring a superposition of atomistic-level properties. In comparison, the mechanical measures were made at the whole bone scale. Even though these properties were normalized by bone size and represent tissue-level measurements, the properties were still based on a continuum approximation and ignore all of the hierarchical structures at smaller size scales. Another possible reason for poor regressions may stem from the sampling sites used. Although both sets of data came from the same bone, the compositional measures had to be taken outside of the mechanical testing region in the bone to avoid the effects that mechanical load may have on the Raman spectra.11 Since both composition and mechanical integrity may vary as a function of axial location in the bone, the ability to directly regress the data sets could be reduced.
An important observation in this study is that alterations in compositional properties in all four exercise groups occurred without changes in cross-sectional size, suggesting that the effects of exercise were exerted primarily through alterations in pre-existing tissue and that changes in structural-level properties were driven by tissue-level changes. This observation is consistent with other studies using this exercise model22,39 and further supports the notion that beneficial effects of mechanical loading do not require increased formation activity. Histomorphometric measurements performed in the tibiae of mice from the current study confirm the lack of significantly increased bone formation in any group (data not shown).
There were significant compositional changes with exercise in all four groups in the current study, but the changes were both inbred strain and genotype specific (Table 4). The mechanism leading to changes in composition in response to exercise is not completely clear. Exercise increased the degree of mineralization of the tissue (mineral/matrix ratio, Fig. 3b) in C3H WT mice, consistent with other studies.1,30,37 A more consistent compositional change was the significant increase in carbonate/phosphate ratio noted in 3 of 4 groups (with a trend in the C3H KO group, Fig. 3a). Direct physical stresses imposed by exercise may induce a phase change in the mineral.11,35 The in vivo microenvironment of the mineral crystals may also be responsible. Exercise increases the concentration of mineral ions in the blood47 as well as fluid flow through the microporosity of the bone.9,16 An ionic fluid flowing near mineral crystals that possess large surface-to-volume ratios24,42 could expose the mineral to a newly replenished solution that is potentially rich in ions, leading to the changes in mineral chemistry noted in this study. This fluid based mechanism does not rule out the role of cellular-mediated mechanisms such as local control of mineralization by osteocytes.32 Other potential mechanisms involve the interaction between collagen and mineral and/or changes in structural water that exists at their interface.43,44 Although the idea of a ductile collagen matrix with brittle mineral inclusions has gained acceptance over time, structural water may also be a key to understanding mechanical adaptation of bone as evidenced by recent work at the microstructural and molecular levels.6,18 The role of collagen is interesting to consider given that the collagen cross-linking ratio was only significantly increased with exercise in WT and KO C3H mice, the two groups which failed to exhibit mechanical changes with exercise. In the absence of new bone formation, exercise could lead to a structural rearrangement of the tissue which is beneficial to mechanical properties.20 An exercise-induced increase in collagen crosslink maturity may prevent this rearrangement and, therefore, the beneficial increase in mechanical integrity that these changes may precede.
Bgn-deficient mice exhibit a defect in the growth and differentiation of osteoblast precursor cells12–14 resulting in a decrease in the amount of bone and changes in the compositional quality of the tissue.36,38,46 Since altered tissue quality and low peak bone mass in trabecular and cortical locations are hallmarks of human osteoporosis, the bgn-deficient mouse constitutes a good model for studying the development of this condition and the efficacy of potential treatments.4,46 The bgn-deficient phenotype also shares characteristics with other human diseases including Turner Syndrome4 and Ehlers-Danlos Syndrome.15 The ability to alter pre-existing tissue composition and mechanical integrity in this model of disease suggest that mechanical stimulation may be a possible therapy for deficiencies in tissue quality that are associated with other diseases of bone. Specifically, the increase in yield stress in B6;129 KO mice with exercise raised the property back to its WT control level, supporting the possible therapeutic applications of exercise. Given the background strain-specific changes in tissue composition in this study, the effects of mechanical treatment may be genetically dependent.
It is important to point out that limitations do exist when deriving tissue-level mechanical properties from structural-level tests using beam-bending theory. The use of bending theory requires a number of assumptions which were adhered to as well as possible. These include using as large an aspect ratio as possible, having a constant cross-sectional shape throughout the beam length and testing a material that is isotropic and homogeneous. Further, the equations are only valid in the pre-yield regime. These assumptions may play a role in the properties that are ultimately measured at the tissue level. Because tissue-level properties are calculated directly from measured structural-level properties using geometry as the normalizing parameter, subtle differences in geometry which may not be statistically significant could contribute to changes observed at the tissue-level making it difficult to establish relationships between properties.
In summary, this study reveals inbred strain-specific effects of exercise in wild type and bgn-deficient mice and shows evidence of genotype-specific changes in bone in response to mechanical loading in a gene disruption model. Exercise elicits alterations in bone composition and/or mechanical integrity without changes in geometry. Together, these data suggest that mechanical stimulation may be a viable but genetically dependent means to alter tissue quality and mechanical deficiencies that are associated with many diseases of bone.
ACKNOWLEDGMENTS
Funding Sources: DoD/US Army DAMD17-03-1-0556; NIH R01 AR050210; NIH P30-AR46024; NIH IPA Agreement; NIH Regenerative Sciences Training Grant R90-DK071506.
REFERENCES
- 1.Adami S, Gatti D, Braga V, Bianchini D, Rossini M. Site-specific effects of strength training on bone structure and geometry of ultradistal radius in post-menopausal women. J. Bone Miner. Res. 1999;14:120–124. doi: 10.1359/jbmr.1999.14.1.120. [DOI] [PubMed] [Google Scholar]
- 2.Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. Bone response to in vivo mechanical loading in two breeds of mice. Calcif. Tissue Int. 1998;63:442–449. doi: 10.1007/s002239900554. [DOI] [PubMed] [Google Scholar]
- 3.Amblard D, Lafage-Proust MH, Laib A, Thomas T, Ruegsegger P, Alexandre C, Vico L. Tail suspension induces bone loss in skeletally mature mice in the C57BL/6J strain but not in the C3H/HeJ strain. J. Bone Miner. Res. 2003;18:561–569. doi: 10.1359/jbmr.2003.18.3.561. [DOI] [PubMed] [Google Scholar]
- 4.Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice leads to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 5.Baig A, Fox J, Young R, Wang Z, Hsu J, Higuchi W, Chhettry A, Zhuang H, Otsuka M. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 1999;64:437–449. doi: 10.1007/pl00005826. [DOI] [PubMed] [Google Scholar]
- 6.Bhowmik R, Katti KS, Katti DR. Mechanics of molecular collagen is influenced by hydroxyapatite in natural bone. J. Mater. Sci. 2007;42:8795–8803. [Google Scholar]
- 7.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J. Histochem. Cytochem. 1990;38:1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- 8.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J. Bone Miner. Res. 1999;14:330–335. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- 9.Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–S112. [PubMed] [Google Scholar]
- 10.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J. Bone Joint Surg. Am. 1975;57:956–961. [PubMed] [Google Scholar]
- 11.Carden A, Rajachar RM, Morris MD, Kohn DH. Ultrastructural changes accompanying the mechanical deformation of bone tissue: a Raman imaging study. Calcif. Tissue Int. 2003;72:166–175. doi: 10.1007/s00223-002-1039-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen XD, Allen MR, Bloomfield S, Xu T, Young M. Biglycan-deficient mice have delayed osteogenesis after marrow ablation. Calcif. Tissue Int. 2003;72:577–582. doi: 10.1007/s00223-002-1101-y. [DOI] [PubMed] [Google Scholar]
- 13.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 14.Chen XD, Shi S, Xu T, Robey PG, Young MF. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J. Bone Miner. Res. 2002;17:331–340. doi: 10.1359/jbmr.2002.17.2.331. [DOI] [PubMed] [Google Scholar]
- 15.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J. Bone Miner. Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 16.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif. Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 17.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Fritsch A, Hellmich C, Dormieux L. Ductile sliding between mineral crystals followed by rupture of collagen crosslinks: experimentally supported micromechanical explanation of bone strength. J. Theor. Biol. 2009;260:230–252. doi: 10.1016/j.jtbi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Hoshi A, Watanabe H, Chiba M, Inaba Y. Bone density and mechanical properties in femoral bone of swim loaded aged mice. Biomed. Environ. Sci. 1998;11:243–250. [PubMed] [Google Scholar]
- 20.Isaksson H, Tolvanen V, Finnilä MAJ, Iivarinen J, Tuukkanen J, Seppänen K, Arokoski JPA, Brama PA, Jurvelin JS, Helminen HJ. Physical exercise improves properties of bone and its collagen network in growing and maturing mice. Calcif. Tissue Int. 2009;85(3):247– 256. doi: 10.1007/s00223-009-9273-3. [DOI] [PubMed] [Google Scholar]
- 21.Kodama Y, Umemura Y, Nagasawa S, Beamer WG, Donahue LR, Rosen CR, Baylink DJ, Farley JR. Exercise and mechanical loading increase periosteal bone formation and whole bone strength in C57BL/6J mice but not in C3H/Hej mice. Calcif. Tissue Int. 2000;66:298–306. doi: 10.1007/s002230010060. [DOI] [PubMed] [Google Scholar]
- 22.Kohn DH, Sahar ND, Wallace JM, Golcuk K, Morris MD. Exercise alters mineral and matrix composition in the absence of adding new bone. Cells Tissues Organs. 2009;189:33–37. doi: 10.1159/000151452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn JL, Goldstein SA, Feldkamp LA, Goulet RW, Jesion G. Evaluation of a microcomputed tomography system to study trabecular bone structure. J. Orthop. Res. 1990;8:833–842. doi: 10.1002/jor.1100080608. [DOI] [PubMed] [Google Scholar]
- 24.Landis WJ. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone. 1995;16:533–544. doi: 10.1016/8756-3282(95)00076-p. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Gu W, Masinde G, Hamilton-Ulland M, Rundle CH, Mohan S, Baylink DJ. Genetic variation in bone-regenerative capacity among inbred strains of mice. Bone. 2001;29:134–140. doi: 10.1016/s8756-3282(01)00497-5. [DOI] [PubMed] [Google Scholar]
- 26.Marusic A, Katavic V, Grcevic D, Lukic IK. Genetic variability of new bone induction in mice. Bone. 1999;25:25–32. doi: 10.1016/s8756-3282(99)00095-2. [DOI] [PubMed] [Google Scholar]
- 27.Miller LM, Little W, Schirmer A, Sheik F, Busa B, Judex S. Accretion of bone quantity and quality in the developing mouse skeleton. J. Bone Miner. Res. 2007;22:1037–1045. doi: 10.1359/jbmr.070402. [DOI] [PubMed] [Google Scholar]
- 28.Mosekilde L, Thomsen JS, Orhii PB, McCarter RJ, Mejia W, Kalu DN. Additive effect of voluntary exercise and growth hormone treatment on bone strength assessed at four different skeletal sites in an aged rat model. Bone. 1999;24:71–80. doi: 10.1016/s8756-3282(98)00169-0. [DOI] [PubMed] [Google Scholar]
- 29.Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 30.Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med. Sci. Sports Exerc. 2002;34:196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Takagi M, Yamada T, Kamiya N, Kumagai T, Yamaguchi A. Effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of decorin and biglycan by cultured osteoblastic cells. Histochem. J. 1999;31:403–409. doi: 10.1023/a:1003704425809. [DOI] [PubMed] [Google Scholar]
- 32.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–16. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Timlin JA, Carden A, Morris MD, Rajachar RM, Kohn DH. Raman spectroscopic imaging markers for fatigue-related microdamage in bovine bone. Anal. Chem. 2000;72:2229–2236. doi: 10.1021/ac9913560. [DOI] [PubMed] [Google Scholar]
- 34.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–607. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya S, Karunakaran C, Pande B, Gupta N, Iyer R, Karweer S. Pressure-induced crystalline to amorphous transition in hydroxylapatite. J. Mater. Sci. 1997;32:3213–3217. [Google Scholar]
- 36.Wallace JM, Golcuk K, Morris MD, Kohn DH. Inbred strain-specific response to biglycan deficiency in the cortical bone of C57BL6/129 and C3H/He mice. J. Bone Miner. Res. 2009;24:1002–1012. doi: 10.1359/jbmr.081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH. Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone. 2007;40:1120–1127. doi: 10.1016/j.bone.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace JM, Rajachar RM, Chen XD, Shi S, Allen MR, Bloomfield SA, Les CM, Robey PG, Young MF, Kohn DH. The mechanical phenotype of biglycan-deficient mice is bone- and gender-specific. Bone. 2006;39:106–116. doi: 10.1016/j.bone.2005.12.081. [DOI] [PubMed] [Google Scholar]
- 39.Wallace JM, Ron MS, Kohn DH. Short-term exercise in mice increases tibial post-yield mechanical properties while two weeks of latency following exercise increases tissue-level strength. Calcif. Tissue Int. 2009;84:297–304. doi: 10.1007/s00223-009-9228-8. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Li X, Bank RA, Agrawal CM. Effects of collagen unwinding and cleavage on the mechanical integrity of the collagen network in bone. Calcif. Tissue Int. 2002;71:186–192. doi: 10.1007/s00223-001-1082-2. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 42.Weiner S, Traub W. Bone structure: from angstroms to microns. FASEB J. 1992;6:879–885. [PubMed] [Google Scholar]
- 43.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW. Three structural roles for water in bone observed by solid-state NMR. Biophys. J. 2006;90:3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW. Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J. Bone Miner. Res. 2005;20:625–634. doi: 10.1359/JBMR.041217. [DOI] [PubMed] [Google Scholar]
- 45.Wolff J, Maquet P, Furlong R. The Law of Bone Remodelling. Springer-Verlag Berlin and Heidelberg GmbH & Co. K; Berlin, New York: 1986. p. 126. [Google Scholar]
- 46.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 47.Yeh JK, Aloia JF, Yasumura S. Effect of physical activity on calcium and phosphorus metabolism in the rat. Am. J. Physiol. 1989;256:E1–E6. doi: 10.1152/ajpendo.1989.256.1.E1. [DOI] [PubMed] [Google Scholar]