Abstract
Epidemiological and animal studies indicate that disruption of circadian rhythm increases breast cancer risk. Previously, we demonstrated that methylselenocysteine (MSC) reduced the incidence of N-nitroso-N-methylurea (NMU)-induced mammary carcinomas in Fischer 344 rats by 63%. MSC also increased the expression of Period 2 (Per2) and D-binding protein (DBP), providing evidence for a link between circadian rhythm and chemoprevention. Here, we report that NMU disrupted the expression of core circadian genes (Per1, Per2, Cry1, Cry2, and RevErbAα) and circadian-controlled genes (CCGs), including melatonin receptor 1α (MTNR1A), estrogen receptors (ER a and β), and growth regulatory genes (Trp53, p21, Gadd45α, and c-Myc) in mammary glands of F344 rats. By contrast, dietary MSC (3 ppm Selenium) given for 30 days, significantly enhanced the circadian expression of these genes (except for Cry1 and Cry2). The largest effect was on the levels of the Per2, MTNR1A, and ERβ mRNAs, which respectively showed 16.5-, 4.7-, and 9.5-fold increases in their rhythm-adjusted means, and 44.5-, 6.5-, and 9.7-fold increases in amplitude as compared to the control diet. MSC also shifted the peak expression times of these genes to Zeitgeber Time 12 (ZT12; light off). MSC also induced rhythmic expression of Trp53, p21, and Gadd45α mRNAs with peak levels at ZT12, when c-Myc expression was at its lowest level. However, MSC had no significant impact on the circadian expression of these genes in liver. These results suggest that dietary MSC counteracted the disruptive effect of NMU on circadian expression of genes essential to the normal mammary cell growth and differentiation.
Keywords: N-nitroso-N-methylurea, methylselenocysteine, chemoprevention, circadian rhythm, melatonin receptor 1α, estrogen receptor β
INTRODUCTION
In mammals, circadian rhythm is controlled by the interaction of positive and negative biochemical feedback loops comprising a molecular oscillator. Heterodimers of brain and muscle arnt-like protein 1 (Bmal13, known as Arnt1 in rat) and Clock or neuronal PAS domain protein 2 (Npas2) activate transcription by binding to E-box elements present in the promoters of circadian genes, including Period (Per), Cryptochrome (Cry), Rev-ErbAα, retinoid-related orphan nuclear receptors (ROR), and numerous circadian-controlled genes (CCGs). The Rev-ErbAα and ROR proteins respectively inhibit and activate Bmal1 transcription (1). Postranscriptionally modified Per:Cry heterodimers in turn repress Clock:Bmal1 transcriptional activity, thus limiting their own transcription and the expression of CCGs (2). The Clock protein was shown to have histone acetyltransferase activity (HAT) (3), suggesting that the activation of E-box containing promoters is modulated at the level of chromatin structure. The molecular oscillator functions in most peripheral tissues and maintains a periodicity of ~24 hours that persists in the absence of external cues (1, 2, 4, 5). To synchronize the periodicity among cells in peripheral organs in vivo and adjust the phase to changing environmental signals, the intrinsic molecular oscillator is entrained by external signals that includes melatonin and glucocorticoids (6). In vitro studies on fibroblasts indicate that the intracellular mediators of circadian gene expression include Ca2+, protein kinase C, protein kinase A, mitogen-activated protein kinase, and glucocorticoid receptor signaling pathways (6, 7). In mammals, the central pacemaker for the autonomous oscillators in peripheral organs is melatonin, a hormone whose secretion from the pineal gland is synchronized by light-induced signaling from the suprachiasmatic nucleus (SCN) (8, 9). In this way, SCN coordinates the behavior, physiological and biological functions of organisms and cells with light-dark cycles, food availability, and a variety of environmental signals.
Circadian rhythms also play a critical role in normal cell growth, differentiation, and cellular responses to genotoxic stressors (2). Disruption of normal circadian rhythm by environmental exposures is associated with an increased risk of several cancer types. For example, disruption of circadian rhythm by constant light exposure or pinealectomy increased the incidence of mammary adenocarcinoma in rodents (10, 11). Moreover, mice lacking Per2 show a significantly elevated incidence of carcinomas after exposure to genotoxic stress (1), while over-expression of either Per1 or Per2 in cancer cells inhibits their neoplastic cell growth and increases apoptotic rates (12-14). Clinical studies also reveal an association between the deregulation of Per genes and various human cancers (15-17). In addition, epidemiological studies indicate that disruption of circadian rhythm by shift work (exposure to light at night) increases breast and prostate cancer risk (18), prompting the International Agency for Cancer Research to classify shift work as a probable human carcinogen (Type 2A).
Both epidemiological and animal studies have suggested that various forms of selenium, a dietary trace element, reduce cancer risk at multiple organ sites, including breast and prostate (19). Methylselenocysteine (MSC), an organic form produced by plants, mediates its chemopreventive effects at the early stages of carcinogenesis (20-22). Our recent studies were the first to suggest an association between circadian rhythm and chemoprevention. We demonstrated that dietary MSC, given for 30 days after exposure to a single carcinogenic dose of NMU, reduced the incidence of mammary carcinomas in Fischer 344 (F344) rats by 63% at 36 weeks. Gene expression profiles of normal mammary tissue indicated that 30 days of dietary supplementation with MSC significantly increased the levels and rhythmic expression of the core circadian gene, Period 2 (Per2), and the circadian output gene, D-binding protein (DBP)(23). In the present study, we extended these observations, by demonstrating that a single carcinogenic dose of NMU significantly disrupted rhythmic expression of most of core circadian genes and CCGs during the early stages of carcinogenesis. By contrast, chemopreventive MSC reset and enhanced the circadian gene expression in mammary tissues of NMU-treated rats. The circadian and CCGs affected by MSC included core circadian genes (Per2 and Rev-ErbAα, estrogen and melatonin receptors, and DNA damage responsive genes. We also found that the ablation and resetting of circadian expression of these genes by NMU and MSC, respectively, were independent of serum melatonin levels.
MATERIALS AND METHODS
Animal Maintenance and Diet Preparation
All protocols were reviewed by and received the approval of the Institutional Animal Care and Use Committees. Animal experiments were performed in AAALAC accredited facilities at the Fred Hutchinson Cancer Research Center (Seattle, WA) and the University of Medicine and Dentistry of New Jersey (Piscataway, NJ). Female F344 rats were from Harlan Laboratories (Indianapolis, IN). For acclimatization to a powdered ration from Harlan Tekland (Madison, WI), animals were maintained on a powdered standard AIN-76A diet containing 0.1 ppm Selenium (sodium selenite). The MSC-enriched diet was made by admixing L-Se-methylselenocysteine (Selenium Technologies, Lubbock, TX) with the standard powdered AIN-76A diet to a final concentration of 3 ppm Selenium. Animals were housed under controlled climate conditions and a 12 h light/12 h dark cycle (7 AM on / 7 PM off). The 7 AM is referred to Zeitgeber Time (ZT) 0, indicating the time of light on; and 7 PM is ZT12, indicating the time of light off.
Animal Treatment and Sample Collection
Forty-two female F344 rats between 50 and 55 days of ages received a single intra-peritoneal (i.p.) injection of NMU (Ash Stevens Inc., Detroit, MI) at a dose of 50 mg/kg body weight. NMU was dissolved in acidified saline at a final concentration of 10 mg/ml just prior to injection. Rats were then randomized to two groups and maintained on either the standardized control diet or the MSC-enriched diet for 30 days. An additional 21 age-matched female F344 rats injected with the vehicle and maintained on the standardized diet served as control group. Three rats of each group were sacrificed every 4 h during 24 h, beginning at 7 AM (ZT0). Blood samples were collected into heparinized-BD Vacutainers by cardiac punctures after CO2 asphyxiation. Plasma samples were separated by sequential centrifuge (at 1300 rcf for 10 min first and then at 2400 rcf for 15 min) and stored at -80 °C. All mammary glands from each side of individual rat were carefully dissected, combined into a pool of left or right mammary glands, frozen on dry ice, and stored at -80 °C. Liver of each rat was also dissected, frozen on dry ice and stored at -80 °C.
Quantitative Real-Time PCR
Total RNA was extracted from a small piece (~50 mg) of mammary tissue from the left side pool of mammary glands and liver of each rat using Tri Reagent (Sigma, Saint Louis, MO) and Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA), and then digested with Qiagen RNAse-free DNAse. Relative mRNA expression levels were determined with real-time quantitative RT-PCR using specific primers (Supplemental Table 1), designed with Primer Express Software V 3.0 (Applied Biosystems, Foster City, CA), and SYBR as the reporter. For reverse transcription, 1 μg of RNA was reversely transcribed to cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). For real-time PCR, cDNA was amplified with the SYBR Universal PCR Master Mix (Applied Biosystems) in ABI Prism 7900 Sequencing Detector according to the manufacture’s instruction. A no-template control was included in each assay. β-Actin was used as an endogenous control. The expression level of each gene in mammary tissue of the rat sacrificed at ZT0 was used as a calibrator in each group. Three independent samples were analyzed for each time point. The comparative Ct method was used to analyze the relative mRNA expression levels (24).
Western Blot
Total protein was extracted from a small piece (~200 mg) of mammary tissue from the left side pool of mammary glands of each animal using RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Sodium-deoxycholate, 0.1% SDS) supplemented with a cocktail of protease inhibitor (1:100), phosphatase inhibitor I (1:100), and phosphatase inhibitor II (1:100) (Sigma-Aldrich). Forty μg of protein sample was separated on 7.5% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. After blocking, the membrane was incubated with primary antibody, anti-rat Per2 (G-19) (Santa Cruz Bio, Santa Cruz, CA, 1:200), anti-rat ERβ (Abcam, Cambridge, MA, 1:2000), or anti-rat MTNR1A antibody (R-18) (Santa Cruz Bio, 1:200) overnight at 4°C, followed by incubation with IR Dye 800 CW anti-goat or anti-rabbit IgG (LI-COR, Lincoln, NE). Targeted protein signals with fluorescence were detected using Odyssey Infrared Imaging System (LI-COR). Whole tissue protein from rat brain was used as a positive control for Per2 and MTNR1A; rat ovary tissue protein was used for ERβ. The intensities of bands were determined using densitometry, and β-Actin was used as an internal control for normalization.
Immunohistochemistry
A small piece (0.5 cm2) of frozen mammary tissue from the left side pool of mammary glands of each rat was embedded in OTC medium and stored at -80°C. Cryo-sections (15 μm) were fixed in 4% paraformaldehyde for 20 min. Immunohistochemical staining was performed with anti-rat Per2 (1:50), ERβ (1:200), or MTNR1A (1:50) antibody used in Western blot, using avidin-biotin-peroxidase complex (ABC) staining systems (Santa Cruz Bio) (25). PBS containing 1.5% normal serum instead of primary antibody was used as negative control in immunohistochemistry.
Determination of Plasma Melatonin Levels
Plasma was separated from blood by centrifugation and stored at -80°C. Melatonin levels were determined using Melatonin ELISA Kit (GenWay Biotech, San Diego, CA) and SpectraMax V5 microplate reader (Molecular Devices, Sunnyvale, CA).
Sequence Analyses of Promoter Regions
The sequences of the promoter regions (10k-bp 5’ up-stream, exon 1, and 2k-bp intron 1) of MTNR1A, ERβ, Per1, Per2, Cry1, Rev-ErbAα, DBP genes were obtained from NCBI Map Viewer. The E-box motif (CACGTG) of Bmal1 was identified within the promoter regions of these genes. The 66-bp sequences including the motif in the promoter regions of MTNR1A in different species were analyzed using Multiple Sequence Alignment algorithm of F. Corpet (http://bioinfo.genotoul.fr/multalin/).
Statistic Analyses
Intergroup differences were evaluated using multivariate ANOVA. Statistical significance of circadian rhythmicity was documented by Cosinor analysis using Time Series Single Cosinor 6.3 (Expert Soft Tech., Richelieu, France) (26). Period s = 24 hours or s = 12 hours was considered a priori. The rhythm characteristics estimated by this linear least squares method include mesor (rhythm-adjusted mean) and amplitude (a half of the difference between minimum and maximum of fitted cosine function). A rhythm was detected if the null hypothesis was rejected with P < 0.05. Significance of differences in protein expression levels between MSC-enriched vs control diet group were analyzed with Student t-test (mean ± SE, n=3).
RESULTS
Effects of NMU and MSC on the expression of circadian genes
In our previous study, we demonstrated that a chemopreventive regimen of dietary MSC-enriched diet significantly increased the level and rhythm of Per2 mRNA in mammary gland, but not in liver, of NMU-treated rats. To determine if NMU was directly responsible for the ablation of circadian gene expression, we used quantitative RT-PCR to generate circadian expression profiles of circadian and CCGs in mammary gland and liver tissues of rats treated with a carcinogenic dose of NMU or the vehicle. Our results showed that NMU significantly reduced the circadian expression of Per2, Cry1, Cry2, and Rev-ErbAα genes in mammary tissues (Fig. 1A), but had only a modest effect in liver tissues (Fig. 1B) (Table 1). By contrast, the circadian expression of Per1 was significantly reduced by NMU in liver, but not in mammary gland.
Figure 1. Effects of NMU and MSC on the mRNA expression of core circadian genes.
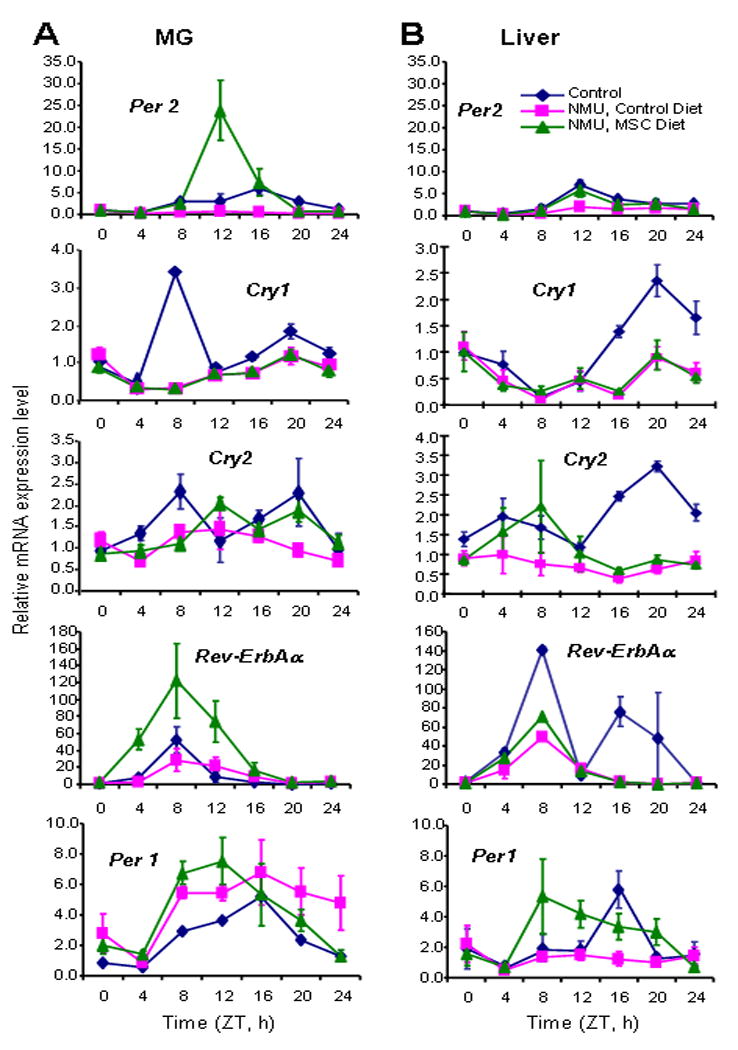
Untreated pubescent female F344 rats were maintained on a standardized diet (Control, diamond). NMU-treated rats were maintained on either standardized diet (Control Diet, square) or MSC-enriched diet (MSC Diet, triangle) for 30 days after exposure. Three animals were sacrificed every 4 h over a 24 h period, beginning at 7 AM (ZT0). (A&B) Quantitative analysis of Per1, Per2, Cry1, Cry2, and Rev-ErbAα mRNA expression. Quantitative real-time RT-PCR was performed with total RNA samples from mammary gland (MG) (A) or liver tissues (B). The results were analyzed using a comparative Ct method. β-Actin was used as an endogenous control. The expression level of the first sample at ZT0 was arbitrary set as 1 in each group. X-axis: Zeitgeber time (light on at 7 AM and off at 7 PM); Y-axis: relative mRNA level, shown in mean ± SE (n=3).
Table 1.
Circadian expression of circadian and circadian-controlled genes in MSC-enriched vs control diet groups in NMU-treated rats.
| Gene | Mammary Gland | Liver | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Change of Rhythm* | Change of Level (Fold Change)§ | Peak Time‡(ZT, h) | Change of Rhythm | Change of Level (Fold Change) | Peak Time (ZT, h) | |||||
| Control | MSC | Mesor** | Amplitude† | MSC | Control | MSC | Mesor | Amplitude | MSC | |
| Per1 | Nc | P-4 | -0.06 | 0.39 | 12 | D | R/P-8 | 1.14 | 14.25 | 8 |
| Per2 | D | R/P-4 | 16.49 | 44.53 | 12 | Nc | Nc | 0.86 | 1.28 | 12 |
| Cry1 | D | D | -0.04 | -0.29 | 20 | Nc | Nc | 0.13 | -0.33 | 20 |
| Cry2 | D | D | 0.24 | 0.64 | 12 | D | D | 0.69 | 1.30 | 8 |
| Rev-ErbAα | D | R | 2.99 | 3.55 | 8 | R/P | Nc | 0.33 | 0.44 | 8 |
| Arntl | Nc | Nc | 0.48 | 0.06 | 0 | Nc | Nc | 0.08 | -0.04 | 0 |
| Clock | Nc | Nc | -0.41 | 0.22 | 8 | Nc | Nc | 0.18 | -0.09 | 0 |
| Npas2 | Nc | Nc | -0.13 | -0.33 | 0 | Nc | Nc | 0.24 | 0.44 | 0 |
| MTNR1A | D | R/P+4 | 4.67 | 6.50 | 12 | D | D | 4.92 | 1.70 | 0 |
| ERα | D | R/P+4 | 0.50 | 3.63 | 12 | |||||
| ERβ | Nr/Nc | I | 9.48 | 9.71 | 12 | Nr/Nc | Nr/Nc | 1.5 | 3.58 | 0 |
| c-Myc | Nr/Nc | I | -0.36 | 0.00 | 0 | Nc | Nc | 0.12 | 0.09 | 4 |
| Trp53 | D | R | 1.68 | 9.67 | 12 | Nc | Nc | -0.13 | 0.24 | 8 |
| P21 | D | R | 3.05 | 7.11 | 12 | D | D | 0.02 | 0.60 | 12 |
| Gadd45α | D | R | 2.29 | 18.00 | 12 | R | D | 0.52 | 5.07 | 8, 12 |
The rhythmicity was determined by Time Series Single Cosinor 6.3 software. The significance of the circadian rhythm was tested by a zero-amplitude test (p<0.05). The rhythm of each gene per group was compared to that of normal control rat. Changes of rhythms were described as D, significant disruption of rhythm (p>0.05); R, significant restoration of rhythm (p<0.05); I, induction of rhythm; P, phase resetting with advanced (-) or delayed (+) peak; N, no significant change (Nc) or no rhythm (Nr).
Mesor indicates rhythm-adjusted mean of relative expression.
Amplitude indicates the half of the difference between minimum and maximum of the fitted cosine function.
Peak times present the times of the maximum values on the graphs of “NMU, MSC Diet” group in the figures, with light onset as phase reference.
Fold change = (MSC diet-Control diet)/Control diet
The present study confirmed that in NMU-treated rats, MSC induced the rhythmic expression of Per2, with a 16.5-fold increase of rhythm-adjusted mean and a 44.5-fold increase in amplitude of Per2 mRNA expression relative to the control diet (Fig. 1A and Table 1), and a 1.5-fold increase in rhythm-adjusted mean and 2.9-fold increase in amplitude as compared to the untreated control rats (Supplemental table 2). Western blot analysis indicated that MSC also induced a statistically significant increase in Per2 protein expression in mammary tissues as compared to the control diet (p<0.05). Since circadian expression profiles could differ among cell types, and the ratio of cell types could vary among the tissue specimens used for the analyses, we also compared Per2 expression levels by immunohistochemical staining. Results indicated that increased expression of Per2 in mammary tissue resulted primarily from increased expression in mammary epithelial cells (Fig. 2, left panel).
Figure 2. Determination of Per2 (left), MTNR1A (middle), and ERβ (right) protein expression levels in mammary tissues of rats sacrificed at ZT12.
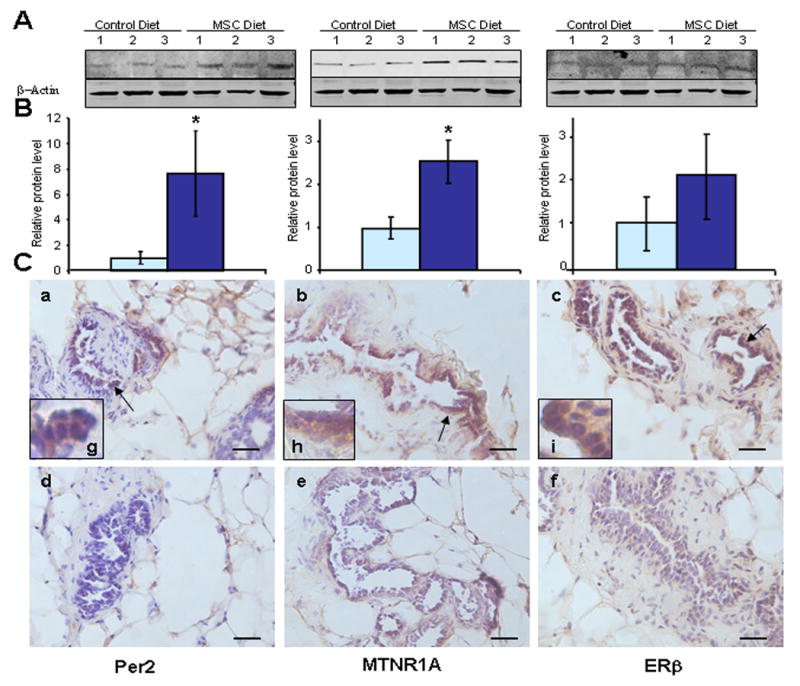
(A) Western blot results with whole tissue protein. β-Actin was used as an internal control. (B) Quantitative analysis of protein expression levels. Mean expression levels were calculated from densitometric intensities of bands determined with Western blot and normalized to β-Actin. * indicates significant difference with p<0.05. (C) Representative immunohistochemical staining on frozen sections of mammary tissues (200X, scale bar=0.1 mm). a-c: NMU, MSC diet; d-f: NMU, control diet; g-i: representative epithelial cells with positive staining are indicated by arrows and magnified photomicrograph (400X),
In addition to enhancing Per2 expression in NMU-treated rats, dietary MSC seemed to reset the phase of Per2 expression with peak level at ZT12 (right after the light turned off), which was advanced 4 hours from the peak at ZT16 in normal control rats. MSC also increased the rhythm-adjusted mean and amplitude of Rev-ErbAα mRNA expression by 2.99- and 3.55- fold, respectively (Fig. 1A and Table 1), exceeding those seen in normal mammary tissues of untreated rats (2.76-fold of rhythm-adjusted mean and 2.05-fold of amplitude) (Supplemental table 2). MSC increased the mRNA expression level and advanced the circadian phase for Per1 expression in both mammary and liver tissues although the effect of NMU on this gene was somehow different between mammary gland and liver. MSC did not show any effect on Cry1 and Cry 2 in either organ. Importantly, neither NMU treatment nor MSC-enriched diet significantly altered the mRNA expression patterns of genes encoding the heterodimeric circadian transcription factors (Arntl, Npas2 and Clock) (Supplemental Fig 1).
Effects of NMU and MSC on plasma melatonin and melatonin receptor expression
To investigate the mechanisms by which NMU and MSC alter the expression levels and phases of circadian genes, we first investigated their effects on the entrainment of circadian rhythm. We compared plasma melatonin levels of control rats over a 24-hour period to those of NMU-treated rats with or without MSC-enriched diet. All three groups showed normal circadian patterns of plasma melatonin, with nighttime levels attaining peak concentrations (~111.7 pg/ml) at ZT20, and the minimum daytime levels (~59.9 pg/ml) occurring at the ZT4 (Fig. 3A). These results excluded the possibility that either compound altered the production or stability of the hormone. Since the melatonin-mediated responses in peripheral organs are mediated through the MTNR1A (27, 28), we next asked if NMU and/or MSC affected the expression level of MTNR1A in mammary tissue. The results showed that MTNR1A expression is normally under circadian control in rat mammary gland with peak expression level at ZT 8 (Fig. 3B). A single carcinogenic dose of NMU completely disrupted MTNR1A expression in mammary gland. By contrast, MSC restored the levels of the MTNR1A mRNA expression to the normal levels of untreated control rats, increasing in rhythm-adjusted mean (4.67 fold) and amplitude (6.5 fold) in mammary gland (Fig. 3B) relative to those in NMU-treated rats on control diet (Table 1 and Supplemental table 2). Dietary MSC also shifted the phase of peak MTNR1A mRNA expression by 4 hours from ZT8 to ZT 12 (Fig. 3B). The MSC-enriched diet also significantly increased expression levels of MTNR1A protein in mammary tissues of NMU-treated rats compared to the control diet (p<0.05). Immunohistochemistry confirmed that the differences observed by Western blot were primarily due to the increased MTNR1A protein expression in mammary epithelial cells (Fig. 2, middle panel) in MSC vs control diet group. By contrast, the rhythmic MTNR1A expression in normal liver was much lower, and neither NMU nor MSC had an appreciable impact on its expression levels in liver (Fig. 3C).
Figure 3. Effects of NMU and MSC on the plasma melatonin concentrations and MTNR1A mRNA expression levels in mammary gland and liver.
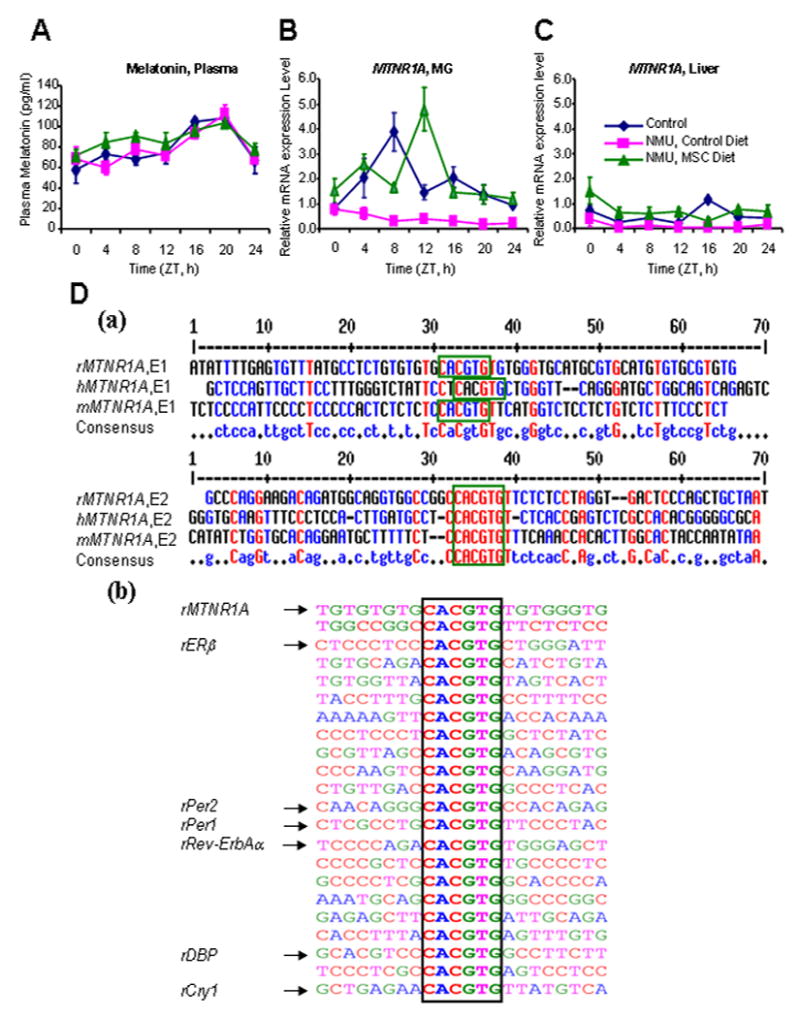
(A) Plasma melatonin levels were measured by ELISA in plasma samples of rats in different groups as described in Fig. 1 legend; (B and C) Relative mRNA expression levels were determined using quantitative real-time RT-PCR with total RNA samples from mammary gland (MG) (B) and liver tissues (C) of rats as described previously; (D) Conservation of Bmal1 E-box elements (framed) in the promoter regions of rat MTNR1A and circadian genes. The E-boxes within the 10-kb of 5’ up-stream, exon 1, and 2-kb of intron 1 region were analyzed. r, rat; m, mouse; h, human (a) Alignment of the proximal MTNR1A promoter regions from rat, mouse, and human. (b) Sequence comparison of the E-boxes found in MTNR1A promoter with those in the promoter regions of the indicated circadian genes in rat.
Many circadian genes and CCGs are transcriptionally regulated by the binding of Bmal1:Clock heterodimeric transcription factors to the E-box binding elements in their promoter regions. To examine the possibility that the rhythmic expression of the melatonin receptor is under Bmal1:Clock mediated transcriptional control, we analyzed the sequence of the promoter region of rat MTNR1A gene and compared its conservation across species. DNA sequence comparisons identified two well-conserved E-box binding motifs (CACGTG) of Bmal1 in the promoter regions of human (at -2926 and -2509), mouse (at -4505 and -237), and rat (at -8949 and -7047) MTNR1A genes (Fig. 3D (a)). We also found the numerous copies of the E-box binding motifs of Bmal1 in the promoter regions of ERβ and various circadian genes in rat, as reported previously in mouse (Fig. 3D (b)) (29). These findings indicated that the MTNR1A might be a CCG, whose expression could be entrained by exogenous signals that alter Bmal1 and Clock levels or activity.
MSC induced circadian rhythm of ERβ and reset the phase of ERα expression
Recent studies indicated that both MTNR1A-mediated melatonin signaling and Per2 regulate the expression levels of estrogen receptors in vitro (29-32). We therefore asked if NMU and MSC altered the circadian expression profiles of both ERα and ERβ genes in mammary and liver tissues. We observed that the expression levels of ERβ mRNA in both tissues were normally low, with a relatively weak circadian rhythm. Nonetheless, a carcinogenic dose of NMU reduced the expression of ERβ mRNA in both tissues. More importantly, dietary MSC dramatically induced circadian expression of ERβ mRNA in mammary gland (Fig. 4A), but not in liver (Fig. 4B). In mammary glands of NMU-treated rats, the MSC-enriched diet induced a 9.48-fold increase in the rhythm-adjusted mean level and a 9.71-fold increase in amplitude of ERβ mRNA expression, as compared to the control diet (Table 1). Western blot results indicated that dietary MSC only resulted in a marginal, statistically insignificant increase of ERβ protein expression relative to the control diet. However, immunohistochemical staining showed that ERβ protein expression was dramatically enriched in mammary epithelial cells of rats on MSC-enriched diet as compared to the rats on control diet. ERβ protein also expressed in stromal cells of mammary glands in both groups (Fig. 2, right panel). Although the effect was less dramatic, MSC also restored NMU-disrupted circadian expression of ERα mRNA in mammary tissues and shifted its peak expression to ZT12 (Fig. 4C).
Figure 4. Effects of NMU and MSC on the expression of estrogen receptors in rat mammary gland and liver.
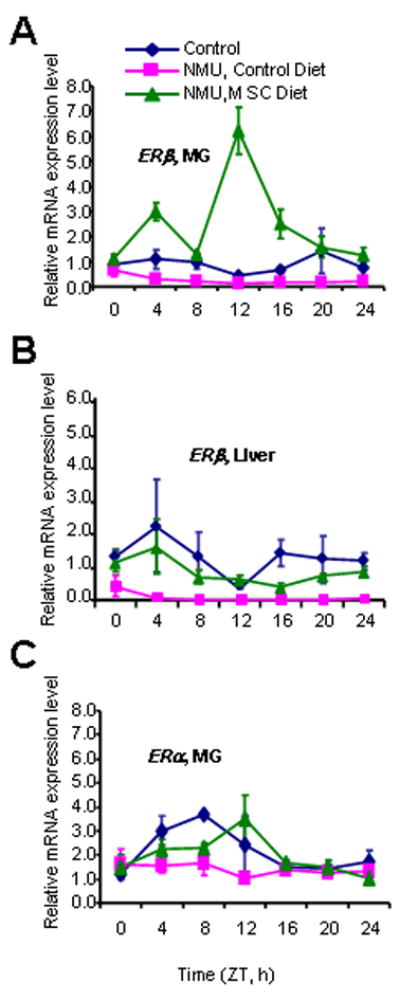
Relative mRNA expression levels of ERβ (A, MG, mammary gland and B, liver) and ERα (C) were determined using quantitative real-time RT-PCR as described in Fig.1 legend.
MSC reset the circadian expression of growth regulatory genes
Our analysis further indicated that NMU significantly reduced the circadian expression of c-Myc, Trp53, p21, and Gadd45α genes in mammary tissues. As was the case for Per2, MTNR1A and ERβ, dietary MSC also reset the phase and increased the circadian expression of these growth regulatory genes in mammary gland, but not in liver (Fig. 5) (Table 1). The expression levels of the Trp53, p21, and Gadd45α were at their peak levels at ZT12, immediately after the light was turned off, while the peak expression of the c-Myc proto-oncogene was at its lowest level (Fig. 5). These results indicate that NMU disrupted, while a MSC-enriched diet reset, the circadian expression of these genes involved in cellular responses to genotoxic stress and cell cycle control.
Figure 5. Effects of NMU and MSC on the circadian expression of growth regulatory genes in rat mammary gland (MG) (A) and liver (B).
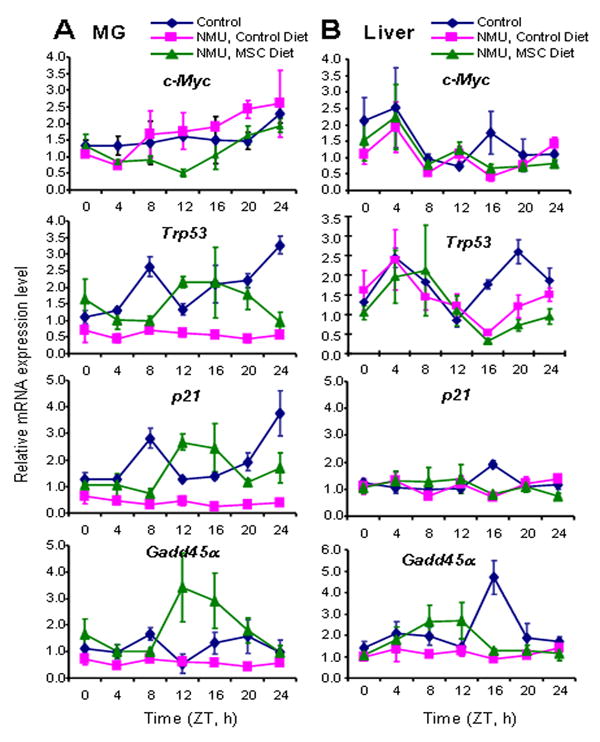
Relative mRNA expression levels of c-Myc, Trp53, p21, and Gadd45α were determined using quantitative real-time RT-PCR as described in Fig, 1 legend.
DISCUSSION
Epidemiological and animal studies indicate that disruption of circadian rhythm is associated with an increased risk of breast cancer (18). In the present study, we demonstrated that exposure of pubescent female F344 rats to a carcinogenic dose of NMU significantly reduced the rhythmic expression of most of core circadian genes (especially Per2 and Rev-ErbAα) and numerous CCGs, including hormone receptor genes (MTNR1A and ERs) and DNA damage responsive and growth regulatory genes (Trp53, p21, Gadd45α and c-Myc) in the mammary glands. The effect of NMU on circadian gene expression was much less pronounced in liver, which is not a major target organ in the NMU-induced rat mammary tumor model. Our preliminary results further indicated that NMU exposure has little effect on circadian gene expression in mammary glands of the resistant Copenhagen strain (data not shown). Together, these findings link NMU-induced disruption of circadian gene expression, which altered the DNA damage response and growth control pathways, to the mechanism of mammary carcinogenesis. It is interesting to note that expression of the melatonin receptor MTNR1A, which could be a CCG in mammary tissue, displayed little rhythmicity in the liver. This finding is consistent with the observation that food availability, rather than the photoperiod, is the major regulator of circadian rhythm in liver (33). This tissue-specific regulation of circadian rhythm explains how the liver is able to maintain rhythmic expression of Per2 in the absence of rhythmic MTNR1A expression. Moreover, the fact that disruption of circadian rhythm by NMU during mammary carcinogenesis might be mediated via reduced MTNR1A expression, in part, explains the tissue specificity of NMU in this animal tumor model.
Our results also indicated that a chemopreventive regimen of MSC counteracted the effect of NMU by enhancing and resetting the circadian expression of these genes during the early stages of carcinogenesis. To investigate how NMU and MSC altered the regulation of circadian rhythm, we first examined their effects on the entrainment of circadian gene expression in peripheral tissues. In mammals, entrainment is primarily mediated by light entering the eye. By modulating the number and the synchronicity of post-synaptic signals reaching the pineal gland responding to the light, the SCN regulates the synthesis and secretion of melatonin into the circulation as a function of light/dark cycles (28). Serum melatonin is rapidly metabolized with a half-life less than 20 min (27), permitting rapid adjustment of circulating levels of melatonin to the amount of light entering the eyes. Circulating melatonin binds to MTNR1A on the surface of peripheral cells, producing a long-lasting sensitization to adenyl cyclase that induces the synchronization of the circadian gene expression in peripheral cells with the central rhythm (34, 35). Normally there is an inverse relationship between serum melatonin and melatonin receptor levels in peripheral organs, indicative of a regulatory feedback loop (36). However, we found that neither NMU nor MSC had effect on circulating melatonin levels, indicating that neither compound altered melatonin secretion from the pineal gland, or its metabolism in serum. These finding suggest that the effects of NMU and MSC were mediated at the level of melatonin signaling in peripheral cells. To test this hypothesis, we first confirmed that rhythmic expression of MTNR1A in mammary tissues of normal rats is normally linked to the photoperiod. We next demonstrated that a single carcinogenic dose of NMU ablated while MSC restored the circadian expression of MTNR1A in mammary tissue. MSC-mediated restoration on melatonin receptor signaling may reset the rhythmic expression of circadian and CCGs in peripheral tissues, which lack an innate capacity for self-recovery of circadian rhythm. Significantly, MSC not only restored MTNR1A levels in mammary tissues of NMU-treated rats to normal levels as seen in untreated control rats, but also induced a shift of peak expression time of the MTNR1A gene from ZT8 to ZT12 (light off), responding to the light change. This phase shift resulted in a closer temporal alignment of peak serum melatonin levels (ZT20) with peak cellular melatonin receptor levels (ZT12). If the observed realignment of ligand concentrations and receptor expression levels results in increased MTNR1A-mediated melatonin signaling, the MSC-induced phase shift could contribute to the enhanced expression of many core circadian genes and CCGs relative to those seen in normal control rats.
However, since our results showed that expression of MTNR1A is itself under circadian control, the effects of NMU and MSC on MTNR1A expression also could be an indirect consequence of altered circadian rhythm. For example, MSC could restore circadian gene expression by increasing the activity of the Clock: Bmal1 heterodimeric transcription factors. The Clock protein, which has HAT activity, can regulate Clock: Bmal1-dependent transcriptional activity directly by acetylating the Bmal1 protein or indirectly by acetylating histone proteins associated with promoter regions of core circadian genes (3). Other studies have shown that HAT activity of Clock protein can be counteracted by Sirt1, a NAD+-dependent histone deacetylase (HDAC), thereby linking cellular energy balance to Clock: Bmal1-regulated transcription of circadian genes and CCGs (37). Moreover, the inhibition of Sirt1 associated HDAC activity with splitomicin or nicotinamide increased histone 3 acetylation levels, and further increased the circadian oscillation of the circadian gene expression (37). Based on these findings, we hypothesize that NMU and MSC regulate the circadian expression of circadian genes and CCGs at epigenetic level during carcinogenesis and chemoprevention. This hypothesis is supported by several lines of evidence. Inorganic selenium, which has antioxidant activity, alters NADP/NADPH and NAD/NADH coupled redox cycling (21). MSC could therefore alter the redox state of these cofacors to inhibit the HDAC activity of Sirt1 (37, 38), further enhancing the rhythmic expression of circadian genes. Consistent with this possibility, our previous studies suggested that NMU may mediate part of its carcinogenic effects through epigenetic mechanism that alters DNA conformation (39, 40). In addition, the majority circadian genes deregulated in human cancers harbor increased DNA methylation and/or deacetylated histones in their promoter regions (15-17). Recent studies also demonstrated that selenite can inhibit DNA hypermethylation and histone deacetylation in prostate cancer cells in vitro (41). More importantly, our preliminary in vitro experiments also demonstrated that MSC increases histone acetylation in mammary epithelial cells (data not shown).
The present study also provides insight into the mechanisms by which resetting of circadian gene expression by MSC can contribute to chemoprevention. Previous studies demonstrated that dietary MSC supplement did not prevent the initiation of carcinogenesis, but suppressed the outgrowth of premalignant lesions (21). Other studies demonstrated that ovariectomy after carcinogen exposure prevents mammary carcinogenesis in pubescent female rats, implicating estrogens in the promotion of initiated cells (42). Significantly, the core circadian protein Per2 binds to and increases the degradation of ERα protein and also indirectly regulates the transcription of ERβ (29, 31). These findings suggest that disruption of circadian gene expression could enhance, while restoration of circadian rhythm could inhibit, estrogen mediated promotion of mammary carcinogenesis. ERα and ERβ have opposing effects on estrogen-mediated cell growth of mammary epithelial cells, while over-expression of Per2 inhibited breast cancer cell growth (43)(44). Consistent with these in vitro study results, we found that MSC dramatically enhanced circadian expression of Per2 and ERβ at mRNA and protein levels in mammary epithelial cells in vivo. Although we observed a less dramatic effect of MSC on ERα, the large increase in ERβ levels would elevate the ERβ/ERα ratio in mammary cells. It has been demonstrated that when co-expressed in breast cancer cells, ERβ antagonizes the expression of ERα-mediated expression of growth regulatory genes, including c-Myc and cyclin D1. Our in vivo studies also found that MSC-enriched diet inhibited expression of c-Myc in mammary tissues in vivo, with the lowest level of c-Myc expression occurred at ZT12, while Per2 and ERβ expression levels were at their highest levels. Taken together, these findings suggest that enhanced circadian expression of ERβ protein might suppress the promotion phase of mammary carcinogenesis by inhibiting estrogen-induced cell growth.
Another mechanism, by which MSC-mediated effects on circadian rhythm can contribute to suppression of mammary carcinogenesis, is through altered responses to genotoxic stressors (45, 46). Disrupting normal circadian rhythm though shift work, exposure to chemical carcinogens, or genetic manipulation would therefore be expected to negatively impact the ability of cells to respond to and repair DNA damage. Consistent with such a mechanism, our data showed that disruption of circadian rhythm by NMU inhibited the rhythmic expression of DNA damage responsive and growth regulatory genes (Tpr53, p21, Gadd45α, and c-Myc). By contrast, a dietary regimen of MSC reset and enhanced the rhythmic expression of core circadian genes, Per2 and Rev-ErbAα, and CCGs, including these DNA damage responsive genes. Interestingly, the peak expression levels of the Trp53, p21, and Gadd45α genes corresponded temporally with the lowest level of c-Myc gene expression, suggesting that enhanced circadian gene expression by MSC also plays a role in coordinating cellular growth with DNA damage responses.
Together, our findings suggest that disruption and resetting of circadian rhythm by NMU and chemopreventive MSC, respectively, are linked to the mechanisms of mammary tumor promotion (Fig. 6). Disruption of circadian rhythm by shift work, exposure to light at night, and blindness has also been associated with increased rates of breast, prostate, and colon cancers (18). Our findings thus have potential implications for mitigating the increased risk of breast and prostate cancers associated with shift work (47). Numerous studies have suggested that normal dietary levels of MSC can reduce the risk of several cancers without excess tissue accumulation or toxicity (19). MSC is a naturally occurring amino acid synthesized in plant grown in selenium containing soil. The highest levels are found in Brazil nuts (up to 200 μg each), garlic, wild leeks, onions, and broccoli. Lower levels are found in some meat and seafood. Our chemoprevention studies in rats indicated that dietary MSC at 3ppm selenium for up to 8 months yielded no apparent toxicity (23). This level of dietary supplementation with MSC corresponds to a daily dose of ~200-300 μg in human, which is well below recommended maximal daily intake level of selenium (400 μg/ day) established by Institute of Medicine (48). Significant toxicity in human is typically seen only after prolonged intake exceeding 800 μg per day (49). The average dietary intake of selenium in Americans and Europeans is ~100 μg daily, with many populations around the world routinely ingesting 400 to 600 μg of selenium daily without apparent adverse effects (50). Multiple clinical trials with 200 and 400 μg per day of inorganic selenium for months to years have shown no toxicity (51, 52). Another study reported no obvious toxicity with doses from 1600 to 3200 μg per day given for 12 months (53). We therefore expect that a dietary supplement of ~200 μg MSC /day, a level currently found in many multivitamin preparations, would be effective in resetting circadian rhythm in humans. This prediction is currently being tested in shift workers in preparation for a prospective intervention trial to evaluate the efficacy of MSC in mitigating the increased risk of cancer associated with the disruption of circadian rhythm by shift work.
Figure 6. Hypothetical model for the interaction of NMU and MSC with circadian control network during the early stages of carcinogenesis.
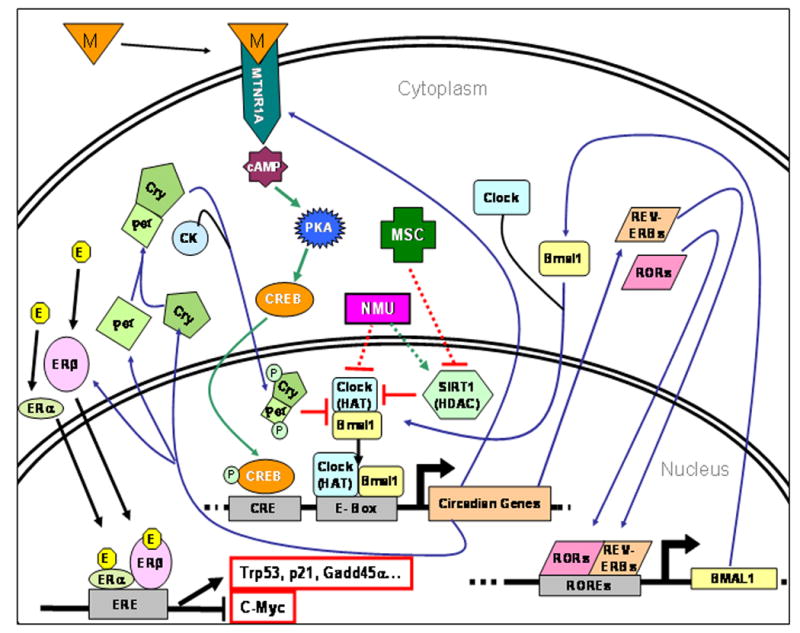
The proposed model combines the changes in gene expression observed in the present study with known regulating network (blue arrows), comprising and modulating the activity of the molecular oscillator in peripheral mammalian cells (5). The rhythmic expression of the melatonin receptor (MTNR1A), itself a circadian-controlled gene that plays a crucial role in the entrainment and synchronization of peripheral circadian rhythm with central pacemaker, was abolished by NMU and reset by MSC without altering the expression of Bmal1 and Clock. These data are consistent with the possibility that NMU and MSC regulate rhythmic expressions of circadian and circadian-controlled genes by modifying the activity of Clock: Bmal1. Altered Bmal1: Clock activity would also modulate the expression of ERβ and other growth regulatory genes. Hypothetical positive or negative inputs from NMU and MSC are indicated by dotted red or green lines, respectively. E, estrogen; ERE, estrogen-response element; M, melatonin; CK, casein kinase; PKA, Protein kinase A; CREB, cAMP-response element binding protein; HAT, histone acetyltransferase; HDAC, histone deacetylase.
Supplementary Material
Acknowledgments
We thank Dr. Michael Gallo for helpful discussion and Ms. Jennifer Williams for critical reading of the manuscript.
This work was supported by NIH Grants: U19ES011387, P30ES005022, P30ES007033, and funds from the Environmental and Occupation Health Sciences Institute, a joint institute of UMDNJ and Rutgers, the State University of New Jersey.
Footnotes
Abbreviations: Bmal1, brain and muscle arnt-like protein 1; Npas2, neuronal PAS domain protein 2; Per, period; Cry, cryptochromes; CCGs, circadian-controlled genes; SCN, suprachiasmatic nucleus; MSC, methylselenocysteine; NMU, N-nitroso-N-methylurea; ER, estrogen receptor; MTNR1A, melatonin receptor 1α.
References
- 1.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–61. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 2.Chen-Goodspeed M, Lee CC. Tumor suppression and circadian function. J Biol Rhythms. 2007;22:291–8. doi: 10.1177/0748730407303387. [DOI] [PubMed] [Google Scholar]
- 3.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 5.Mackey SR. Biological rhythms workshop IA: Molecular Basis of Rhythms Generation. In: Stillman B, S D, Grodzicker T, editors. Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 7–19. [DOI] [PubMed] [Google Scholar]
- 6.Balsalobre A. Clock genes in mammalian peripheral tissues. Cell Tissue Res. 2002;309:193–9. doi: 10.1007/s00441-002-0585-0. [DOI] [PubMed] [Google Scholar]
- 7.Shim HS, Kim H, Lee J, et al. Rapid activation of CLOCK by Ca2+-dependent protein kinase C mediates resetting of the mammalian circadian clock. EMBO Rep. 2007;8:366–71. doi: 10.1038/sj.embor.7400920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Leersnyder H, Bresson JL, de Blois MC, et al. Beta 1-adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2003;40:74–8. doi: 10.1136/jmg.40.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton T. Influence of environmental light and melatonin upon mammary tumour induction. Br J Surg. 1969;56:764–6. doi: 10.1002/bjs.1800561018. [DOI] [PubMed] [Google Scholar]
- 11.Aubert C, Janiaud P, Lecalvez J. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J Neural Transm. 1980;47:121–30. doi: 10.1007/BF01670163. [DOI] [PubMed] [Google Scholar]
- 12.Gery S, Gombart AF, Yi WS, Koeffler C, Hofmann WK, Koeffler HP. Transcription profiling of C/EBP targets identifies Per2 as a gene implicated in myeloid leukemia. Blood. 2005;106:2827–36. doi: 10.1182/blood-2005-01-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua H, Wang Y, Wan C, et al. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97:589–96. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canaple L, Kakizawa T, Laudet V. The days and nights of cancer cells. Cancer Res. 2003;63:7545–52. [PubMed] [Google Scholar]
- 15.Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–6. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 16.Yeh KT, Yang MY, Liu TC, et al. Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol. 2005;206:111–20. doi: 10.1002/path.1756. [DOI] [PubMed] [Google Scholar]
- 17.Yang MY, Chang JG, Lin PM, et al. Downregulation of circadian clock genes in chronic myeloid leukemia: alternative methylation pattern of hPER3. Cancer Sci. 2006;97:1298–307. doi: 10.1111/j.1349-7006.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254–8. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- 19.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–63. [PubMed] [Google Scholar]
- 20.El-Bayoumy K, Sinha R. Mechanisms of mammary cancer chemoprevention by organoselenium compounds. Mutat Res. 2004;551:181–97. doi: 10.1016/j.mrfmmm.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–26. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 22.Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: a new compound for chemoprevention of breast cancer. Nutrition and Cancer. 2001;40:12–7. doi: 10.1207/S15327914NC401_5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Zarbl H. Chemopreventive Doses of Methylselenocysteine Alter Circadian Rhythm in Rat Mammary Tissue. Cancer Prevention Research. 2008;1:119–27. doi: 10.1158/1940-6207.CAPR-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang MZ, Chen D, Sun Y, Jin Z, Christman JK, Yang CS. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41. doi: 10.1158/1078-0432.CCR-05-0406. [DOI] [PubMed] [Google Scholar]
- 25.Fang MZ, Jin Z, Wang Y, et al. Promoter hypermethylation and inactivation of O(6)-methylguanine-DNA methyltransferase in esophageal squamous cell carcinomas and its reactivation in cell lines. International Journal of Oncology. 2005;26:615–22. [PubMed] [Google Scholar]
- 26.Iurisci I, Filipski E, Reinhardt J, et al. Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res. 2006;66:10720–8. doi: 10.1158/0008-5472.CAN-06-2086. [DOI] [PubMed] [Google Scholar]
- 27.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–53. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Stehle JH, von Gall C, Korf HW. Melatonin: a clock-output, a clock-input. J Neuroendocrinol. 2003;15:383–9. doi: 10.1046/j.1365-2826.2003.01001.x. [DOI] [PubMed] [Google Scholar]
- 29.Cai W, Rambaud J, Teboul M, et al. Expression levels of estrogen receptor beta are modulated by components of the molecular clock. Mol Cell Biol. 2008;28:784–93. doi: 10.1128/MCB.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cos S, Gonzalez A, Martinez-Campa C, Mediavilla MD, Alonso-Gonzalez C, Sanchez-Barcelo EJ. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect Prev. 2006;30:118–28. doi: 10.1016/j.cdp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–20. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- 32.Horard B, Rayet B, Triqueneaux G, Laudet V, Delaunay F, Vanacker JM. Expression of the orphan nuclear receptor ERRalpha is under circadian regulation in estrogen-responsive tissues. J Mol Endocrinol. 2004;33:87–97. doi: 10.1677/jme.0.0330087. [DOI] [PubMed] [Google Scholar]
- 33.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 34.Jilg A, Moek J, Weaver DR, Korf HW, Stehle JH, von Gall C. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci. 2005;22:2845–54. doi: 10.1111/j.1460-9568.2005.04485.x. [DOI] [PubMed] [Google Scholar]
- 35.von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci. 2005;1040:508–11. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- 36.Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006;41:297–305. doi: 10.1111/j.1600-079X.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–40. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–4. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 39.Jin Z, Houle B, Mikheev AM, Cha RS, Zarbl H. Alterations in H-ras1 promoter conformation during N-nitroso-N-methylurea-induced mammary carcinogenesis and pregnancy. Cancer Res. 1996;56:4927–35. [PubMed] [Google Scholar]
- 40.Zarbl H. Toxicogenomic analyses of genetic susceptibility to mammary gland carcinogenesis in rodents: implications for human breast cancer. Breast Disease. 2007;28:87–105. doi: 10.3233/bd-2007-28109. [DOI] [PubMed] [Google Scholar]
- 41.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–81. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukamoto R, Mikami T, Miki K, et al. N-methyl-N-nitrosourea-induced mammary carcinogenesis is promoted by short-term treatment with estrogen and progesterone mimicking pregnancy in aged female Lewis rats. Oncology Reports. 2007;18:337–42. [PubMed] [Google Scholar]
- 43.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–32. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 44.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Molecular Interventions. 2003;3:281–92. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 45.Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van der Horst GT. Phase resetting of the mammalian circadian clock by DNA damage. Curr Biol. 2008;18:286–91. doi: 10.1016/j.cub.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 46.Kondratov RV, Antoch MP. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol. 2007;17:311–7. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–7. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. Journal of the American Dietetic Association. 2000;100:637–40. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 49.Yang G, Zhou R. Further observations on the human maximum safe dietary selenium intake in a seleniferous area of China. Journal of Trace Elements and Electrolytes in Health and Disease. 1994;8:159–65. [PubMed] [Google Scholar]
- 50.Combs GF., Jr Impact of selenium and cancer-prevention findings on the nutrition-health paradigm. Nutrition and Cancer. 2001;40:6–11. doi: 10.1207/S15327914NC401_4. [DOI] [PubMed] [Google Scholar]
- 51.Orzano AJ, Strickland PO, Tallia AF, et al. Improving outcomes for high-risk diabetics using information systems. J Am Board Fam Med. 2007;20:245–51. doi: 10.3122/jabfm.2007.03.060185. [DOI] [PubMed] [Google Scholar]
- 52.Tsavachidou D, McDonnell TJ, Wen S, et al. Selenium and vitamin E: cell type- and intervention-specific tissue effects in prostate cancer. Journal of the National Cancer Institute. 2009;101:306–20. doi: 10.1093/jnci/djn512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orzano AJ, Scott J, Hudson SV, et al. Strategies for conducting complex clinical trials in diverse community practices. Medical Care. 2007;45:1221–6. doi: 10.1097/MLR.0b013e31814847a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


