Abstract
Chronic stress is associated with more rapid tumor progression, and recent evidence suggests that stress may contribute to social and ethnic disparities in the incidence and mortality of breast cancer. We evaluated the p53+/- FVB/N mouse as a model to investigate effects of chronic social stress on mammary gland development, gene expression and tumorigenesis. We individually housed (IH) wild type and p53+/- female FVB/N mice, starting at weaning. At 14 weeks of age, both wild type and p53+/- IH mice showed strikingly reduced mammary development compared to group-housed (GH) controls, with IH mice having significantly fewer pre-terminal end buds. This morphological difference was not reflected in levels of mammary transcripts for estrogen receptor alpha or progestin receptor. However, IH increased levels of mRNA for the kisspeptin receptor in the medial preoptic area of the hypothalamus, associated with reduced duration of estrous cycles. Further, IH altered mammary transcripts of genes associated with DNA methylation; transcripts for methyl binding protein 2 and DNA methyltransferase (DNMT) 3b, but not DNMT 1 and DNMT3a, were reduced in IH, compared to GH females. Interestingly, glands of p53+/- females displayed reduced expression of all these mediators, compared to wildtype females. However, contrary to our initial hypothesis, IH did not increase mammary tumorigenesis. Rather, p53+/- GH females developed significantly more mammary tumors than IH mice. Together, these data suggest that social isolation initiated at puberty may confound studies of tumorigenesis by altering mammary development in mouse models.
Keywords: breast cancer, mouse models, p53, social environment, epigenome, stress, isolation
Introduction
Breast cancer is a complex disease in which multiple endocrine, paracrine and intracellular systems interact in both the development and the progression of tumors. Because these systems are sensitive to social experience, particularly social stress, it has been suggested that one component of the observed social and ethnic disparities in breast cancer incidence and mortality might be higher levels of social stress in vulnerable populations. Several epidemiologic studies have associated life stress with breast cancer incidence, although a 2003 meta-analysis showed that only the death of a spouse significantly increases risk (1). In contrast, progression of breast cancer has been more definitively linked with stress (for reviews, 2, 3). Depression has been correlated with reduced cell-mediated immunity, and higher risk for metastasis (4). Biomarkers that are sensitive to social stress were predictive of breast cancer recurrence in a large, multi-ethnic cohort of women (5). African American women have a higher incidence of early-onset breast cancer and are more likely to die from the disease (6). African American women also report more stressful life events, higher levels of perceived stress and lower social support than Caucasian women (7). A retrospective analysis of data from the Black Women's Health Study documented a positive correlation between experience of racial bias and risk for breast cancer in this population (8). While it seems likely that stressful experiences may play a role in the increased risk and mortality of African American women, the underlying mechanisms are poorly understood. Many factors impede the study of social stress and physiology in human populations, including long lifespans, inaccuracy of self-reporting, ethical considerations, and high levels of genetic variation.
Animal models circumvent many of these complications, and provide more tractable systems for studying the interplay of social factors and breast cancer. Studies in rats have shown a relationship between social isolation and mammary tumors (9, 10), and social isolation of murine recipients of ovarian tumor xenografts increases tumor progression (11). Genetically defined mouse models play critical roles in investigation of tumorigenesis (12), as well as studies of behavior and stress (13). They allow us to interrogate the net outcome of complex interactions in response to specific perturbations, including not only physiologic stimuli, but also environmental factors, such as social experiences. This capacity to investigate the impact of experience on physiology is especially important in the context of addressing health disparities, where it is likely that interactions between genes and environment contribute to differences in disease incidence and progression (14). Mice, like humans, are exquisitely sensitive to changes in their environment, and therefore are excellent models for investigating underlying mechanisms of these interactions.
In this study, we evaluated a mouse model to study the effect of social stress on mammary development and tumorigenesis. To model social stress, we chose individual housing (IH), a well-validated paradigm for modeling the effects of chronic stress in female mice (15). In order to examine interactions between chronic stress and tumorigenesis, we compared wildtype FVB/N females to those heterozygous for the p53 gene, modeling the Li Fraumeni syndrome in women (16, 17). Mutations of this gene predispose to tumorigenesis in multiple organs, including the breast. p53 is commonly mutated in “triple negative” breast tumors, the aggressive tumor subtype that predominates in young African-American women. We examined the interplay between social isolation and p53 heterozygosity on mammary gland morphology and gene expression in young-adult mice, and followed a subset of animals to tumor development or natural death. Our results suggest important interactions between chronic isolation, and mammary development, tumorigenesis and gene expression in this model.
Materials and Methods
Genotyping and maintaining mice
p53 heterozygous (p53+/-) mice (18) were crossed into the FVB/N background for more than 10 generations, and were maintained in the FVB/N genetic background. Experimental p53+/- females were generated by crossing p53+/- and wild type (WT)-FVB/N animals from our existing colony. Tail biopsies were collected at weaning and offspring were screened for the p53 mutation, as described previously (19) (primer sequences in Table 1). Mice were housed and handled in accordance with the Guide for Care and Use of Laboratory Animals in facilities accredited by the Association for the Assessment and Accreditation for Laboratory Animal Care. All procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee.
Table 1.
Primer Sequences
| Target | Accession or Genbank # | Forward | Reverse |
|---|---|---|---|
| ATP citrate lyase | NC_000077 | AGC GAT CCG AAG AGT TGG | GTT CTT TGC CGG TCT GCT |
| Cytokeratin 8 | NM_031170 | TGA ACA ACA AGT TCG CCT CCT T | TCC ACT TGG TCT CCA GCA TCT |
| DNMT1 | NM_010066 | ACG GAA ACC CAA GGA AGA GT | TTC CGG TCT TGC TTC TCT GT |
| DNMT3a | NM_007872 | ACT TGA CAG GTG ACC CAA GG | CTT TCC CAG TCT GCT CAA GG |
| DNMT3b | NM_010068 | ACT TGG TGA TTG GTG GAA GC | CCA GAA GAA TGG ACG GTT GT |
| ERα | NM_007956.4 | GCC AGA ATG GCC GAG AGA | TCA TTG CAC ACG GCA CAG T |
| Kiss1r | NM_053244 | CAG TCC CAG GAC ACA ATC CT | ACC AAT GAG TTT CCG ACC AG |
| Mecp2 | NM_001081979 | AAC AGA GAG GAG CCT GTG GA | ACT TCT GGC CCT GGT TAG GT |
| PR | NM_008829 | GGA CAC TGG CTG TGG AAT TT | ATG GCA CAC CAC AAG ATT CA |
| p53 | AF367373 | (m) CTA TCA GGA CAT AGC GTT GG (wt) ACA GCG TGG TGG TAC CTT AT |
TAT ACT CAG AGC CGG CCT |
Social isolation and behavioral testing
At 21 days, female mice were weaned and either housed singly or in groups of 3-4. Mice remained in their housing condition until sacrifice or natural death. A subset of the group- and singly-housed females were sacrificed at 14 weeks of age to examine effects of social isolation and p53 heterozygosity on physiology prior to the onset of disease. On that morning, these mice were given a brief social behavior test consisting of a 5 minute interaction with a novel wildtype, group-housed female mouse in a clean cage in a different room from the colony. This interaction was videotaped and the following behaviors scored offline by an observer blind to the test condition of the mice: latency to first contact with the novel mouse (with contact defined as placement of the focal animal's nose on the novel animal), number of contacts, total time spent in contact, number of rears (with rearing defined as the animal rising onto its hind legs), total time spent rearing, and number of digs (with digging defined as the animal rearranging bedding material with its front paws). Individually housed mice were alternated with group housed mice. Following the 5 minute test, each animal was returned to its home cage and the home cage returned to the colony room.
Collection of tissue – 14 week cohort
Two to three hours after the completion of the social behavior test, animals were killed with CO2 gas. The brain and one caudal mammary gland were rapidly dissected, frozen and stored at -80° C. Trunk blood was collected at the same time, centrifuged and the resulting serum stored at -20° C. The contralateral chain of glands was dissected, mounted on a glass slide and fixed in 10% neutral buffered formalin overnight and stored in 70% ethanol until processed. This “whole mounted” tissue was stained with carmine alum, dehydrated with graded ethanol, cleared of fat with xylenes, and stored in glycerol until analysis. Glands were viewed through a dissecting microscope, and numbers of visible hyperplastic lesions counted.
Serum corticosterone assay
Plasma levels of corticosterone were measured using an enzyme immunoassay (Cayman, USA) according to manufacturer's instructions.
Cytology
To assess effects of housing condition on estrous cycling, we examined vaginal cytology daily from both group-housed (n = 7) and individually-housed (n = 6) FVB/N WT mice for 21 consecutive days, following established protocols (20).
Collection of tissue – end stage cohort
The remainder of the group- and singly-housed mice remained in their housing condition until they reached end stage, defined as a tumor of 1.5 cm or deteriorating health status. All animals underwent necropsy, and all mammary glands were examined for tumors. One chain of mammary glands, contralateral to a tumor (if present), was whole-mounted, and processed as above for analysis of epithelial morphology. Invasive mammary carcinomas were confirmed by a board certified pathologist. In addition, the opposite cranial gland was evaluated histologically for microscopic lesions.
Assessment of mammary gland morphology
Digital photos were taken of whole-mounted caudal mammary glands of 14 week old females using a digital camera mounted on a dissecting microscope. An observer blind to experimental treatment identified a 7mm × 3mm rectangle extending caudally from the lymph node, along the gland margin, and counted the number of terminal end-buds (defined as club-shaped structures at the end of a duct or branch), pre-terminal end-buds (defined as outgrowths not at the ends of ducts or branches), and established ductal branching points within this rectangle.
Tissue processing
Brains were defrosted to -10° C, and sectioned at 300μm on a cryostat. Micropunches were taken from the appropriate sections for the medial preoptic area (MPOA) at -0.30 mm from Bregma (21), rapidly re-frozen and stored at-80°C (22). RNA was extracted from brain and mammary gland samples using the AllPrep DNA/RNA Mini Kit from Qiagen (Qiagen, USA). Concentrations of RNA in each sample were measured using the Qubit quantitation platform (Invitrogen, USA).
Quantitative RT-PCR
cDNA was generated, and quantitative RT-PCR (qRT-PCR) was carried out essentially as described (23), using 18S RNA as an internal control. qRT-PCR results were calculated using the delta delta CT method. Estrogen receptor alpha (ERα) primers were synthesized by Integrated DNA Technologies (San Diego, CA). All other primers were synthesized by Invitrogen (Carlsbad, CA). Primer accession numbers and sequences are listed in Table 1.
Statistical Analyses
Behavioral tests and mammary morphological measurements were analyzed using two-way ANOVAs with genotype and housing condition as factors. Serum corticosterone and vaginal cytology were analyzed using a one-way ANOVA or unpaired student's t-test, unless otherwise noted. These values were analyzed using two-way ANOVAs, unless otherwise noted. Where data was square root transformed to achieve equal variance and/or normal distribution, this is indicated in the figure legend. The Holm-Sidak test was used for all post hoc analyses. Survival was analyzed using the Gehan-Breslow test and curves drawn using the Kaplan-Meier method. Mammary tumor and lesion rates were analyzed using the Fisher Exact test. All statistical analyses were performed using SigmaStat software (Systat, Inc.).
Results
Behavioral effects of isolation
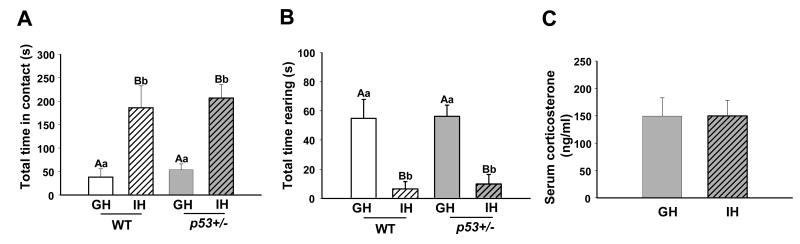
Individual housing (IH) has been demonstrated to elevate indices of stress and anxiety in female mice (15), including reducing exploratory behavior. After 14 weeks of IH, both wildtype (WT) and p53+/- female mice in our study displayed significantly different behavioral responses from group-housed (GH) mice, indicating altered social function and a reduction in exploratory behavior. In a social interaction test, IH mice reared less often (p < 0.001) and for less time (p < 0.001), made more contacts with a novel intruder (p = 0.01) and spent more time in contact with the intruder (p < 0.001) than GH mice (Figure 1A,B). These findings replicate those of other studies (15, 24, 25), and demonstrate that the IH treatment altered behavioral systems associated with the stress response. Serum, collected from p53+/- GH and IH females between 1400 and 1600 hours during the animals' light cycle, did not contain different levels of corticosterone (p = 0.868) (Figure 1C). This lack of altered corticosterone may be strain-dependent; the FVB/N strain has been shown to have lower levels of corticosterone in response to chronic stress (26).
Fig. 1.
Group- and individually-housed mice respond differently to the presence of a novel conspecific, but show no difference in serum corticosterone. Mice were placed in a clean cage and then a novel, female mouse was introduced to the cage for 5 minutes. A) IH mice of both genotypes spent significantly more time in contact with the novel mouse, B) GH mice spent significantly more time rearing up on their hind legs, a behavior associated with investigation of the environment. n = 5 for each group in the novel conspecific test. C) Serum corticosterone (Cort) collected 2-3 hours after the novel conspecific test showed no difference between IH and GH animals. Cort from group- and individually-housed p53+/- animals was compared using a standard EIA kit. GH n = 8, IH n = 8. WT = wild type, p53+/- = p53 heterozygote, GH = group-housed, IH = individually housed. Different uppercase letters denote overall statistical differences in housing or genotype (p≤0.05). Different lowercase letters denote statistical differences found in post-hoc comparisons between experimental groups (p≤0.05). Bars show means ± SEM.
Mammary gland morphology and individual housing
At puberty, activation of epithelial ERα initiates elongation of the ductal rudiment throughout the mammary fat pad (27). The ductal tree develops increasing complexity throughout subsequent estrous cycles in nonparous females, which may be accompanied by alveolar budding in some mouse strains, including FVB/N (28). In order to examine effects of housing conditions on mammary structures, we examined whole mounted mammary glands of both WT and p53+/- young adult females at 14 weeks of age. Even at this age, we found that IH mice of both genotypes displayed marked differences from GH animals. Although mammary glands of females in both housing conditions were similar in gross weight, and exhibited completely elongated ducts and comparable numbers of remaining terminal end buds (data not shown), glands of IH females had significantly fewer pre-terminal end buds than GH females (p = 0.041) (Figure 2). Examination of mammary glands of end stage IH and GH animals indicated that this hypo-development persisted throughout adulthood in most IH females (data not shown). In order to determine if mammary epithelial cells were substantially altered by p53 status or housing, we quantitated mammary transcripts for cytokeratin 8, which is expressed by luminal, but not myoepithelial cells. As shown in Figure 2F, levels of this transcript were not significantly different among groups. However, group-housed p53+/- glands displayed a trend (p=0.071) toward lower levels than the others.
Fig. 2.
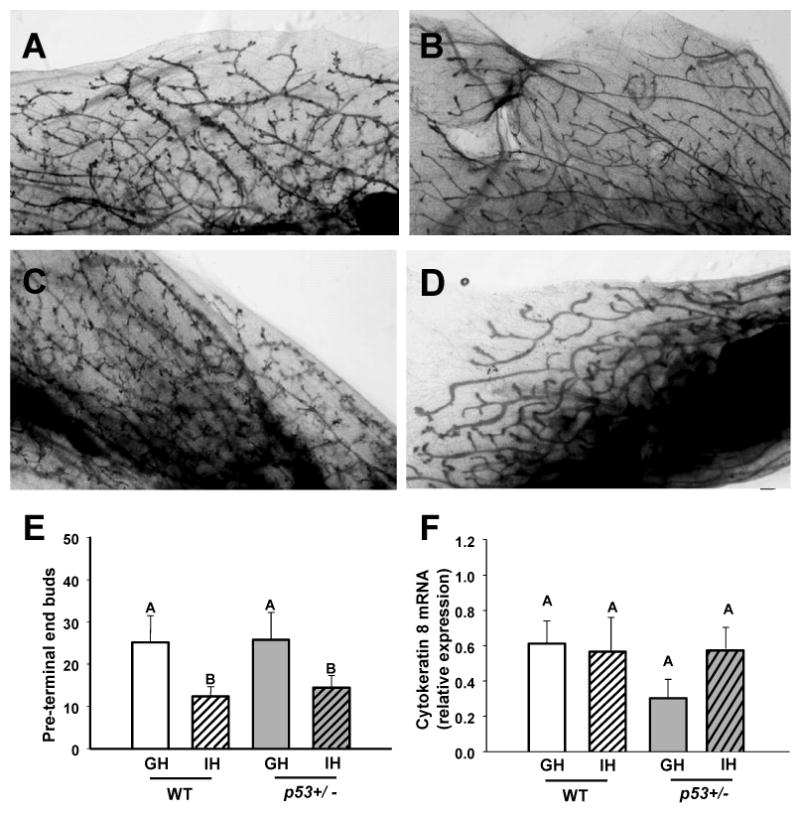
Individually housed mice develop fewer mammary pre-terminal end buds. Photomicrographs of carmine-stained whole-mounted caudal mammary glands of A) WT group-housed, B) WT individually-housed, C) p53+/- group-housed and, D) p53+/- individually-housed female mice at 14 weeks of age. E) Housing significantly affected the number of pre-terminal end buds (defined as all buds not located at the tip of a duct). WT GH n = 6, IH n = 5; p53+/- GH n = 10, IH n = 10. F) Housing does not alter transcripts for cytokeratin 8. WT GH n = 5, IH n = 5; p53+/- GH n = 8, IH n = 9. WT = wild type, p53+/- = p53 heterozygote, GH = group-housed, IH = individually-housed. Different uppercase letters denote overall statistical differences in housing or genotype (p≤0.05). Bars show means ± SEM.
Estrous cycling and individual housing
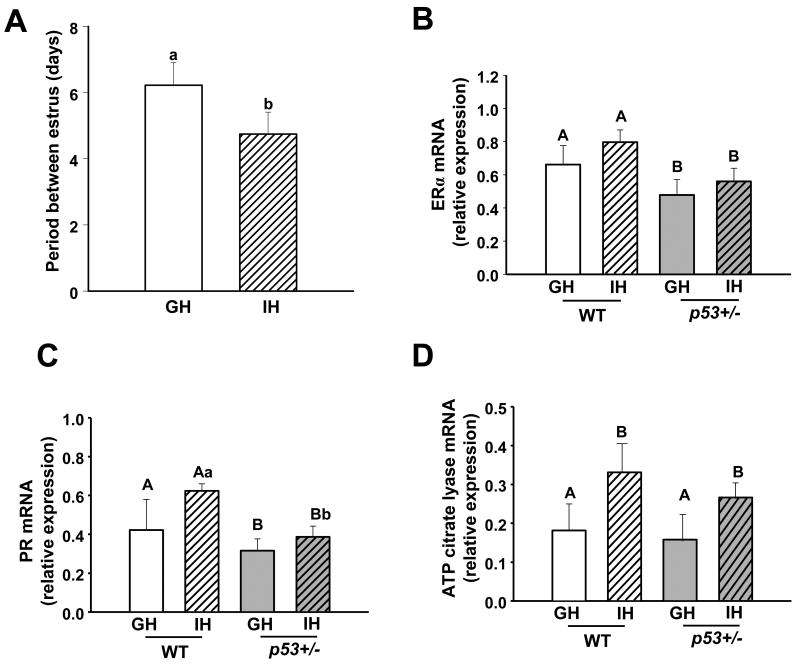
Reduced preterminal budding is associated with reduced progesterone action (29), and suggests altered estrous cycles. Therefore, we examined the effects of housing on estrous cycling, by daily monitoring of vaginal smears. Periodicity was somewhat irregular in both GH and IH mice, with no significant differences in number of days in estrus (p = 0.271), number of days in diestrus (p = 1.0, Mann Whitney rank sum), or number of days in proestrus (p = 0.428). However, isolated mice had a significantly shorter average number of days between the onset of estrous cytology than did GH mice (p = 0.002) (Figure 3A). Given the effects of IH on gland morphology and cycling, we compared levels of mammary transcripts for estrogen receptor alpha (ERα) and progesterone receptor (PR) among the 4 groups. Housing did not significantly affect ERα or total PR mRNA (Figure 3B,C) nor PRb transcripts (not shown). However, p53 heterozygosity significantly reduced transcript levels for both receptors. In order to determine if social isolation altered expression of genes governing metabolic processes, as recently reported in female mice similarly isolated (25), we quantitated mRNA for ATP citrate lyase (Acyl). As shown in Figure 4C, transcripts for this gene were elevated in individually housed females of both genotypes (p=0.028).
Fig. 3.
Individual housing alters estrous cycling; p53 heterozygosity reduces expression of ERα, but not PR in mammary glands of 14 week old female mice. A) Isolation decreased days between the onset of estrus. Vaginal cytology from group- and individually-housed wild type mice was evaluated daily over a 21 day period. While cycling was variable in both groups, IH mice had fewer days between the onset of consecutive appearances of estrous smears. GH n = 7, I n = 6. B) Absence of one p53 allele reduces mammary ERα transcripts. WT GH n = 5, I n = 5; p53+/- GH n = 10, IH n = 10. C) Absence of one p53 allele reduces total mammary PR transcripts. Data square-root transformed to achieve normal distribution. WT GH n = 5, IH n = 5; p53+/- GH n = 9, IH n = 10. D) Individual housing raises mammary transcripts for ATP citrate lyase. WT GH n = 5, IH n = 5; p53+/- GH n = 8, IH n = 9. WT = wild type, p53+/- = p53 heterozygote, GH = group-housed, IH = individually-housed. Different uppercase letters denote overall statistical differences in housing or genotype (p≤0.05). Different lowercase letters denote statistical differences found in post-hoc comparisons between experimental groups (p≤0.05). Bars show means ± SEM.
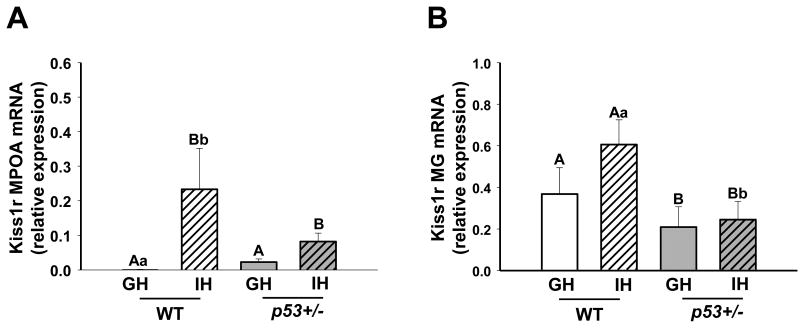
Fig. 4.
Kiss1r transcript levels in the brain and mammary gland of 14 week old females are modulated by housing and genotype. A) Kiss1r transcript levels in the medial preoptic area (MPOA) of the hypothalamus. Levels were higher in individually- compared to group-housed animals. WT GH n = 2, IH n = 5, p53+/- GH n = 8, IH n = 6. B) Kiss1r transcript levels in the mammary glands (MG). p53+/- status also drove lower expression of Kiss1r, with WT IH animals showing higher levels of expression than p53+/- IH animals. WT G n = 5, I n = 5, p53+/- G n = 9, I n = 10. WT = wild type, p53+/- = p53 heterozygote, GH = group-housed, IH = individually-housed. Different uppercase letters denote overall statistical differences in housing or genotype (p≤0.05). Different lowercase letters denote statistical differences found in post-hoc comparisons between experimental groups (p≤0.05). Bars show means ± SEM.
p53 status, individual housing and kisspeptin receptor expression
Kisspeptin and its cognate receptor, Kiss1r, have been shown to modulate the hypothalamic-pituitary-gonadal axis (30, 31), and mediate some effects of stress on secretion of LH and FSH (32). We therefore wondered if the Kisspeptin system might be altered in response to IH. We focused on the receptor because ligand expression in the brain is sensitive to reproductive state (30), and examined kiss1r transcripts in the brains and mammary glands of IH and GH mice of both genotypes (Figure 4). Both housing and p53 status affected levels of kiss1r mRNA in the medial pre-optic area of the hypothalamus (MPOA, Figure 4A). Overall, IH animals showed higher levels of kiss1r mRNA than GH mice (p = 0.043).
In the mammary gland, kiss1r mRNA was strongly reduced in p53+/- females (p = 0.019) (Figure 4B). This effect was focused in the IH groups; mammary kiss1r transcripts were twice as high in WT IH mice, compared to p53+/- IH females (p = 0.03).
Impact of housing and p53+/- status on epigenetic regulators
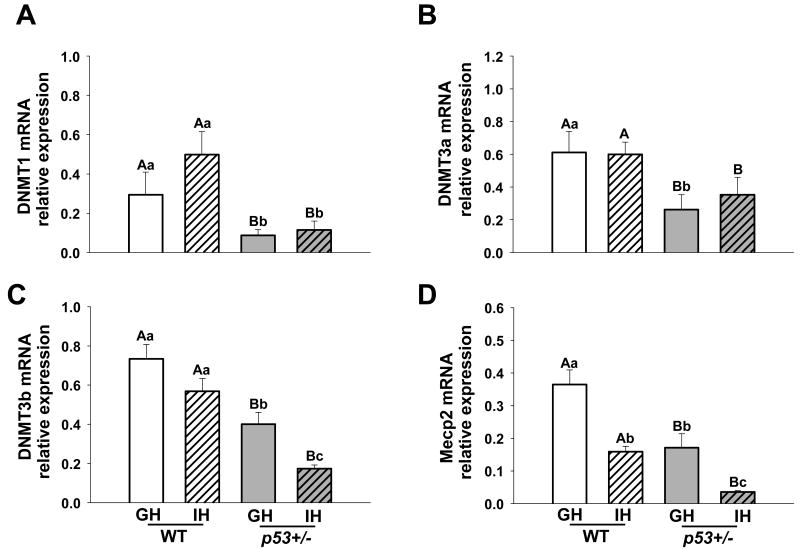
We hypothesized that IH might alter neoplastic processes in our model by altering expression of genes implicated in methylation of DNA. As shown in Figure 5, we found striking effects of housing and/or p53 status on mRNA levels of DNA methyltransferase (DNMT) 1, DNMT3a, DNMT3b and methyl binding protein (Mecp) 2. Mammary glands of IH females, both wildtype and p53+/-, contained significantly reduced Mecp2 transcripts (Figure 5D, p < 0.001). DNMT3b transcripts were similarly altered by housing conditions (Figure 5C, p = 0.002). There was a trend of an effect of IH on DNMT3b mRNA in WT females (p = 0.08), which was highly significant in p53+/- females (p <0.001). In contrast, neither DNMT1 nor DNMT3a transcripts were impacted by housing (Figure 5A,B).
Fig. 5.
Housing and p53 status differentially modulate transcripts for DNA methyltransferase (DNMT) and methyl-binding protein (Mecp2) in mammary glands of 14 week old wild type and p53+/- females. A) Both GH and IH p53+/- mice had lower levels of DNMT1 mRNA, and there was a trend towards an effect of isolation in WT animals, B) loss of a p53+/- allele led to reduced DNMT3a mRNA, C) both isolation and loss of a p53+/- allele diminished expression of DNMT3b mRNA, and D) both isolation and loss of a p53+/- allele diminished expression of MeCP2. WT GH n = 5, WT IH n =5, and p53+/- GH n = 10, and p53+/- IH n = 10.WT = wild type, and p53+/- = p53 heterozygote, GH = group-housed, IH = individually-housed. Different uppercase letters denote overall statistical differences in housing or genotype (p≤0.05). Different lowercase letters denote statistical differences found in post-hoc comparisons between experimental groups (p≤0.05). Bars show means ± SEM.
Notably, p53 heterozygosity reduced transcripts for all of these epigenetic regulators (p<0.001; Figure 5), indicating a strong effect of this genetic modification on this system.
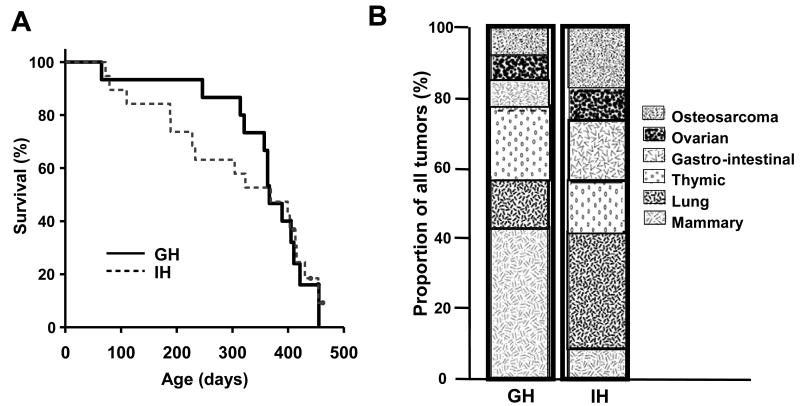
Social isolation, survival and tumorigenesis in p53+/- mice
Initially, the IH mouse cohort appeared to sicken or develop tumors more rapidly than the GH controls (Figure 6A). Indeed, survival of IH at 1 year of age was significantly reduced (P<0.021). The earliest deaths were primarily due to thymic lymphomas in both housing groups. However, this difference disappeared as the study progressed, and there was no significant effect of housing on overall survival (p = 0.96). Likewise, the proportion of females that had developed tumors at end stage was not significantly different for IH and GH females (GH: 93%, n = 15; IH: 82%, n = 16; Fishers exact test respectively). However, housing did significantly impact the incidence of mammary tumors (Figure 6B); IH mice were less likely than GH mice to develop mammary tumors (p = 0.012). Indeed, only a single invasive mammary carcinoma was found in one end stage IH female, compared to 6 single tumors in GH females. Nonetheless, IH p53+/- females did develop some mammary histologic abnormalities, and the quantity was not statistically different from GH glands. Housing did not significantly affect the incidence of any other tumor type.
Fig. 6.
Effects of housing on survival and tumor distribution in p53+/- females. A) While there was an early effect of individual housing on survival, rates of death eventually converged; survival curves of GH and IH groups did not differ (GH, n=15; IH, n=16; p=0.96). B) Although GH and IH p53+/- mice developed tumors with similar frequency, housing significantly affected the distribution of these tumors. IH females were less likely to develop mammary tumors (p = 0.012). GH = group-housed, IH = individually-housed.
Discussion
In this study, we examined the effects of social isolation on development and tumorigenesis of the mammary gland. Using this established model for chronic stress combined with p53 heterozygosity, mimicking the Li Fraumeni syndrome in women, we demonstrated that social experience alters mammary gene expression, development and tumorigenesis. Females housed individually beginning at puberty, regardless of genotype, displayed underdeveloped mammary ductal trees, which correlated with reduced estrous cycle length, significantly elevated kiss1r transcripts in the MPOA of the hypothalamus, and reduced mammary transcripts for the epigenetic regulators, Mecp2 and DNMT3b. Despite morphologic similarity to mammary glands of wildtype female controls, p53 heterozygotes, regardless of housing status, displayed a very different profile of gene expression, characterized by reduced mammary transcripts for ERα, PR, Kiss1r, and all 4 epigenetic regulators. Contrary to our hypothesis, IH reduced the incidence of endstage mammary tumors in p53+/- females. Our findings indicate that social isolation initiated at puberty inhibits mammary development and alters its epigenome, which may confound studies of mammary tumorigenesis.
Both wildtype and p53+/- IH females displayed a significant and unpredicted lack of mammary development. Although ductal elongation was complete, glands of IH young adult females of both genotypes exhibited reduced numbers of pre-terminal end buds. This phenotype suggests altered progesterone responsiveness (29), but we found no significant difference in either ERα or PR mRNA levels in IH animals. However, differences in estrous cycling in IH and GH mice suggest altered function of the hypothalamic-pituitary-gonadal axis, which may lead to these differences in morphology.
p53 is a powerful tumor suppressor, and exerts its actions via multiple pathways, including regulation of the cell cycle and apoptosis (33,34). Genetic defects in one allele result in the Li Fraumeni syndrome in humans (16, 17). Afflicted individuals are at high risk for multiple cancers, including a 55% chance of breast cancer in women by age 45 (35). Here we employed a mouse model heterozygous for a well-studied germline mutation of this gene (18). We backcrossed this mutation, originally developed in 129/SV × C57BL/6 mice, into the FVB/N genetic background, in order to study its behavior in the context of a mouse strain that is extensively used for transgenic models of cancer. This change in strain background increased overall tumor incidence (>90%), and reduced latency, compared to the original report (18). Interestingly the distribution of tumor types in group-housed p53+/- FVB/N females also shifted toward a predominance of mammary tumors, more similar to the human syndrome.
Social isolation, conferred by individual housing of normally social species, is a stress paradigm which is relevant to human disease. Indeed, use of this stressor increases progression of ovarian tumors in mice (11), and two recent studies also linked this stress to progression of mammary tumors in rat (10) and mouse (25) models. In the latter study, Williams and colleagues used a very similar experimental paradigm and the same mouse strain (FVB/N) as we employed in our study. In seeming contrast to our observed reduced incidence of invasive mammary carcinomas in IH p53+/- females, they demonstrated that isolation increased mammary lesions and tumor burden induced by the C3(1)/SV40 T-antigen (Tag) transgene (25).
The C3(1)/SV40 Tag model differs from p53 heterozygotes in several important respects. Transformation induced by SV40-Tag also involves loss of p53 function, but retinoblastoma protein is also inactivated (36). Moreover, C3(1) targets Tag expression to only epithelial cells, in contrast to the germline p53+/- model employed in our study. The consequences of these differences on tumor incidence and progression in the context of social isolation merit additional investigation. However, the impact of IH on mammary development in both wildtype and p53+/- females found in our study re-emphasizes the importance of a long understood consequence of isolation: IH exerts complex effects on estrous cycling (37,38), and consequently steroid hormone action. Particularly when housing treatments are initiated at puberty, this can disrupt normal mammary development, which may impede tumorigenic processes (e.g., 39). Furthermore, mammary epithelial populations and potential tumor precursors expand rapidly at this time (40); manipulations that interfere with these events may also inhibit oncogenesis. In addition, ovarian steroids are potent mitogenic stimuli for hormonally responsive mammary lesions, which include those induced by the absence of p53 in other strain backgrounds (41). By reducing mammary development and ovarian steroid action, individual housing may mask possible effects of stress on tumor incidence from stimuli, such as p53 heterozygosity, which may be relatively weak compared to viral oncogenes. Thus experimental social isolation, particularly in the vulnerable peri-pubertal period, may confer additional complications in efforts to tease out any relationship between psycho-social factors and breast tumor incidence, as opposed to cancer progression. Techniques that reliably induce stress without depriving subjects of pheromonal cues that drive estrous cycling may be more appropriate for studies of mammary cancer.
Contrary to our expectations, IH also did not alter the overall survival of p53+/- females in our study, despite behavioral and physiologic evidence of increased stress. Larger studies examining cell specific loss of p53 alleles may reveal effects of isolation on individual tumor types. However, in the initial months after weaning, IH animals died at a more rapid rate. The convergence of the survival rates with time suggests that some, but not all, mice could “adapt” to the higher stress level. This is consistent with a growing body of literature suggesting that individual temperaments play a role in the experience of stress and risk for disease (42,43).
Kisspeptin was originally identified as an anti-metastatic agent in breast and other cancers (44). This ligand and its cognate receptor, Kiss1r, also play critical roles in regulating maturation and function of the HPG axis in humans, non-human primates and rodents (31, 45). Kisslr transcripts in the hypothalami and MPOA of adults have been shown to be modulated by several stressors (32, 46), supporting the hypothesis that kisspeptin mediates the effect of stress on the estrous cycle by modulation of gonadotropin secretion. The altered cycling and higher kiss1r mRNA levels in the MPOA of IH females in our study, regardless of genotype, suggest that IH may be exerting its effects on mammary development in part via kisspeptin action on the HPG. A similar mechanism may also mediate the delayed mammary development reported in isolated Sprague Dawley rats (10).
Studies exploring the impact of maternal care have demonstrated that social experience in infancy can alter methylation of genes in the brain (47, 48). Similarly, studies in adult animals show that injury to the central nervous system induces changes in the epigenome (49). Here we showed that postpubertal social isolation can also result in lower expression of genes regulating methylation in the mammary gland. The reduced DMNT3b and Mecp2 mRNA that we observed may reflect the relatively underdeveloped glands; DNA methylation and Mecp2 both play a role in some (50), but not all (51), aspects of mammary development. Alternatively, IH may alter this system regardless of mammary maturation. Examination of the effect of IH on fully mature glands would further illuminate this relationship.
Mammary glands of p53+/- females exhibited reduced transcripts for all four epigenetic regulators. This is surprising in light of the association of heterozygosity of p53 with regional hypermethylation of CpG islands, repression of DNMT1 transcription by p53, and corepression of some promoters by p53 and DNMTs (52,53). However, global hypomethylation as well as localized hypermethylation are hallmarks of cancer (54). DNA hypomethylation and reduced DMNT3b, in particular, increase chromosomal instability (54, 55), and global 5-methylcytosine levels are reduced in breast as well as prostatic disease (56,57). Hypomethylation may be particularly important for early neoplastic events (54). Together, these observations indicate a need for more study of the role of epigenetic changes in tumorigenesis secondary to mutations in p53.
Our study demonstrates that social isolation initiated at puberty exerts complex physiological effects, including dysregulation of mammary development and expression of epigenetic modulators. In the context of p53 heterozygosity, this experimental paradigm unexpectedly reduces the incidence of mammary tumors, highlighting the neglected interplay between social experience, mammary development and tumor biology. These studies in the context of the literature underscore the need for further investigation of the impact of social experience on the risk and progression of mammary cancer.
Acknowledgments
The authors are grateful to Debra Rugowski, Dylan Coss and Nneka Akubeze for their assistance with these studies.
Grant support: This work was supported by CDMRP W81XWH-08-1-0504 (L.A.S.), T32 HD049302 (N.S.H.) and R01 MH072956 (A.P.A.).
References
- 1.Duijts SF, Zeegers MP, Borne BV. The association between stressful life events and breast cancer risk: a meta-analysis. Int J Cancer. 2003;107:1023–1029. doi: 10.1002/ijc.11504. [DOI] [PubMed] [Google Scholar]
- 2.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 4.Sephton SE, Dhabhar FS, Keuroghlian AS, Giese-Davis J, McEwen BS, Ionan AC, Spiegel D. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1153. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joslyn SA, Foote ML, Nasseri K, Coughlin SS, Howe HL. Racial and ethnic disparities in breast cancer rates by age: NAACCR Breast Cancer Project. Breast Cancer Res Treat. 2005;92:97–105. doi: 10.1007/s10549-005-2112-y. [DOI] [PubMed] [Google Scholar]
- 7.Paul K, Boutain D, Agnew K, Thomas J, Hitti J. The relationship between racial identity, income, stress and C-reactive protein among parous women: implications for preterm birth disparity research. J Natl Med Assoc. 2008;100:540–546. doi: 10.1016/s0027-9684(15)31300-6. [DOI] [PubMed] [Google Scholar]
- 8.Taylor TR, Williams CD, Makambi KH, Mouton C, Harrell JP, Cozier Y, Palmer JR, Rosenberg L, Adams-Campbell LL. Racial discrimination and breast cancer incidence in US Black women: the Black Women's Health Study. Am J Epidemiol. 2007;166:46–54. doi: 10.1093/aje/kwm056. [DOI] [PubMed] [Google Scholar]
- 9.Hermes GL, McClintock MK. Isolation and the timing of mammary gland development, gonadarche, and ovarian senescence: implications for mammary tumor burden. Dev Psychobiol. 2008;50:353–360. doi: 10.1002/dev.20295. [DOI] [PubMed] [Google Scholar]
- 10.Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc Natl Acad Sci. 2009;106:22393–22398. doi: 10.1073/pnas.0910753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 12.Bennett CN, Green JE. Unlocking the power of cross-species genomic analyses: identification of evolutionarily conserved breast cancer networks and validation of preclinical models. Breast Cancer Res. 2008;10:213. doi: 10.1186/bcr2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Brower V. Cancer disparities: disentangling the effects of race and genetics. J Natl Cancer Inst. 2008;100:1126–1129. doi: 10.1093/jnci/djn302. [DOI] [PubMed] [Google Scholar]
- 15.Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav. 2001;73:411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- 16.Royds JA, Iacopetta B. p53 and disease: when the guardian angel fails. Cell Death Differ. 2006;13:1017–1026. doi: 10.1038/sj.cdd.4401913. [DOI] [PubMed] [Google Scholar]
- 17.Lozano G, Zambetti GP. What have animal models taught us about the p53 pathway? J Pathol. 2005;205:206–220. doi: 10.1002/path.1704. [DOI] [PubMed] [Google Scholar]
- 18.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERα-positive and ERα-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–4674. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Franklin KBJ. The mouse brain in steriotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 22.McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, Rodd ZA. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: a proteomics study. Pharmacol Biochem Behav. 2009;92:304–313. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–178. doi: 10.4161/epi.2.3.4841. [DOI] [PubMed] [Google Scholar]
- 24.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 25.Williams JB, Pang D, Delgado B, Kocherginsky M, Tretiakova M, Krausz T, Pan D, He J, McClintock MK, Conzen SD. A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev Res. 2009;2:850–861. doi: 10.1158/1940-6207.CAPR-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibarguen-Vargas Y, Surget A, Touma C, Palme R, Belzung C. Multifaceted strain-specific effects in a mouse model of depression and of antidepressant reversal. Psychoneuroendocrinology. 2008;33:1357–1368. doi: 10.1016/j.psyneuen.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imagawa W, Bandyopadhyay GK, Nandi S. Regulation of mammary epithelial cell growth in mice and rats. Endocrine Rev. 1990;11:494–523. doi: 10.1210/edrv-11-4-494. [DOI] [PubMed] [Google Scholar]
- 29.Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:205–214. doi: 10.1023/a:1025952924864. [DOI] [PubMed] [Google Scholar]
- 30.Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KG. Kisspeptins: regulators of metastasis and the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2005;17:519–525. doi: 10.1111/j.1365-2826.2005.01328.x. [DOI] [PubMed] [Google Scholar]
- 32.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O'Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21:20–29. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 33.Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 34.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 35.Allain DC. Genetic counseling and testing for common hereditary breast cancer syndromes: a paper from the 2007 William Beaumont hospital symposium on molecular pathology. J Mol Diagn. 2008;10:383–395. doi: 10.2353/jmoldx.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green JE, Shibata MA, Yoshidome K, Lui M, Jorcyk C, Anver MR, Wigginton J, Wiltrout R, Shibata E, Kaczmarczyk S, Wang W, Lui Z, Calvo A, Couldrey C. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial targeting with multistage progression to carcinoma. Oncogene. 2000;19:1020–1027. doi: 10.1038/sj.onc.1203280. [DOI] [PubMed] [Google Scholar]
- 37.Whitten WK. Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J Endocrinol. 1956;13:399–404. doi: 10.1677/joe.0.0130399. [DOI] [PubMed] [Google Scholar]
- 38.Whitten WK. Occurrence of anoestrus in mice caged in groups. J Endocrinol. 1959;18:102–107. doi: 10.1677/joe.0.0180102. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd T, Hassell JA. Role of Ets transcription factors in mammary gland development and oncogenesis. J Mammary Gland Biol Neoplasia. 2001;6:129–140. doi: 10.1023/a:1009576801226. [DOI] [PubMed] [Google Scholar]
- 40.LaMarca HL, Rosen JM. Minireview: hormones and mammary cell fate--what will I become when I grow up? Endocrinology. 2008;149:4317–4321. doi: 10.1210/en.2008-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina D, Kittrell FS, Shepard A, Contreras A, Rosen JM, Lydon J. Hormone dependence in premalignant mammary progression. Cancer Res. 2003;63:1067–1072. [PubMed] [Google Scholar]
- 42.Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun. 2008;22:662–667. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Charbonneau AM, Mezulis AH, Hyde JS. Stress and emotional reactivity as explanations for gender differences in adolescents' depressive symptoms. J Youth Adolesc. 2009;38:1050–1058. doi: 10.1007/s10964-009-9398-8. [DOI] [PubMed] [Google Scholar]
- 44.Harms JF, Welch DR, Miele ME. KISS1 metastasis suppression and emergent pathways. Clin Exp Metastasis. 2003;20:11–18. doi: 10.1023/a:1022530100931. [DOI] [PubMed] [Google Scholar]
- 45.Tena-Sempere M. Kisspeptin signaling in the brain: Recent developments and future challenges. Mol Cell Endocrinol. 2010;314:164–169. doi: 10.1016/j.mce.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O'Byrne KT. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocrinol. 2009;21:683–689. doi: 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 48.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 49.Westberry JM, Prewitt AK, Wilson ME. Epigenetic regulation of the estrogen receptor alpha promoter in the cerebral cortex following ischemia in male and female rats. Neuroscience. 2008;152:982–989. doi: 10.1016/j.neuroscience.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–132. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Tsellou E, Michailidi C, Pafiti A, Troungos C. DNA methylation-independent regulation of p16 in epithelial cells during mouse mammary gland development. Epigenetics. 2008;3:143–148. doi: 10.4161/epi.3.3.6371. [DOI] [PubMed] [Google Scholar]
- 52.Taylor SM. p53 and deregulation of DNA methylation in cancer. Cell Science Reviews. 2006;2 [Google Scholar]
- 53.Sansom OJ, Maddison K, Clarke AR. Mechanisms of disease: methyl-binding domain proteins as potential therapeutic targets in cancer. Nat Clin Pract Oncol. 2007;4:305–315. doi: 10.1038/ncponc0812. [DOI] [PubMed] [Google Scholar]
- 54.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta. 2007;1775:138–162. doi: 10.1016/j.bbcan.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Dodge JE, Okano M, Dick F, Tsujimoto N, Chen T, Wang S, Ueda Y, Dyson N, Li E. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization. J Biol Chem. 2005;280:17986–17991. doi: 10.1074/jbc.M413246200. [DOI] [PubMed] [Google Scholar]
- 56.Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- 57.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–831. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]