Abstract
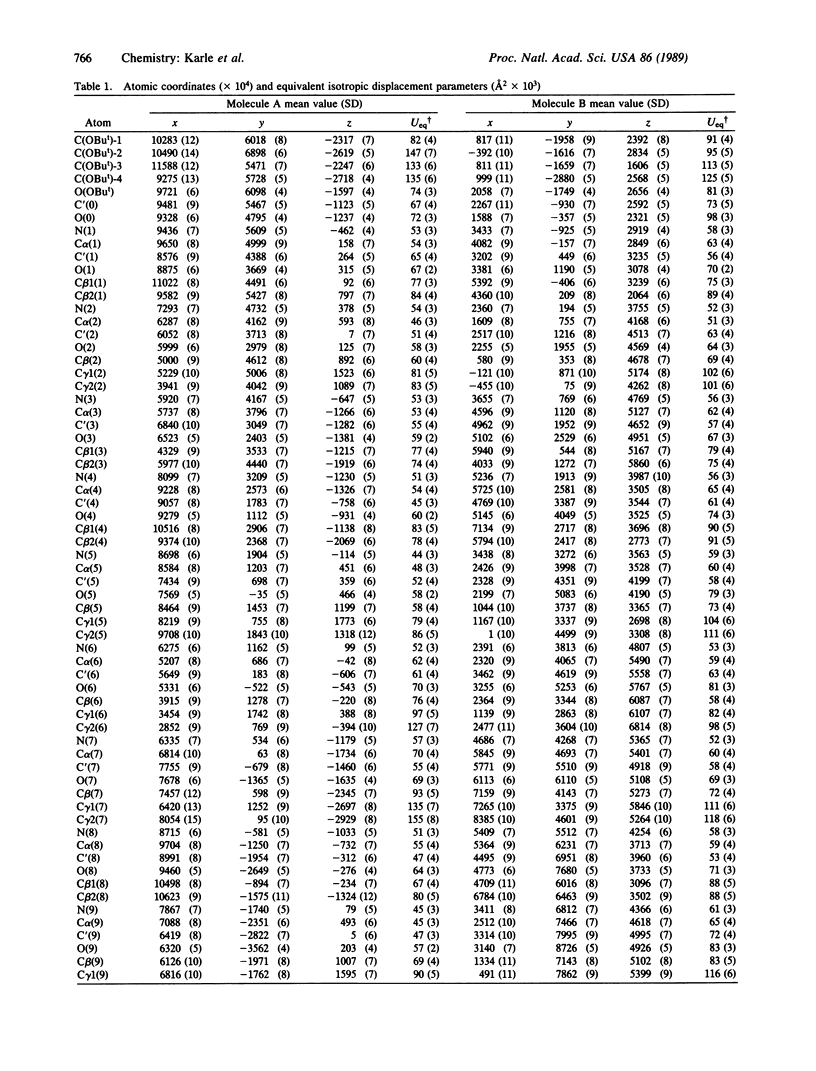
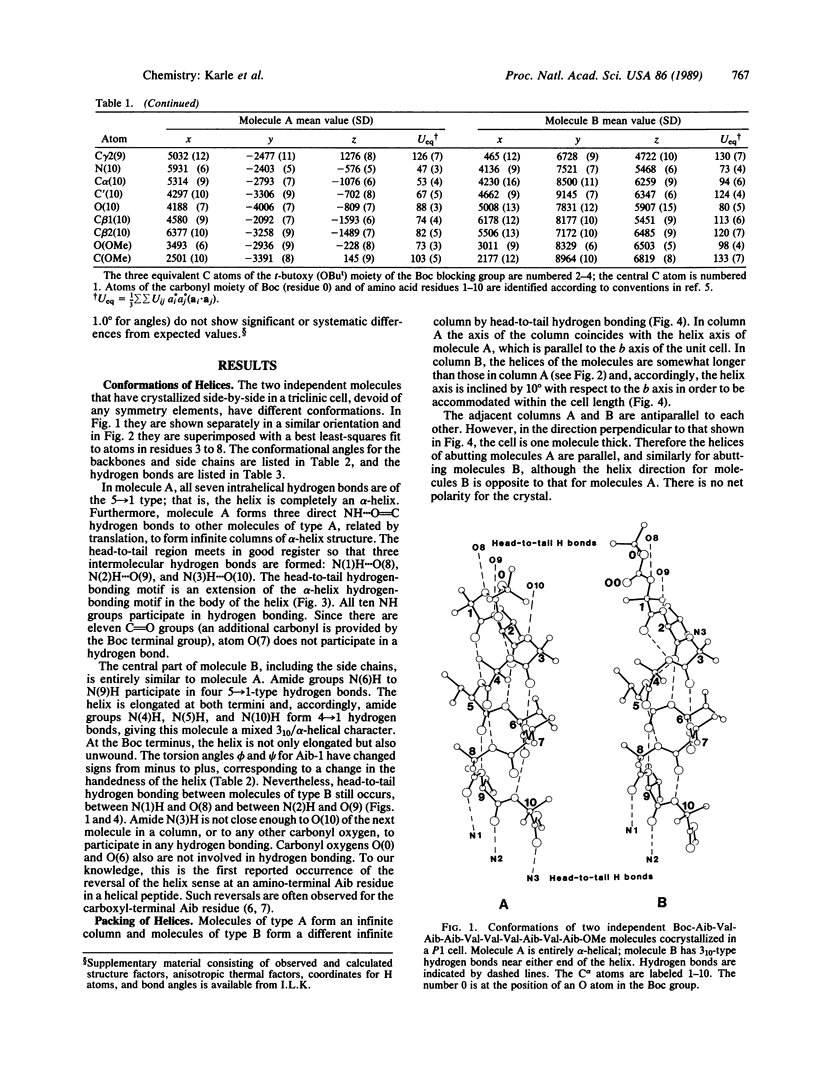
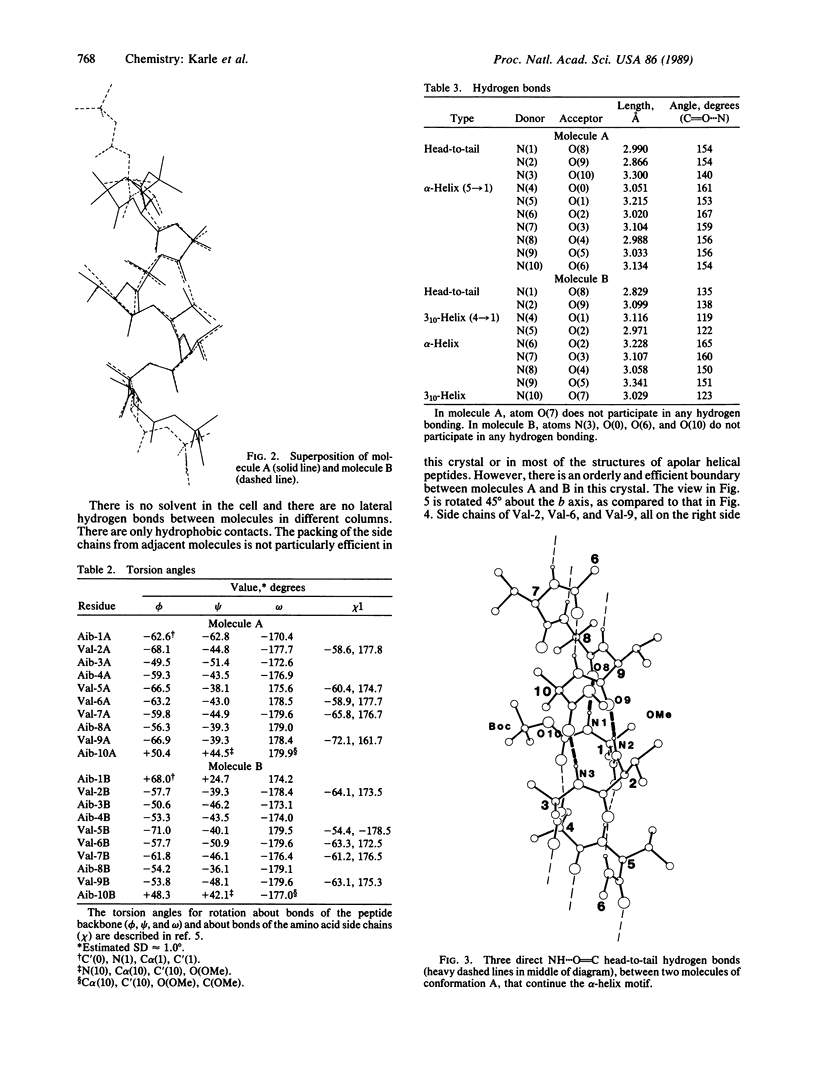
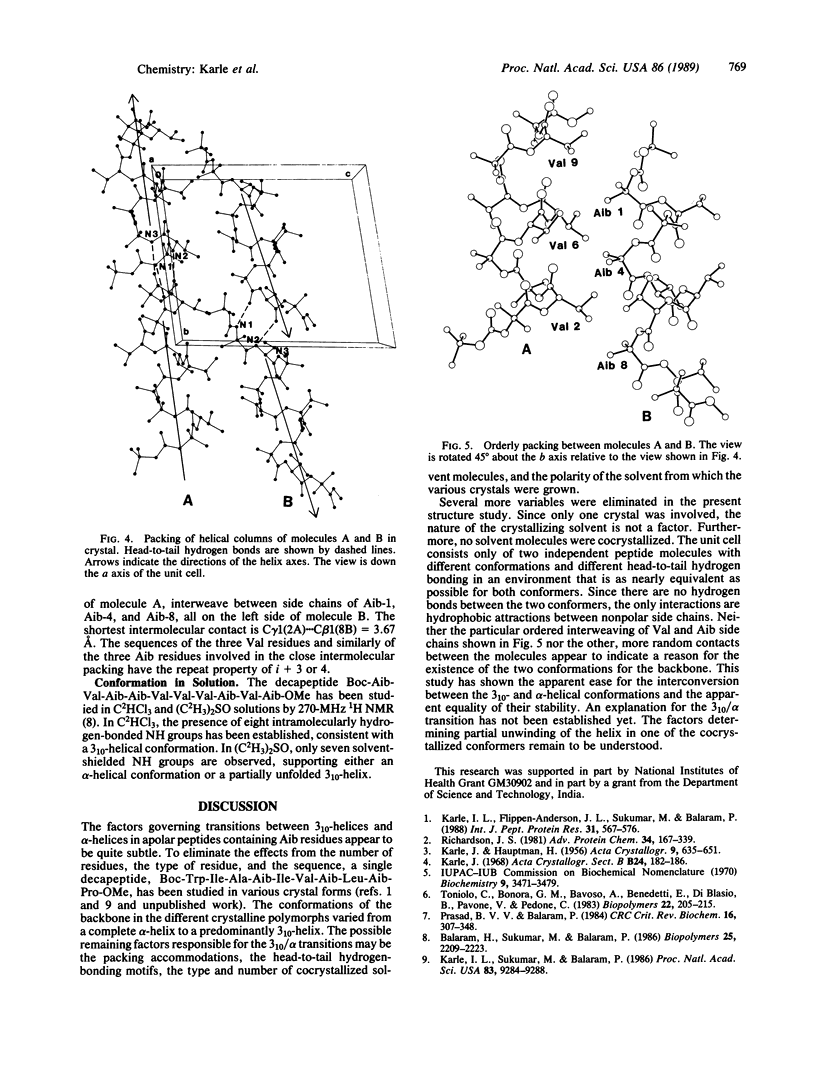
Two molecules of Boc-Aib-Val-Aib-Aib-Val-Val-Val-Aib-Val-Aib-OMe (where Boc is t-butoxycarbonyl and Aib is alpha-aminoisobutyryl) cocrystallize in a triclinic cell with different helical conformations. One molecule is completely alpha-helical with seven 5----1 intramolecular hydrogen bonds. It forms three head-to-tail NH...O = C hydrogen bonds to other molecules of the same conformation. The second molecule has a mixed 3(10)/alpha-helix conformation with three 4----1 hydrogen bonds and four 5----1 hydrogen bonds; furthermore, there is a helix reversal at both termini. The second molecule forms only two head-to-tail hydrogen bonds with molecules of the same type, and the N(3)H group does not participate in any hydrogen bonding. The two different types of helices occur in alternate sheets in the crystal, where each sheet is composed of adjacent rods of helices formed by head-to-tail hydrogen bonding. Within each sheet, containing helices of only one type of conformation, the helices aggregate in a parallel mode. Between the sheets of different helices, the aggregation is antiparallel. The peptide, with formula C51H92N10O13, crystallizes in space group P1 with Z = 2 and cell parameters a = 10.047 +/- 0.002 A, b = 16.684 +/- 0.003 A, c = 19.198 +/- 0.004 A, alpha = 80.30 degrees +/- 0.01 degrees, beta = 85.74 degrees +/- 0.01 degrees, and gamma = 83.03 degrees +/- 0.01 degrees; overall agreement factor R = 6.7% for 6053 data ([F0] greater than 3 sigma) and 0.96-A resolution.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Karle I. L., Flippen-Anderson J. L., Sukumar M., Balaram P. Monoclinic polymorph of Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe(anhydrous). Parallel packing of 3(10)-/alpha-helices and a transition of helix type. Int J Pept Protein Res. 1988 Jun;31(6):567–576. doi: 10.1111/j.1399-3011.1988.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Sukumar M., Balaram P. Parallel packing of alpha-helices in crystals of the zervamicin IIA analog Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe.2H2O. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9284–9288. doi: 10.1073/pnas.83.24.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Balaram P. The stereochemistry of peptides containing alpha-aminoisobutyric acid. CRC Crit Rev Biochem. 1984;16(4):307–348. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]



