Abstract
Polymorphisms associated with prostate cancer include those in three genes encoding major secretory products of the prostate: KLK2 (encoding kallikrein-related peptidase 2; hK2), KLK3 (encoding prostate-specific antigen; PSA), and MSMB (encoding beta-microseminoprotein). PSA and hK2, members of the kallikrein family, are elevated in serum of men with prostate cancer. In a comprehensive analysis which included sequencing of all coding, flanking, and 2kb of putative promoter regions of all 15 kallikrein (KLK) genes spanning ≈280 Kb on chromosome 19q, we identified novel SNPs and genotyped 104 SNPs in 1419 cancer cases and 736 controls in CAPS1, with independent replication in 1267 cases and 901 controls in CAPS2. This verified prior associations of SNPs in KLK2 and in MSMB (but not in KLK3) with prostate cancer. Twelve SNPs in KLK2 and KLK3 were associated with levels of PSA forms or hK2 in plasma of control subjects. Based on our comprehensive approach, this is likely to represent all common KLK variants associated with these phenotypes. A T allele at rs198977 in KLK2 associated with increased cancer risk and a striking decrease of hK2 levels in blood. We also found a strong interaction between rs198977 genotype and hK2 levels in blood in predicting cancer risk. Based on this strong association, we developed a model for predicting prostate cancer risk from standard biomarkers, rs198977 genotype, and rs198977 x hK2 interaction; this model had greater accuracy than did biomarkers alone (AUC 0.874 vs 0.866), providing proof in principle to clinical application for our findings.
Keywords: prostate cancer, prostate-specific antigen, human kallikrein-related peptidase 2, genetic variation, case-control study
Introduction
Prostate cancer is among the most heritable of the common cancers; one estimate of its heritability, from a twin study, was 42% (1). Based on this high heritability, several genome-wide association studies (GWAS) for prostate cancer susceptibility loci have been performed, and numerous polymorphisms have been associated with prostate cancer (2–7). The implicated genes include KLK3 (encoding prostate-specific antigen; PSA) (3, 8–10) and MSMB (encoding beta-microseminoprotein; MSP or PSP94) (3, 7). In addition, SNPs in KLK2, a gene that encodes kallikrein-related peptidase 2 (hK2) and a homolog of KLK3, have also been reported to be associated with prostate cancer (11).
All three genes (KLK2, KLK3, and MSMB) code for highly abundant secretory products of the prostate. PSA and hK2 are both kallikreins, a family of serine proteases that are all encoded in a gene cluster on chromosome 19q. PSA is a commonly used biomarker for detection of prostate cancer and monitoring after treatment. There is debate as to whether SNPs in KLK3 identified by GWAS are true prostate cancer susceptibility alleles, or whether these SNPs were detected due to association with PSA levels and because an elevated PSA increases the likelihood of diagnosis of asymptomatic prostate cancer (12, 13). hK2 is also used as a biomarker for prostate cancer. While SNPs in KLK2 have been associated with prostate cancer risk (11), this association has not been consistently replicated (14). Also, interpretation of prior associations between SNPs in KLK2 and hK2 levels in blood is complicated by both lack of replication and that all study subjects had elevated PSA and/or abnormal findings on digital rectal examination in these studies. Taken together, prior data suggest that the KLK locus may influence prostate cancer risk (15). We therefore undertook a detailed analysis of association across the entire kallikrein locus. We performed comprehensive resequencing of all KLK-genes, genotyped known and newly discovered kallikrein SNPs, as well as an MSMB SNP, in a large prostate cancer case/control cohort from Sweden. By examining the relationship between SNP genotype, serum levels of PSA and hK2, and disease status, we found that several SNPs at this locus are associated with PSA and hK2 levels in the blood and that an interaction between one SNP in KLK2 and hK2 levels in plasma is associated with prostate cancer.
MATERIAL AND METHODS
SNP discovery
Ninety-four men referred for prostate biopsy at the University Hospital, Malmö, Sweden and 47 male patients at the University Hospital with no signs of prostate cancer were resequenced for the KLK2 and KLK3 genes. A subset of the men referred for prostate biopsy was sequenced for the other genes (Supplementary Table 2). Genomic DNA was isolated from whole blood, PCR-amplified, and sequenced using Big Dye Terminator chemistry (Applied Biosystems 3730). For each gene, all coding regions, flanking intronic sequences, and 2kb of the putative promoter were sequenced. The generally bidirectional sequence data were assembled and compared using SeqScape v2.5 (Applied Biosystems) with manual confirmation of candidate heterozygotes followed by independent genotyping to confirm all detected polymorphisms. This sequencing strategy is expected to detect 95% of SNPs with an allele frequency ≥ 1% given a sample size of 48 individuals (16).
Case-control study population
Subjects were recruited for Cancer Prostate in Sweden (CAPS), a population-based case-control study (17, 18), in two stages (Supplementary Table 1). Due to the setup of Swedish regional oncology centers, the individual cohorts of patients contributed by them are genuinely population-based. Patients aged 35–65 were enrolled in the southern regions, compared to ages 35–79 in the northern regions. Concurrent with recruitment of case subjects, controls subjects were randomly selected from the Swedish Population Registry, matched by geographic region and the expected age distribution of cases (within 5-years). Clinical tumor characteristics and treatment information were collected from the National Prostate Cancer Register (19).
Immunodetection of biomarkers
Levels of hK2, free and total PSA measured in EDTA-anti-coagulated blood plasma from cases and controls were performed in Dr. Lilja’s laboratory at Dept Laboratory Medicine, Lund University, University Hospital UMAS during 2005–2006. For most cases, blood samples were collected after initiation of treatment for prostate cancer; hence, these plasma levels generally reflect treatment effects. Free and total PSA were simultaneously measured with a dual-label assay (DELFIA Prostatus PSA F/T, PerkinElmer Life Sciences, Turku, Finland) (20). Total hK2 was measured according to a three-step in-house assay (21, 22).
Genotyping
All SNPs discovered in our resequencing and selected KLK locus SNPs identified in dbSNP (www.ncbi.nlm.nih.gov/SNP) were genotyped on parent-child trios to check data quality, confirm Mendelian segregation, and check for segregation patterns consistent with deletions. For the current study, DNA suitable for genotyping was available from 2686 cases and 1637 controls in CAPS1+2. Genotypes were determined using the MassARRAY MALDI-TOF system (Sequenom), using the Homogenous MassEXTEND protocol. We initially assessed 137 probes corresponding to 112 SNPs on the CAPS1 samples, using the Sequenom system. 124 probes corresponding to 104 SNPs passed quality control. Where one SNP had multiple probes, only the probe with the fewest missing observations was used for statistical analysis. Based on results from CAPS1, 36 probes corresponding to 34 SNPs were chosen for replication in CAPS2. After quality control, 33 probes remained, each corresponding to a unique SNP. Technical sample replicates showed greater than 99.9% genotype concordance and multiple probes for the same SNP showed over 99% concordance. The final sets of SNPs for CAPS1 and CAPS2 had on average 1.9% and 2.8% missing observations (maximum: 6.7% and 7.8%).
For confirming the genotype of the SNP marker rs198977, we used a predesigned TaqMan assay (C_736084; Applied Biosystems). The genotypes were called automatically using an ABI 7900HT SDS and verified manually. Agreement between Sequenom and TaqMan assays for non-missing measurements was 99.93% across CAPS1 and CAPS2.
Statistical analysis
Case-control study
Statistical analyses were generally performed using R version 2.3.1 (http://www.r-project.org). Association with case/control status was performed using Pearson’s χ2 or Fisher’s exact test on a 3×2 contingency table. Stratified unconditional logistic regression was used to test for association between genotype and disease status controlling for age and geographic area; recessive, dominant, codominant, and additive models were tested. Haploview version 3.3.2 with default parameters was used for evaluation of multimarker haplotypes and for permutation testing. For tests of Hardy-Weinberg equilibrium, a χ2 test with one degree of freedom was used to assess deviation of observed from expected allele frequencies.
Quantitative variables
Kallikrein levels in plasma were analyzed for association with SNP genotypes in controls. Free PSA, tPSA, and hK2 levels were normalized by taking the log. Association was tested using linear regression with an additive model (23). 10,000 permutations of the quantitative variables were used to assess the cutoff for an empirical p<0.05 across all quantitative association tests. Conditional haplotype-based testing was performed for all pairs of significant SNPs at the KLK locus. For each pair of SNPs, independent association was tested for each conditional on the other using whap, version 2.09 (24).
Interaction analysis
To test for interaction between hK2 levels and rs198977, we removed all case individuals for whom hK2 was measured in a blood sample obtained after any type of treatment and individuals with missing rs198977 or hK2 data, resulting in 189 CAPS1 cases, 143 CAPS2 cases, and 1600 controls. Using logistic regression, we tested for association of any T allele at rs198977 with prostate cancer in a univariate manner both solely and after controlling for hK2 and also in interaction with hK2 levels. To prevent bias due to an age difference between treated and untreated cases, sensitivity analyses were conducted adjusting for age, which confirmed that our results were not affected by the exclusion of treated cases (data not shown).
Predictive models
Log-transformed values for all plasma biomarkers were used to model their non-linear association with outcome. These analyses included those participants with complete data for all biomarkers, rs198977, rs10993994, and rs2271094. The models were constructed using 178 cases and 661 controls from CAPS1, and independently validated by calculating the area under the receiver operating characteristics curve (AUC) among 136 cases and 819 controls from CAPS2.
RESULTS
SNP Discovery
Sequencing in all 15 kallikrein genes identified 140 polymorphisms (Supplementary Tables 2–3), including 38 novel SNPs. We designed and successfully validated genotyping assays for 13 novel SNPs and 89 previously described SNPs in this region. These assays capture most of the common variation in the entire region (r2>0.5 for over half of HapMap tag SNPs).
SNP association analysis
The 102 SNPs were genotyped in 1419 prostate cancer cases (Supplementary Table 1) and 736 controls in the CAPS1 study (17). We did not find any significant association between SNP and prostate cancer risk under a variety of genetic models after correcting for the number of SNPs tested. Therefore no novel cancer susceptibility associations were revealed.
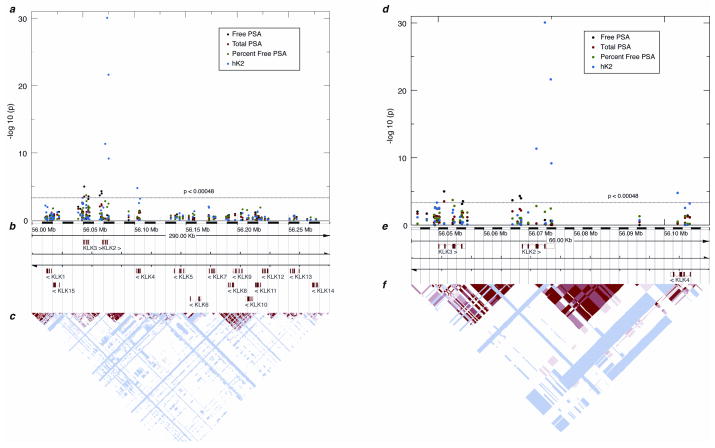
Previous studies have noted associations between KLK locus SNPs and PSA or hK2 levels which could confound the clinical use of PSA or hK2 measurements. Therefore, we next asked if any of the 102 SNPs were associated with plasma levels of hK2 and PSA (free PSA [fPSA], total PSA [tPSA], and the ratio of free to total PSA [%fPSA]). We restricted this analysis to controls to avoid any bias from measuring post-treatment PSA and hK2 levels. We identified 13 associations with p<0.00048, a threshold determined through permutation testing (Table 1). One associated SNP was in KLK4; all others were in the KLK2 KLK3 region (Fig. 1; Supplementary Table 4). Levels of hK2 were clearly associated with several SNPs in the KLK2 region. The most striking association involved rs198977 (P<0.0001; Fig. 1a; Table 1). Several other SNPs were associated with fPSA, tPSA, or %fPSA (Table 1); most SNPs were in or adjacent to a haplotype block that includes KLK3 and the KLK3-KLK2 intergenic region (Fig. 2). On retesting in CAPS2, almost all significant associations were replicated with p<0.05 (Table 1).
Table 1.
Significant associations between plasma kallikrein values and SNPs in the kallikrein locus in the CAPS study. Trait values (ng/ml) are given for CAPS1+CAPS2 when available. Otherwise, trait values are for CAPS1.
| Mean trait value in ng/ml (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | SNP | Location | Position on chr 19 (build 36) | CAPS1 p* | CAPS2 p† | CAPS1 + CAPS2 p | Common homozygote | Heterozygote | Rare homozygote |
| Free PSA (ng/mL) | rs2271094 | KLK3 exon 2 | 56,051,309 | <0.0001 | 0.002 | <0.0001 | 0.62 (0.081–2.1) | 0.69 (0.086–2.5) | 0.98 (0.094–3.6) |
| rs11084039 | KLK2 promoter | 56,068,031 | <0.0001 | ND | ND | 0.075 (0.069–2.9) | 0.075 (0.10–2.7) | 0.96 (0.13–3.5) | |
| rs11670728 | KLK2 promoter | 56,068,301 | 0.0001 | 0.024 | <0.0001 | 0.66 (0.084–2.3) | 0.67 (0.085–2.5) | 1.0 (0.092–3.9) | |
| rs3760728 | KLK2 promoter | 56,066,404 | 0.0002 | 0.17 | <0.0001 | 0.66 (0.083–2.3) | 0.68 (0.085–2.5) | 1.0 (0.094–3.8) | |
| rs6998 | KLK3 exon 5 | 56,055,473 | 0.0003 | 0.4 | <0.0001 | 0.66 (0.083–2.3) | 0.74 (0.084–2.6) | 0.87 (0.094–3.5) | |
| Total PSA (ng/mL) | rs2271094 | KLK3 exon 2 | 56,051,309 | 0.0003 | 0.031 | <0.0001 | 2.6 (0.20–10) | 2.8 (0.21–12) | 4.00 (2.4–16) |
| %Free PSA | rs61752561 | KLK3 exon 3 | 56,053,194 | 0.0002 | 0.0001 | <0.0001 | 32 (8.1–56) | 36 (9.5–63) | 48 (27–69) |
| hK2 (ng/mL) | rs198977 | KLK2 exon 5 | 56,073,589 | <0.0001 | <0.0001 | <0.0001 | 0.069 (0.013–0.21) | 0.050 (0.0086–0.14) | 0.032 (0.0016–0.071) |
| rs198978 | KLK2 exon 5 | 56,074,884 | <0.0001 | <0.0001 | <0.0001 | 0.068 (0.014–0.20) | 0.062 (0.0091–0.18) | 0.036 (0.0023–0.11) | |
| rs198972 | KLK2 exon 3 | 56,071,705 | <0.0001 | <0.0001 | <0.0001 | 0.065 (0.012–0.20) | 0.060 (0.0078–0.19) | 0.049 (0.0024–0.14) | |
| SNP11 | KLK2 exon 5 | 56,075,012 | <0.0001 | <0.0001 | <0.0001 | 0.062 (0.0096–0.20) | 0.060 (0.0038–0.19) | 0.014 (0.0043–0.033) | |
| rs1654553 | KLK4 intron 4 | 56,102,928 | <0.0001 | <0.0001 | <0.0001 | 0.065 (0.012–0.20) | 0.062 (0.0086–0.21) | 0.057 (0.0052–0.19) | |
| rs17526278 | KLK3 promoter | 56,049,699 | 0.0005 | ND | ND | 0.068 (0.0097–0.22) | 0.056 (0.0047–0.22) | 0.038 (0.0050–0.15) | |
ND, no data
p<0.00048 is considered significant based on permutation testing; p<0.00012 is considered significant based on the Bonferroni correction.
p<0.05 is considered significant.
Figure 1.
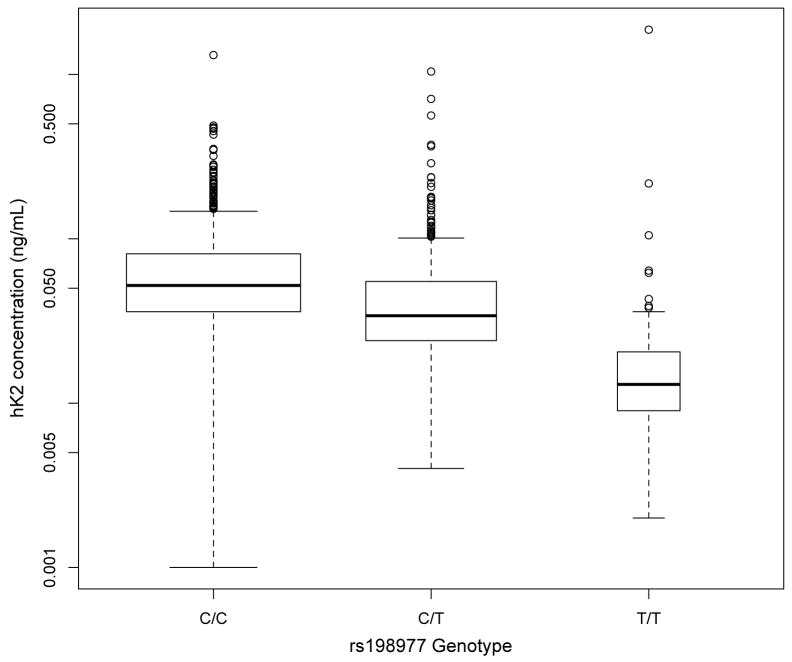
a) Association of rs198977 with hK2 levels. Box-plots show hK2 levels in controls from both CAPS1 and CAPS2 stratified by rs198977 genotype. Each box covers the middle two quartiles, with the bar representing the median. Plot whiskers extend out to 1.5 times the interquartile range from the 25th and 75th percentiles. Widths of boxes are proportional to the square root of the number of observations.
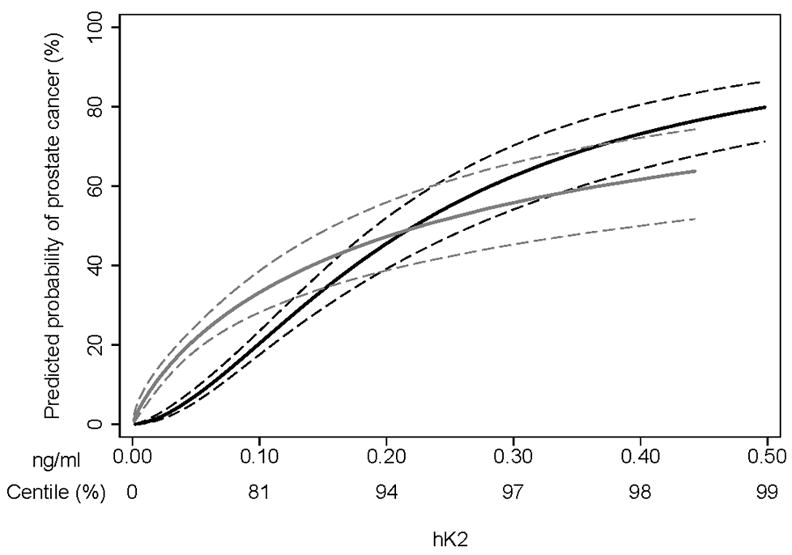
b) Interaction plot. Association between hK2 level in plasma and prostate cancer risk in CAPS is shown for men with C/C genotype at rs198977 (black curve) and for men with at least one T allele (gray curve). Dashed lines show 95% confidence intervals.
Figure 2.
Association of polymorphisms at the kallikrein locus with PSA forms and hK2 in plasma. A) Plot showing strength of association between SNPs and levels of free and total PSA, %fPSA, and hK2. A schematic of the kallikrein locus is below the plot, showing position and orientation of all 15 genes. B) Linkage disequilibrium plot from the CEU population in HapMap. Red indicates high LD as measured by D′. C, D) Similar plots as in A and B, but restricted to the KLK2 KLK3 region.
Associations with previously reported prostate cancer susceptibility alleles
We next considered three SNPs previously associated with prostate cancer susceptibility: rs198977 in KLK2 (11), which was one of the 102 SNPs described above, rs2735839 in the KLK2-KLK3 intergenic region (3, 12, 13), and rs10993994 in MSMB (3, 7). MSMB, like KLK2 and KLK3, encodes a protein for which serum levels are associated prostate cancer (25, 26). The three SNPs were genotyped in the combined CAPS (17, 18). rs2735839 was not associated with case status (P=0.82). Both rs198977 and rs10993994, however, were significantly associated (rs198977 P=0.029, OR=1.08, 95% CI =0.97–1.19; rs10993994 P=0.0020, OR=1.17, 95% CI=1.07–1.28), thus replicating the previous findings. Though rs198977 showed deviation from Hardy-Weinberg equilibrium in controls (P=0.00013), genotype calls were confirmed with a second genotyping method, ruling out genotyping error. To enhance the weak association of rs198977 with prostate cancer, we conducted a meta-analysis of this SNP using 4 cohorts (Nam et al (11), CAPS1, CAPS2, and CGEMS (27)). Using Fisher’s exact test, rs198977 was significantly associated with prostate cancer risk (P=0.011), even when the Nam et al. replication cohort was excluded (P=0.039). Therefore, rs198977 appears to be weakly associated with prostate cancer susceptibility.
We next examined the three SNPs for association with plasma hK2, fPSA, tPSA, and %fPSA in CAPS controls. Both rs2735839 in the KLK2-KLK3 region and rs10993994 in MSMB were previously associated with tPSA levels among controls (3, 12). The association of rs2735839 with tPSA was not replicated in our study, though this SNP was associated with %fPSA (Table 3). We did, however, replicate the association of the MSMB SNP rs10993994 with tPSA, and also found stronger associations with fPSA and with hK2. As noted above, the T allele of rs198977 in KLK2 was strikingly associated with lower hK2 level, and it was also associated with higher %fPSA.
Table 3.
Associations of three selected SNPs (rs198977, rs2735839, and rs10993994) with prostate cancer risk and plasma kallikrein values in the CAPS study.
| Measure | CAPS1 p | CAPS2 p | Combined CAPS p | CAPS1 p | CAPS2 p | Combined CAPS p | CAPS1 p | CAPS2 p | Combined CAPS p |
|---|---|---|---|---|---|---|---|---|---|
| rs198977 (KLK2) | rs2735839 (KLK2-KLK3intergenic region) | rs10993994 (MSMB) | |||||||
| Cancer risk | 0.09 | 0.19 | 0.029 | 0.7 | 0.2 | 0.8 | 0.3 | 0.003 | 0.002 |
| Total PSA | 0.7 | 0.3 | 0.5 | 0.07 | 0.5 | 0.08 | 0.09 | 0.055 | 0.006 |
| Free PSA | 0.18 | 0.3 | 0.13 | 0.2 | 0.7 | 0.5 | 0.011 | 0.024 | 0.0006 |
| % Free PSA | 0.011 | <0.0001 | <0.0001 | 0.014 | 0.006 | 0.0002 | 0.18 | 0.86 | 0.5 |
| hK2 | <0.0001 | <0.0001 | <0.0001 | 0.8 | 0.16 | 0.3 | 0.015 | 0.006 | 0.0002 |
Bold type indicates statistically significant results (p≤0.05).
Independence of the discovered associations
To remove redundancies in the SNP/kallikrein associations due to LD between SNPs, we tested for the independence of each SNP association relative to the other SNPs (24) (Supplementary Table 5). Overall, our data suggest that the observed association with fPSA is explained by rs2271094 and that the observed association with hK2 is explained by rs198977. For the three SNPs associated with %fPSA, conflicting results were obtained from CAPS1, CAPS2, and the combined dataset, and therefore we cannot determine their interdependence in influencing %fPSA.
Prostate cancer risk models
We next tested for a statistical interaction in which the association between hK2 level and disease status (case or control) depends on rs198977. We compared three logistic regression models (Table 4). The association between rs198977 and prostate cancer risk was strengthened by adding hK2 level as an independent factor. When the interaction between rs198977 and hK2 was added, rs198977, hK2, and the interaction term were all strongly associated with disease (Figure 1b). Among men with low hK2 levels, those with a T allele at rs198977 had a greatly elevated probability of prostate cancer, whereas among men with higher hK2 levels, those with and without any T allele had little difference in probability of prostate cancer.
Table 4.
Prediction of prostate cancer: univariate and multivariable analyses of rs198977, hK2 level, and the interaction between rs198977 and hK2 level.
| CAPS1 | CAPS2 | CAPS1 + CAPS2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 707 controls, 189 cases | 893 controls, 143 cases | 1600 controls, 332 cases | |||||||
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Univariate | |||||||||
| Any T allele* | 1.48 | 1.07, 2.04 | 0.018 | 1.15 | 0.81, 1.64 | 0.4 | 1.29 | 1.02, 1.64 | 0.034 |
| Controlling for hK2 | |||||||||
| Any T allele* | 3.10 | 2.11, 4.55 | <0.0005 | 2.07 | 1.38, 3.09 | <0.0005 | 2.55 | 1.94, 3.37 | <0.0005 |
| Log hK2 | 3.24 | 2.57, 4.08 | <0.0005 | 3.63 | 2.82, 4.67 | <0.0005 | 3.43 | 2.90, 4.07 | <0.0005 |
| Interaction analysis | |||||||||
| Any T allele* | 0.34 | 0.10, 1.19 | 0.091 | 0.20 | 0.05, 0.82 | 0.025 | 0.27 | 0.11, 0.68 | 0.005 |
| Log hK2 | 4.98 | 3.50, 7.09 | <0.0005 | 6.14 | 4.03, 9.36 | <0.0005 | 5.51 | 4.21, 7.22 | <0.0005 |
| Interaction | 0.43 | 0.27, 0.69 | <0.0005 | 0.41 | 0.24, 0.69 | 0.0008 | 0.42 | 0.30, 0.60 | <0.0005 |
Heterozygous genotypes were encoded as intermediate between C/C and T/T genotypes.
Various prediction models for prostate cancer use tPSA, fPSA, %fPSA, and hK2 in plasma (28). We tested whether such a prediction model could be further improved by incorporating rs198977, rs10993994, rs2271094, and the SNP x kallikrein interactions. We evaluated the predictive accuracy for a base model (tPSA, fPSA, %fPSA, hK2), full model (base plus rs198977, rs198977 x %fPSA, and rs198977 x hK2), and two exploratory models (model 1: full plus rs10993994, rs10993994 x tPSA, rs10993994 x fPSA, and rs10993994 x hK2; model 2: full plus rs2271094, rs2271094 x tPSA, rs2271094 x fPSA). The area under the receiver operating characteristics curve (AUC) was 0.866 for the base model, slightly increasing to 0.874 for the full model. A small enhancement was observed with the rs10993994 exploratory model (AUC 0.877), but not for the rs2271094 exploratory model (AUC 0.872).
DISCUSSION
Here we analyzed common genetic variation in the kallikrein genes in association with kallikrein levels in plasma and risk of developing prostate cancer. To our knowledge, this represents the first comprehensive analysis of this locus, and we believe that it has identified all common variants in the Swedish population associated with prostate cancer risk and kallikrein levels.
This analysis identified a novel association of rs2271094 with free and total PSA levels. Although rs2271094 is not associated with prostate cancer risk, it still may have clinical importance: because it is associated with tPSA, this SNP may influence whether a patient’s early-stage prostate cancer gets detected.
We were unable to replicate previously reported associations of rs2735839 with prostate cancer risk and tPSA (3, 12). However, we identified an association between rs2735839 and %fPSA, suggesting rs2735839 influences kallikrein levels in blood. Though the nearby SNP rs2271094 was associated with tPSA, LD between this SNP and rs2735839 is low (r2=0.08). While we cannot explain the discrepancy with previous association results with tPSA, our denser genotyping and measures of different PSA isoforms provides finer grained information on the relationship of KLK3 SNPs to PSA levels. The reported association of rs2735839 with prostate cancer could be confounded if this SNP affects PSA levels, as previously noted (12).
Specifically, the association with PSA could introduce selection bias in which individuals with the risk allele tend to have higher PSA levels, and therefore are more likely to have asymptomatic prostate cancer diagnosed. Supporting this notion, we found no significant difference in genotype frequencies of rs2735839 in a cohort of cases not ascertained through PSA screening (3) versus a comparable population sample (29) (nominal p=0.2; see Supplementary Table 6).
We replicated previously observed associations of rs10993994, in the MSMB promoter, with prostate cancer risk and with tPSA. We extended these observations by noting that rs10993994 shows even stronger evidence for association with fPSA and hK2 levels in plasma. These data could suggest that MSP, the MSMB gene product, plays a role in the etiology of prostate cancer. Alternatively, the association between rs10993994 and cancer could result from its association with tPSA causing a selection bias. Arguing against this selection bias, the genotype frequency of rs10993994 does significantly differ between prostate cancer cases ascertained without PSA screening and a comparable population sample (p<0.0005 after correcting for multiple tests; see Supplementary Table 6).
Similarly, we replicated observed associations of rs198977 (in KLK2) with prostate cancer risk and hK2 level. While association between rs198977 and hK2 level was previously observed in a mixed group of cases and controls (11, 30), our results are first to show an association among controls, with remarkably strong effect. We also identified an association between rs198977 and %fPSA. These data again suggest a role of hK2 and/or PSA in the etiology of prostate cancer. The potential selection bias described for SNPs affecting tPSA is implausible for rs198977, as neither hK2 nor %fPSA is routinely used to screen for prostate cancer. Moreover, the allele associated with cancer risk is also associated with lower hK2 and higher %fPSA, both of which are associated with lower tPSA (absolute value of Spearman’s rho >0.5 for both markers in cases and controls separately). Therefore, any selection bias from tPSA would make diagnosis of asymptomatic cancer less, not more, likely in men with the risk allele. We also demonstrated an interaction between rs198977 genotype and hK2 levels in predicting prostate cancer risk. This suggests that it is important to consider SNP-biomarker level interactions in genetic association studies.
Our data suggest that measuring a blood marker can help identify cancer-related SNPs. Assessed in isolation, the association between rs198977 and prostate cancer was small (odds ratio of 1.29 for combined cohorts), and significant in only one cohort. In contrast, after controlling for hK2 levels in plasma, the association was much stronger (odds ratio of 2.55) and highly significant in both cohorts (p<0.0005). Our data suggest that the ability of a marker to predict cancer is importantly enhanced if SNP status is known and vice versa (p<0.0005 for the interaction term). This suggests a potential role for incorporating both genotype and marker levels in predictive models. Further studies should examine the degree to which evaluation of genotype at these SNPs increases the predictive accuracy of PSA or other kallikrein measures in blood.
Supplementary Material
Table 2.
Case/control analysis of rs198977 in the KLK2 gene in three studies of prostate cancer. Data for the combined cohort of CAPS1 and CAPS2 is included for informational purposes in the last column; these two cohorts were considered separately in the meta-analysis.
| Nam et al (11) | CGEMS (27) | CAPS1 | CAPS2 | CAPS1+2 | |
|---|---|---|---|---|---|
| Cases, Number (%) | |||||
| C/C | 335 (52) | 618 (53) | 788 (56) | 670 (55) | 1458 (56) |
| C/T | 260 (40) | 472 (40) | 515 (37) | 460 (38) | 975 (37) |
| T/T | 50 (8) | 80 (7) | 94 (7) | 89 (7) | 183 (7) |
| Controls, Number (%) | |||||
| C/C | 356 (59) | 630 (57) | 443 (61) | 483 (57) | 926 (59) |
| C/T | 216 (36) | 404 (37) | 233 (32) | 287 (34) | 520 (33) |
| T/T | 34 (6) | 63 (6) | 48 (7) | 72 (9) | 120 (8) |
| P value (3×2 table) | 0.038 | 0.079 | 0.088 | 0.19 | 0.029 |
| Heterozygote OR (95% CI) | 1.3 (1.0–1.6) | 1.2 (1.0–1.4) | 1.2 (1.0–1.5) | 1.2 (0.96–1.4) | 1.2 (1.0–1.4) |
| Homozygote OR (95% CI) | 1.6 (1.0–2.5) | 1.3 (0.91–1.8) | 1.1 (0.76–1.6) | 0.89 (0.64–1.2) | 1.0 (0.76–1.2) |
| Hardy-Weinberg P value (controls) | 0.9 | 0.9 | 0.025 | 0.002 | 0.0001 |
CI, confidence interval; OR, odds ratio
Acknowledgments
Funding
National Cancer Institute [1R21CA127768-01A1 to H.L., P30 CA008748 to MSKCC]; National Cancer Institute Specialized Programs of Research Excellence [P50-CA92629]; Swedish Cancer Society [08-0345]; Swedish Research Council [Medicine: K2009-54X-20095-04-3]; Sidney Kimmel Center for Prostate and Urologic Cancers, and David H. Koch through the Prostate Cancer Foundation.
We thank Gun-Britt Eriksson and Kerstin Håkansson for expert assistance with immunoassays.
Footnotes
Conflict of Interest Statement: Dr. Hans Lilja holds patents for free PSA and hK2 assays.
References
- 1.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 2.Amundadottir LT, Sulem P, Gudmundsson J, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–8. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 3.Eeles RA, Kote-Jarai Z, Giles GG, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–21. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- 4.Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068–73. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudmundsson J, Sulem P, Rafnar T, et al. Common sequence variants on 2p15 and Xp11.22 confer susceptibility to prostate cancer. Nat Genet. 2008;40:281–3. doi: 10.1038/ng.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudmundsson J, Sulem P, Steinthorsdottir V, et al. Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet. 2007;39:977–83. doi: 10.1038/ng2062. [DOI] [PubMed] [Google Scholar]
- 7.Thomas G, Jacobs KB, Yeager M, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–5. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- 8.Lai J, Kedda MA, Hinze K, et al. PSA/KLK3 AREI promoter polymorphism alters androgen receptor binding and is associated with prostate cancer susceptibility. Carcinogenesis. 2007;28:1032–9. doi: 10.1093/carcin/bgl236. [DOI] [PubMed] [Google Scholar]
- 9.Severi G, Hayes VM, Neufing P, et al. Variants in the prostate-specific antigen (PSA) gene and prostate cancer risk, survival, and circulating PSA. Cancer Epidemiol Biomarkers Prev. 2006;15:1142–7. doi: 10.1158/1055-9965.EPI-05-0984. [DOI] [PubMed] [Google Scholar]
- 10.Cicek MS, Liu X, Casey G, Witte JS. Role of androgen metabolism genes CYP1B1, PSA/KLK3, and CYP11alpha in prostate cancer risk and aggressiveness. Cancer Epidemiol Biomarkers Prev. 2005;14:2173–7. doi: 10.1158/1055-9965.EPI-05-0215. [DOI] [PubMed] [Google Scholar]
- 11.Nam RK, Zhang WW, Klotz LH, et al. Variants of the hK2 protein gene (KLK2) are associated with serum hK2 levels and predict the presence of prostate cancer at biopsy. Clin Cancer Res. 2006;12:6452–8. doi: 10.1158/1078-0432.CCR-06-1485. [DOI] [PubMed] [Google Scholar]
- 12.Ahn J, Berndt SI, Wacholder S, et al. Variation in KLK genes, prostate-specific antigen and risk of prostate cancer. Nat Genet. 2008;40:1032–4. doi: 10.1038/ng0908-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eeles R, Giles G, Neal D, Muir K, Easton DF. Reply to “Variation in KLK genes, prostate-specific antigen and risk of prostate cancer”. Nat Genet. 2008;40:1035–6. doi: 10.1038/ng0908-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal P, Xi H, Sun G, et al. Tagging SNPs in the kallikrein genes 3 and 2 on 19q13 and their associations with prostate cancer in men of European origin. Hum Genet. 2007;122:251–9. doi: 10.1007/s00439-007-0394-3. [DOI] [PubMed] [Google Scholar]
- 15.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 16.Kruglyak L, Nickerson DA. Variation is the spice of life. Nat Genet. 2001;27:234–6. doi: 10.1038/85776. [DOI] [PubMed] [Google Scholar]
- 17.Lindmark F, Zheng SL, Wiklund F, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. J Natl Cancer Inst. 2004;96:1248–54. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 18.Zheng SL, Sun J, Wiklund F, et al. Cumulative Association of Five Genetic Variants with Prostate Cancer. N Engl J Med. 2008;358:910–9. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 19.Varenhorst E, Garmo H, Holmberg L, et al. The National Prostate Cancer Register in Sweden 1998–2002: trends in incidence, treatment and survival. Scand J Urol Nephrol. 2005;39:117–23. doi: 10.1080/00365590510007793. [DOI] [PubMed] [Google Scholar]
- 20.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20. [PubMed] [Google Scholar]
- 21.Becker C, Piironen T, Kiviniemi J, Lilja H, Pettersson K. Sensitive and specific immunodetection of human glandular kallikrein 2 in serum. Clin Chem. 2000;46:198–206. [PubMed] [Google Scholar]
- 22.Vaisanen V, Eriksson S, Ivaska KK, Lilja H, Nurmi M, Pettersson K. Development of sensitive immunoassays for free and total human glandular kallikrein 2. Clin Chem. 2004;50:1607–17. doi: 10.1373/clinchem.2004.035253. [DOI] [PubMed] [Google Scholar]
- 23.Stranger BE, Forrest MS, Clark AG, et al. Genome-wide associations of gene expression variation in humans. PLoS Genet. 2005;1:e78. doi: 10.1371/journal.pgen.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Daly MJ, Sham PC. WHAP: haplotype-based association analysis. Bioinformatics. 2007;23:255–6. doi: 10.1093/bioinformatics/btl580. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamsson PA, Andersson C, Bjork T, et al. Radioimmunoassay of beta-microseminoprotein, a prostatic-secreted protein present in sera of both men and women. Clin Chem. 1989;35:1497–503. [PubMed] [Google Scholar]
- 26.Nam RK, Reeves JR, Toi A, et al. A novel serum marker, total prostate secretory protein of 94 amino acids, improves prostate cancer detection and helps identify high grade cancers at diagnosis. J Urol. 2006;175:1291–7. doi: 10.1016/S0022-5347(05)00695-6. [DOI] [PubMed] [Google Scholar]
- 27.Yeager M, Orr N, Hayes RB, et al. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–9. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008;6:19. doi: 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genome-wide association study of 14,000 cases of seven common diseases and 3000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam RK, Zhang WW, Trachtenberg J, et al. Single nucleotide polymorphism of the human kallikrein-2 gene highly correlates with serum human kallikrein-2 levels and in combination enhances prostate cancer detection. J Clin Oncol. 2003;21:2312–9. doi: 10.1200/JCO.2003.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.