Abstract
The detection of water and the regulation of water intake are essential for animals to maintain proper osmotic homeostasis1. Drosophila and other insects have gustatory sensory neurons that mediate the recognition of external water sources2-4, but little is known about the underlying molecular mechanism for water taste detection. Here, we identify a member of the Degenerin/Epithelial Sodium Channel family5, ppk28, as an osmosensitive ion channel that mediates the cellular and behavioral response to water. We use molecular, cellular, calcium imaging and electrophysiological approaches to show that ppk28 is expressed in water-sensing neurons and loss of ppk28 abolishes water sensitivity. Moreover, ectopic expression of ppk28 confers water sensitivity to bitter-sensing gustatory neurons in the fly and sensitivity to hypo-osmotic solutions when expressed in heterologous cells. These studies link an osmosensitive ion channel to water taste detection and drinking behavior, providing the framework for examining the molecular basis for water detection in other animals.
To uncover novel molecules involved in taste detection, we performed a microarray-based screen for genes expressed in taste neurons. Proboscis RNA from flies homozygous for a recessive poxn null mutation was compared to RNA from heterozygous controls. poxn mutants have a transformation of labellar gustatory chemosensory bristles into mechanosensory bristles, and therefore lack all taste neurons6, 7. Whole genome microarray comparisons revealed that 256 of ~18,500 transcripts were significantly decreased in poxn mutants (>2 fold enrichment in control relative to poxn, p<0.05, moderated t-test). These included 18 gustatory receptors (representing a 21-fold enrichment in the gene set) and 8 odorant binding proteins (13-fold enrichment) (Supplementary Fig. 1; Supplementary Table 1; accession number GSE19984).
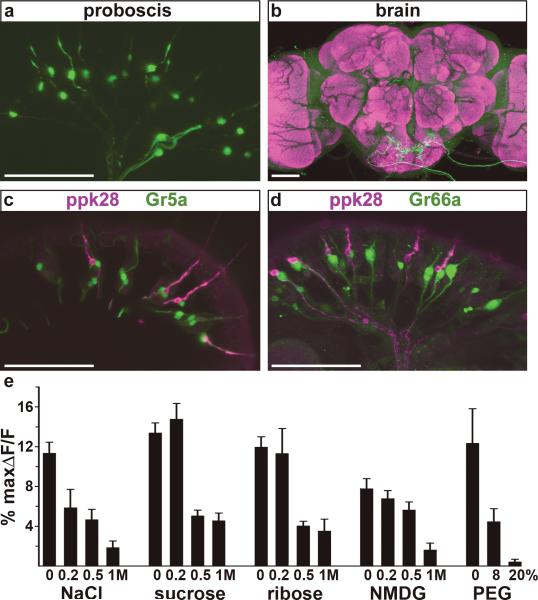
In the mammalian gustatory system, ion channels mediate the detection of sour and salt tastes8, suggesting that ion channel genes may also participate in Drosophila taste detection. We therefore examined the expression pattern of candidate taste-enriched ion channels. The putative promoter of one gene, pickpocket 28 (ppk28), directed robust reporter expression in taste neurons on the proboscis (Fig. 1a). ppk28 belongs to the Degenerin/Epithelial sodium channel family (Deg/ENaC) and these channels are involved in the detection of diverse stimuli, including mechanosensory stimuli, acids and sodium ions5. In the brain, ppk28-Gal4 drives expression of GFP in gustatory sensory axons that project to the primary taste region, the subesophageal ganglion (Fig. 1b; Supplementary Fig. 2). In situ hybridization experiments confirmed that transgenic expression recapitulates that of the endogenous gene, as 48/52 of ppk28-Gal4 neurons expressed endogenous ppk28.
Figure 1.
ppk28-Gal4 labels neurons that respond to water. a, b. ppk28-Gal4 drives GFP in (a) gustatory neurons and (b) their axons in the subesophageal ganglion. ppk28 was previously reported in larval tracheae27. c, d. ppk28 neurons (magenta) do not contain markers for (c) sugar neurons (Gr5a, green) or (d) bitter neurons (Gr66a, green). Scale bar in a-d is 50 μm e. ppk28-Gal4 neurons respond to water. G-CaMP fluorescent changes to water, NaCl, sucrose, ribose, n-methyl-d-glucamine (NMDG) and polyethylene glycol (PEG). Responses different than water by t-test are 0.2M NaCl (P=0.046), 0.5M NaCl (P=0.004), 1M NaCl (P=0.0003), 0.5M sucrose (P=3.27E-5), 1M sucrose (P=1.11E-5), 0.5M ribose (P=0.0008), 1M ribose (P=0.0003), 1M NMDG (P=0.0014), 20% PEG (P=0.028). n=4-11 flies/compound ± s.e.m.
Previous studies have identified different taste cell populations in the proboscis, including cells labeled by the gustatory receptor Gr5a that respond to sugars9-12 and cells marked by Gr66a that respond to bitter compounds10-13. To determine whether these taste neurons express ppk28-Gal4, we performed co-labeling experiments with reporters for Gr5a and Gr66a. These experiments revealed that ppk28 did not co-label Gr5a cells or Gr66a cells, and is thus unlikely to participate in sweet or bitter taste detection (Fig. 1c, d). An enhancer-trap Gal4 line, NP1017-Gal4, labels water-sensing neurons in taste bristles on the proboscis4 and carbonation-sensing neurons in taste pegs14 (Supplementary Fig. 3). ppk28 is expressed in taste bristles but not in taste pegs. Interestingly, ppk28 showed partial co-expression with NP1017-Gal4 (Supplementary Fig. 3), with the majority of ppk28-positive cells containing NP1017-Gal4 (22/30). This correlation suggested the intriguing possibility that ppk28 participates in water taste detection.
To directly investigate the response specificity of ppk28-expressing neurons, we expressed the genetically encoded calcium sensor G-CaMP in ppk28-Gal4 cells, stimulated the proboscis with taste substances and monitored activation of ppk28-Gal4 projections in the living fly by confocal microscopy12. We tested ppk28-Gal4 neurons with a panel of taste solutions, including sugars, bitter compounds, salts, acids and water. ppk28-Gal4 neurons showed robust activity upon water stimulation (Fig. 1e). In addition, ppk28-positive cells responded to other aqueous solutions even in the presence of a wide range of chemically distinct compounds. This response diminished as a function of concentration. Taste compounds such as NaCl, sucrose and citric acid significantly decreased the response (Fig. 1e, Supplementary Fig. 4). In addition, compounds unlikely to elicit taste cell activity such as ribose, a sugar that does not activate Gr5a cells, N-methyl-D-glucamine (NMDG), an impermeant organic cation and the non-ionic high molecular weight polymer polyethylene glycol (PEG, 3350 average molecular weight), all blunted the response in a concentration-dependent manner (Fig. 1e, Supplementary Fig. 4). These data demonstrate that ppk28-expressing neurons respond to hypo-osmotic solutions. This response profile is consistent with previous electrophysiological studies that identified a class of labellar taste neurons activated by water and inhibited by salts, sugars and amino acids4, 15.
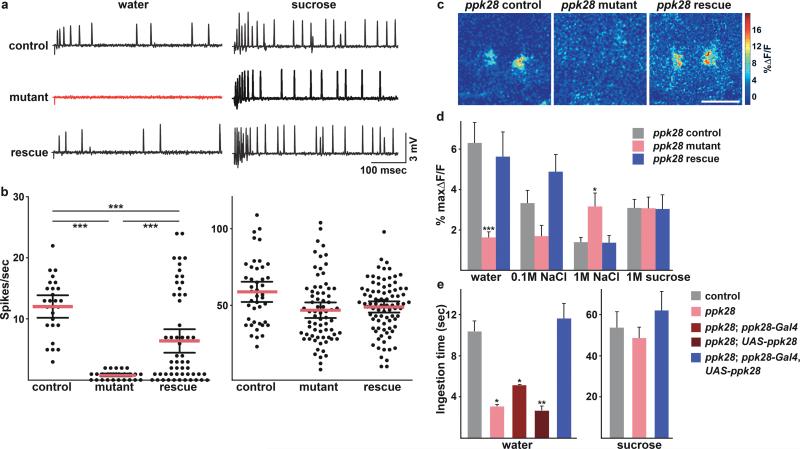
To determine the function of ppk28 in the water response, we generated a ppk28 null mutant by piggybac transposon mediated gene deletion, removing 1.769kb surrounding the ppk28 gene16. We examined the water responses of ppk28 control, mutant and rescue flies by extracellular bristle recordings of l-type labellar taste sensilla. These recordings monitor the responses of the four gustatory neurons in a bristle, including water cells and sugar cells3. Control flies showed 12.0±0.9 spikes/sec when stimulated with water (Fig. 2a, b). Remarkably, ppk28 mutant cells had a complete loss of the response to water (spikes/sec=0.8±0.1). This response was partially rescued by reintroduction of ppk28 into the mutant background (spikes/sec=6.4±1.0), demonstrating that defects were due to loss of ppk28 (Fig. 2a, b). Responses to sucrose were not significantly different among the three genotypes (58.9±3.3 spikes/sec, 46.9±2.6 spikes/sec and 49.0±1.8 spikes/sec, for control, mutant and rescue flies, respectively) (Fig. 2a, b), arguing that the loss of ppk28 specifically eliminates the water response. These results were confirmed by G-CaMP imaging experiments that monitor the response of the entire ppk28 population. As expected, ppk28-Gal4 neurons in the mutant did not show fluorescent increases to water and transgenic re-introduction of ppk28 rescued the water response (Fig. 2c, d). Taken together, the electrophysiological and imaging data demonstrate that ppk28 is required for the cellular response to water.
Figure 2.
The ppk28 gene is necessary for cellular and behavioral water responses. a. Extracellular bristle recordings of ppk28 control, mutant and rescue flies after water (left) or 100 mM sucrose (right) stimulation, showing action potentials. Stimulation begins at recording. b. Scatter plot of water and sugar responses (mean ± s.e.m in bars; data points as dots). Water responses are ***P=0.001 by Dunn's multiple comparison. c. G-CaMP fluorescence increase in ppk28 control, mutant and rescue projections to water (%maxΔF/F) (SOG, scale bar 50μm). d. Fluorescence change summary following water, 0.1M NaCl, 1M NaCl, 1M sucrose (n=8-11 trials/concentration ± s.e.m; t-test, ppk28 control versus mutant, water: ***P=0.0008, 1M NaCl: *P=0.03). e. Behavioral assays measuring water or 500mM sucrose consumption time. Control flies drink more water than ppk28 mutants (*P=0.017), ppk28 mutants + ppk28-Gal4 (*P=0.037) or ppk28 mutants + UAS-ppk28 (**P=0.008). Water consumption of control and rescue is not different (P=0.53). Sucrose consumption is not different (vs control, mutant: P=0.63; rescue: P=0.53). n= 3 ± s.e.m trials, 18-25 flies/trial/genotype, t-test.
The detection of water in the environment and the internal state of the animal may both contribute to drive water consumption1. To evaluate the degree to which water taste detection contributes to consumption, we examined the behavioral responses of ppk28 control, mutant and rescue flies to water. Drinking time rather than drinking volume was used to monitor consumption due to difficulty in reliably detecting small volume changes. When presented with a water stimulus, control flies drank on average 10.3±1.1 seconds, mutants drank 3.0±0.3 seconds and rescue flies drank 11.5±1.5 seconds (Fig. 2e). Additionally, control, mutant and rescue flies ingested sucrose equally, showing that ppk28 mutants do not have general drinking defects. Similar defects in water detection were seen when control, mutant and rescue flies were tested on the proboscis extension reflex to water (Supplementary Fig. 5a) or when genetically ablating ppk28-Gal4 neurons (Supplementary Fig. 5b). Although ppk28 mutants lack water taste cell responses and drink less, they still do consume water, arguing that additional mechanisms must exist to ensure water uptake. These experiments reveal that water taste neurons are necessary for normal water consumption. Moreover, they establish a link between water taste detection in the periphery and the drive to drink water.
We next examined whether ppk28 is directly involved in water detection. If ppk28 is the water sensor, then its expression in non-water sensing cells should bestow responsiveness to water. To test this, we used the Gal4/UAS system to ectopically express ppk28 in Gr66a-expressing, bitter-sensing neurons and monitored taste-induced responses by extracellular bristle recordings and G-CaMP imaging experiments (Fig. 3). For extracellular bristle recordings, responses were recorded from i-type sensilla which contain bitter-sensing, Gr66a-positive neurons but lack water cells17. Expression of ppk28 in Gr66a-Gal4 neurons did not significantly affect the response to denatonium (G-CaMP imaging: control %ΔF/F=11.9±1.2; misexpression %ΔF/F=13.8±0.7) or caffeine (control 18.8±3.0 spikes/sec; misexpression 20.6±2.9 spikes/sec; Fig. 3a, b), endogenous ligands for Gr66a-Gal4 neurons12. In response to water, Gr66a-Gal4 neurons showed no significant activity consistent with previous studies (Fig. 3)12. Notably, misexpression of ppk28 in Gr66a-Gal4 neurons conferred sensitivity to water, as seen by extracellular bristle recordings (Fig 3a, b) and G-CaMP imaging (Fig 3c, d, e). Moreover, the response was blunted as solute concentration was increased. Both NMDG and sucrose (substances that do not activate Gr66a-Gal4 neurons) produced dose-sensitive response decreases. The finding that both activation by water and inhibition by other compounds are conferred by ppk28 strongly suggests that ppk28 senses low osmolarity.
Figure 3.
Ectopic expression of ppk28 confers water sensitivity. a. Extracellular bristle recordings of i-type sensilla (non-water responsive) from Gr66a-Gal4 flies lacking (-) or containing (+) UAS-ppk28 upon water, 0.5M NMDG, 1M NMDG or 0.01M caffeine stimulation (at recording). b. Scatter plot of water responses (mean ± s.e.m in bars; data points are dots) and summary of all responses (mean ± s.e.m.). Responses are different to water (***P=2.52E-16) and 0.5M NMDG (*P=0.016) (t-test; n=7-27). c. G-CaMP fluorescence increase in Gr66a bitter-sensing projections (left) and Gr66a projections expressing ppk28 (right), after water stimulation (%maxΔF/F) (SOG, scale 50μm). d. Responses in Gr66a cells (left) and Gr66a cells expressing ppk28 (right) to water (at arrow). e. Summary of fluorescence changes in Gr66a cells without (grey) or with ppk28 (green) tested with water, 0.5 and 1M NMDG and 0.5, 1 and 2M sucrose. (n=4-5 trials/concentration ± s.e.m; t-test, versus Gr66a control, water: **P=0.013, 0.5M NMDG: *P=0.03; water: **P=0.002, 0.5M sucrose: ***P=0.0002).
To determine if ppk28 requires a taste cell environment to function or confers responsiveness to other cell-types, ppk28 was expressed in HEK293 heterologous cells. A FLAG-tagged ppk28 (inserted after amino acid 222 in the extracellular domain) was expressed in HEK293 cells, confirming that the protein was made and trafficked to the cell surface (Supplementary Fig. 6). For calcium imaging experiments, an untagged version of ppk28 was cotransfected with dsRed. Cells expressing the mammalian trpv4 osmo-sensitive ion channel18 were used as a positive control and cells transfected with the vector alone as a negative control. Cells were grown in a modified Ringers solution at 303 mmol/kg, loaded with Fluo-4 to visualize calcium changes and challenged with Ringers solution of different osmolalities (236, 216 and 174 mmol/kg; 80%, 70% and 60% osmotic strength to the isotonic solution, respectively). Cells transfected with vector alone showed a modest increase at 60% osmotic strength, whereas cells transfected with mammalian trpv4 showed fluorescence increases to all hypo-osmotic solutions, as expected (Fig. 4b, c, d). Importantly, cells transfected with ppk28 significantly responded to decreased osmolality, with dose-sensitive responses elicited by osmolalities of 216 and 174 mmol/kg (Fig. 4a, d). These experiments reveal that ppk28 bestows sensitivity to hypo-osmotic solutions in a variety of non-native environments and argue that the channel itself senses low osmolarity. This work provides a foundation for future studies of the biophysical properties of channel activation. Moreover, the ability to express ppk28 in heterologous cells and study its function creates the opportunity to compare its mechanism of gating with other Deg/ENaC family members involved in mechanosensation or sodium sensing.
Figure 4.
Heterologous cells expressing ppk28 respond to hypo-osmolarity. a-c. Pseudocolor images of maximum fluorescence increases (maxΔF) to isotonic (303mmol/kg) and reduced osmolality (174 mmol/kg) for HEK293 cells expressing ppk28, TRPV4 or vector. Color bar indicates maxΔF ranging from -10 to 80. On the right, plots of fluorescence change per frame over the stimulation (bar) at 80%, 70% and 60% of isotonic osmolality (236, 216 and 174 mmol/kg). d. Concentration curve of responses to osmolalities. (n=4-5 trials/concentration ± s.e.m.; t-test, versus vector, ppk28: 70% **P=0.00583; 60% **P=0.00632; TRPV4: 80% **P=0.00384; 70% ***P=0.000120; 60% **P=0.00615).
Overall, these studies examined the molecular basis for water taste detection in Drosophila and identified an ion channel belonging to the Deg/ENaC family, pickpocket 28 (ppk28), as the water gustatory sensor. Our work demonstrates that an ion channel responding to low osmolarity mediates cellular and behavioral responses to water. Although the taste of water has received relatively little attention as a classic taste modality, water-responsive taste neurons have been described in many other insects, such as the blowfly and mosquitoes2, 19, as well as in mammals, such as cats and rats20, 21. The identification of ppk28 as a water taste receptor provides a framework for examining water taste detection in other animals, including humans.
Osmosensation is important not only for the detection of external water sources by peripheral neurons but also for monitoring the plasma osmolality by central neurons1. Several studies have identified members of the transient receptor potential family as candidate peripheral and central osmosensors18, 22-24, but the role of members of the Deg/ENaC family in osmosensation has received little attention. Our finding that ppk28 is an osmosensitive ion channel raises the possibility that Deg/ENaC ion channels may participate broadly in peripheral and central osmosensation.
Methods Summary
Transgenic flies and ppk28 mutants
The ppk28 promoter-Gal4 construct was generated with a 1.004kb upstream fragment (16699333-16700336, Genbank accession number NC_004354.3). Full-length ppk28 (transcript variant a, NM_132941) was subcloned into pUAST. ppk28 mutants were generated by FLP-FRT mediated recombination between Piggybacs f05788 and e02329.
Immunohistochemistry and in situ hybridization
Labeling was performed as described11. In Fig. 1b, the brain was counterstained with nc8225. In Fig. 1c, d, CD2 and GFP were detected by immunohistochemistry on flies containing ppk28-Gal4, UAS-CD2, Gr66a-GFP-IRES-GFP-IRES-GFP or Gr5a-GFP-IRES-GFP-IRES-GFP transgenes11, 12.
G-CaMP imaging experiments
Imaging studies were performed as described12. Details in Methods.
Behavioral Assays
Control flies were isogenic w1118 fly strain (Exelixis strain A5001, BL-6326) and transgenes were backcrossed seven times to the control strain. 2-5 day old flies were starved ~18-24 hours with water, kept in a humid chamber for 2-3 hours and then stimulated on the proboscis with a taste substance. Flies were allowed to ingest freely until they did not ingest after 5 consecutive stimulations. For water ingestion, flies were stimulated on the proboscis with 1M sucrose afterward, and only responders were kept for data tally.
Electrophysiology
Electrophysiology was performed as described26. Details in Methods.
HEK293 calcium imaging experiments
Measurements in cells were made by using calcium indicator Fluo-4 and a confocal laser scanning microscope (Zeiss LSM510, Carl Zeiss, Jena, Germany). Details in Methods.
Methods
Transgenic flies and ppk28 mutants
The ppk28 promoter-Gal4 construct was generated with a 1.004kb upstream fragment (16699333-16700336, Genbank accession number NC_004354.3). Full-length ppk28 (transcript variant a, NM_132941) was subcloned into pUAST. ppk28 mutants were generated by FLP-FRT mediated recombination between Piggybacs f05788 and e02329.
Immunohistochemistry and in situ hybridization
Labeling was performed as described11. In Fig. 1b, the brain was counterstained with nc8225. In Fig. 1c, d, CD2 and GFP were detected by immunohistochemistry on flies containing ppk28-Gal4, UAS-CD2, Gr66a-GFP-IRES-GFP-IRES-GFP or Gr5a-GFP-IRES-GFP-IRES-GFP transgenes11, 12.
G-CaMP imaging experiments
Imaging studies were performed as described12. Flies were aged ~2-5 weeks. For Fig. 1e, flies were UAS-G-CaMP; ppk28-Gal4; UAS-G-CaMP. For NaCl, sucrose and ribose, flies were stimulated 2-3 times, ending with a positive control (>8% ΔF/F). For NMDG (pH 7.4 with HCl), and PEG (molecular weight 3,350), concentrations were presented randomly, ending with a positive control (>7% ΔF/F). For Fig. 2c, d, genotypes were as follows. Control: UAS-G-CaMP;ppk28-Gal4;UAS-G-CaMP. Mutant: Δppk28, UAS-G-CaMP;ppk28-Gal4;UAS-G-CaMP. Rescue: Δppk28, UAS-G-CaMP;ppk28-Gal4;UAS-G-CaMP, UAS-ppk28. Compounds were presented randomly and experiments were performed blind to genotype. For Fig. 3, genotypes were as follows. Gr66a: UAS-G-CaMP;Gr66a-Gal4;TM2/TM6b. Gr66a + ppk28: UAS-G-CaMP;Gr66a-Gal4;UAS-ppk28. Compounds were presented randomly followed by 10mM denatonium (>8% ΔF/F).
Behavioral Assays
Control flies were isogenic w1118 fly strain (Exelixis strain A5001, BL-6326) and transgenes were backcrossed seven times to the control strain. 2-5 day old flies were starved ~18-24 hours with water, kept in a humid chamber for 2-3 hours and then stimulated on the proboscis with a taste substance. Flies were allowed to ingest freely until they did not ingest after 5 consecutive stimulations. For water ingestion, flies were stimulated on the proboscis with 1M sucrose afterward and only responders were kept for data tally.
Electrophysiology
Electrophysiology was performed as described.26 2-3 day old flies were transferred on fresh medium one day prior to experiment. For recording activity from labellar taste neurons, a reference glass electrode filled with AHL solution12 was placed in the proboscis base and a recording electrode filled with testing taste solution covered the tip of a single taste bristle. All test solutions contain 1 mM KCl as an electrolyte. The signal was amplified (100X total), filtered (low-pass:<2800 Hz) by amplifiers (DTP-2, Syntech, Kirchzarten, Germany; CyberAmp 320, Molecular Devices, Sunnyvale, CA) and stored on a PC. Action potentials were counted for the first 1 second. For Fig. 3, only i-type sensilla were recorded, as they contain the bitter cell but lack the water cell. Statistical analyses were done by two-tailed Student t-test or Kruskal–Wallis analysis of variance (ANOVA) (for comparisons among more than two groups) unless otherwise noted. Significant differences were analyzed using Dunn's multiple comparison test as the post-hoc test (significance level = 0.001).
HEK293 calcium imaging experiments and immunohistochemistry
Measurements in cells were made by using calcium indicator Fluo-4 (Invitrogen) and a confocal laser scanning microscope (Zeiss LSM510, Carl Zeiss, Jena, Germany). Cells were seeded on poly-D lysine coated glass one day prior to transfection (lipofectamine 2000, invitrogen), then incubated for 24-48 hours prior to imaging. Cells were then loaded with 10μM Fluo-4 for 45 min at 37°C in isotonic calcium imaging buffer (76mM NaCl, 5mM KCl, 2mM MgCl2, 2mM CaCl2, 10mM glucose, 10mM HEPES, mannitol, pH 7.4) in dark conditions. Solutions of varying osmolalities (303, 236, 216 and 174 mmol/kg) were prepared by adjusting the mannitol concentration. Osmolality of test solutions was measured using a vapor pressure osmometer (Vapro 5520, Wescor Inc., Logan, UT).
Cells were set in a perfusion chamber with isotonic solution for 3 min prior to stimulating with osmotic test solutions. Solution flow was kept constant at 3.3 mL/min. Fluorescence emission at 480 nm was filtered by 505-530 bandpass filter. Images were analyzed using automated routines written in Matlab. Total fluorescence change for the dsRed-positive cells in the field was calculated and divided by cell area to normalize for cell density. Responses were averaged from 3-5 independent experiments/stimulation/transfected cell line.
Supplementary Material
Acknowledgements
The authors thank Karen Vranizan for assistance with microarray analyses. Kristin Gerhold and Dr. Diana Bautista kindly provided the TRPV4 construct, protocols and advice for HEK293 experiments; the Roelink lab provided tissue culture facilities and advice. Dr. Gautam Agarwaal generated heat map images in Matlab for data presentation. Dr. Walter Fischler generated the NP1017 G-CaMP data in Supplementary Information. We are grateful to Dr. Charles Zuker and members of the Scott lab for comments on the manuscript. This work was supported by a grant from the NIH (NIDCD), a Burroughs-Wellcome CAREER Award and a John Merck Award to K.S. and a NIH predoctoral fellowship to P.C. K.S. is an HHMI Early Career Scientist.
Footnotes
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 2.Evans DR, Mellon D., Jr. Electrophysiological studies of a water receptor associated with the taste sensilla of the blow-fly. J Gen Physiol. 1962;45:487–500. doi: 10.1085/jgp.45.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meunier N, Ferveur JF, Marion-Poll F. Sex-specific non-pheromonal taste receptors in Drosophila. Curr Biol. 2000;10:1583–1586. doi: 10.1016/s0960-9822(00)00860-5. [DOI] [PubMed] [Google Scholar]
- 4.Inoshita T, Tanimura T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc Natl Acad Sci U S A. 2006;103:1094–1099. doi: 10.1073/pnas.0502376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 6.Awasaki T, Kimura K. pox-neuro is required for development of chemosensory bristles in Drosophila. J Neurobiol. 1997;32:707–721. doi: 10.1002/(sici)1097-4695(19970620)32:7<707::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Boll W, Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129:5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- 8.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 13.Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- 15.Meunier N, Marion-Poll F, Lucas P. Water taste transduction pathway is calcium dependent in Drosophila. Chem Senses. 2009;34:441–449. doi: 10.1093/chemse/bjp019. [DOI] [PubMed] [Google Scholar]
- 16.Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 17.Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- 18.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VROAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werner-Reiss U, Galun R, Crnjar R, Liscia A. Sensitivity of the mosquito Aedes aegypti (Culicidae) labral apical chemoreceptors to blood plasma components. J Insect Physiol. 1999;45:485–491. doi: 10.1016/s0022-1910(98)00151-6. [DOI] [PubMed] [Google Scholar]
- 20.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 21.Gilbertson TA. Hypoosmotic stimuli activate a chloride conductance in rat taste cells. Chem Senses. 2002;27:383–394. doi: 10.1093/chemse/27.4.383. [DOI] [PubMed] [Google Scholar]
- 22.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 25.Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 26.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Johnson WA, Welsh MJ. Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc Natl Acad Sci U S A. 2003;100:2128–2133. doi: 10.1073/pnas.252785099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.