Summary
Dendritic cells (DCs) orchestrate the innate and adaptive immune systems to induce tolerance and immunity. DC plasticity and subsets are prominent determinants in the regulation of immune responses. Our recent studies suggest that humoral and cellular immunity is regulated by different myeloid DC subsets with distinct intrinsic properties in humans. While antibody response is preferentially mediated by CD14+ dermal DCs, cytotoxic T cell response is preferentially mediated by Langerhans cells (LCs). Thus, mechanisms whereby DCs induce humoral and cellular immunity appear to be fundamentally distinct. In this review, we will focus on the role of DCs in the development of humoral immunity. We will also discuss the mechanisms whereby DCs induce CD4+ T cells associated with the help of B cell response, including T follicular helper (Tfh) cells, and why human LCs lack this ability.
Introduction
Dendritic cells (DCs) induce/maintain tolerance to self and immunity to non-self by integrating the innate and adaptive immune systems1, 2. Generating the right type of immune response can be a matter of life and death. In leprosy, for instance, the tuberculoid form of the disease is characterized by a Type 1 response which keeps the disease in check, while the lepromatous form induces an often fatal Type 2 response3.
DCs are endowed with enormous functional plasticity, which permits them to induce different immune responses according to the microenvironment. In addition, The DC system is composed of subsets associated with the induction of different types of immunity. We have recently demonstrated that two myeloid DC subsets in human skin, i.e., Langerhans cells (LCs) and CD14+ dermal DCs, are engaged in the induction of different types of adaptive immunity4. While LCs are very efficient at inducing CTL responses, CD14+ dermal DCs display a unique property to promote the development of antibody responses (Fig. 1). In this review, we will briefly summarize the phenotypical and functional differences between human LCs and CD14+ dermal DCs, and discuss how human DCs are involved in the regulation of humoral responses.
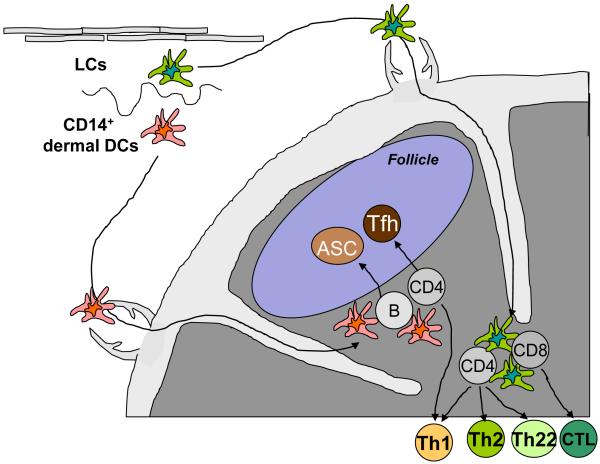
Figure 1. CD14+ dermal DCs preferentially induce humoral immunity, while Langerhans cells induce cellular immunity.
Upon activation, epidermal LCs and CD14+ dermal DCs migrate to the secondary lymphoid organs through afferent lymphatics. Dermal DCs migrate into the outer paracortex, just beneath the B cell follicles, whereas LCs migrate into the T cell rich area. LCs are efficient at inducing high avidity-cytotoxic CD8+ T cell and Th1, Th2, and Th22 responses. In contrast, CD14+ dermal DCs are efficient at inducing the differentiation of naïve B cells into antibody-secreting cells (ASC) and at promoting the development of T follicular helper (Tfh) cells. CD4+ T cells primed by LCs might be efficient at helping the development of CTL responses.
Epidermal LCs and CD14+ dermal DCs
Human skin hosts at least three different mDC subsets. CD1ahighCD14−HLA-DR+ Langerhans cells (LCs) reside in epidermis, while CD1adimCD14−HLA-DR+ DCs (CD1a+ dermal DCs) and CD1a−CD14+HLA-DR+ DCs (CD14+ dermal DCs) are present in dermis 4. CD14+ dermal DCs express CD163 and FXIIIa, which are also expressed by dermal macrophages. However, CD14+ dermal DCs express CD11c, while dermal macrophages do not5.
CD14+ dermal DCs express a broad spectrum of surface C-type lectins including DC-SIGN, DEC-205, LOX-1, CLEC-6, Dectin-1, and DCIR6. In contrast, LCs express a more limited set, including Langerin and DCIR. Neither of the two dermal DC subsets express Langerin, an observation that contrasts with the presence of Langerin+ dermal DCs in mice7-9. CD14+ dermal DCs also express multiple TLRs recognizing bacterial components, such as toll like receptor (TLR)1, 2, 4, 5, 6, 8, and 106, 10, suggesting their involvement in the induction of anti-bacterial immunity. LCs have been reported to express TLR1, 2, 3, 6, (7) and 1010-12, and to respond to ligands of TLR2 (peptideglycan11 and Pam3CysSerLys4 (Pam3CSK4)13) or TLR3 (Poly I:C11, 12). In contrast, a study showed that LCs poorly respond to TLR-ligands derived from bacteria, including TLR2, TLR4, and TLR510. Our microarray studies using of highly purified LCs failed to show much TLR expression6, while CD14+ dermal DCs showed significant expression.
LCs promote CTL responses
Human LCs are remarkable at inducing CTL responses in vitro. For example, upon loading with tumor-derived peptides, LCs effectively prime peptide-specific naïve CD8+ T cells, and induce their differentiation into CTLs that express high levels of cytotoxic molecules and are accordingly efficient at killing tumor cells4. Notably, induction of CTL response by LCs does not appear to be dependent on IL-12 or IFN-α, as neither CD40 nor TLR stimulation do not induce LCs to secrete these cytokines4, 11, 12, 14. Instead, CD40-stimulation induces LCs to secrete IL-154, 14, which we surmise responsible for their capacity to induce potent CTL responses. This hypothesis is partly supported by the observation that externally added IL-15 enhances the ability of CD14+ dermal DCs to develop CTLs with high levels of cytotoxic granules6.
LCs also induce a potent proliferation of allogeneic naïve CD4+ T cells. Naïve CD4+ T cells primed by LCs secrete larger amounts of Type 2 cytokines than those primed by two dermal DCs4. A recent report showed that human LCs also promote the development of IL-22-secreting CD4+ T cells, which do not co-express Th1, Th2 or Th17 cytokines15. Interestingly, IFN-γ-secreting CD4+ T cells are induced at a similar level by LCs and other dermal DC subsets. However, the developmental mechanism of Th1 cells induced by these DC subsets appears be distinct. Consistent with their inability to secrete IL-12, induction of Th1 cells by epidermal LCs was shown to be independent of IL-12 or IL-2312. Considering the potent capacity of LCs to induce CTL responses, such Th1 cells primed by LCs might be efficient helpers for the development of CTL responses.
Role of plasmacytoid DCs in antibody response
In vitro studies with plasmacytoid DCs (pDCs) isolated from human blood and tonsils demonstrate that pDCs are also directly involved in the help B cell responses. Upon stimulation with influenza virus, pDCs promote the differentiation of memory B cells towards antibody-secreting plasma cells through sequential steps. Type I intereferon secreted by pDCs promotes the differentiation of CD40-stimulated B cells into non-antibody-secreting plasmablast. Similar to mDCs, IL-6 secreted by pDCs further induces the transition of non-secreting plasmablasts into antibody-secreting plasma cells16. pDCs activated with TLR9 ligand are also capable of inducing TLR9-triggered naïve B cells to differentiate into IgM-producing plasma cells17.
pDCs also contributes to the humoral immunity through cross-talk with mDCs. Type I IFN secreted by pDCs induces mDCs and monocytes to express BAFF and APRIL18, which promotes B cell survival, proliferation, and class-switching19. Furthermore, a mouse study showed that mDCs exposed to Type I IFN in vivo promote the differentiation of naïve CD4+ T cells towards helper CD4+ T cells which promote antibody responses 20, i.e., T follicular helper (Tfh) cells.
CD14+ dermal DCs directly promote plasma cell development via IL-12
A decade ago, in vitro studies with CD14+ DCs generated from CD34+ hematopoietic precursor cells have shown that CD14+ DCs induce the differentiation of CD40-activated naïve B cells into IgM-producing plasma cells through direct interactions21. In contrast LCs, both in vitro-generated and ex-vivo isolated, lacked this capacity21, 22. Mechanistic studies revealed that IL-12 secreted by CD14+ DCs is critical for the first step of plasma cell differentiation. Co-operation of IL-12 and IL-6 further induces transition from a non-secreting plasmablast to IgM-producing plasma cells23. Indeed, CD14+ DCs, both in vitro-generated and skin-derived, secrete multiple proinflammatory cytokines such as IL-1β, IL-6, IL-10, IL-12, TNFα and GM-CSF in response to stimulation through CD40, while LCs do not4, 11, 12, Thus, the lack of secretion of IL-12 and IL-6 appears to explain the inability of LCs to help naïve B cells through direct interactions.
CD14+ dermal DCs induces development of helper T cells
CD14+ dermal DCs also promote antibody responses indirectly through skewed differentiation of naïve CD4+ T cells. Both in vitro-generated CD14+ DCs and skin-derived CD14+ dermal DCs induce naïve CD4+ T cells to differentiate into effectors capable of helping B cell responses. There, CD4+ T cells primed by CD14+ dermal DCs are efficient at inducing naïve B cells to become antibody secreting plasma cells producing IgM, as well as to switch isotypes towards IgG and IgA4. In contrast, in spite of Th2 cytokine secretion, CD4+ T cells primed by LCs lack the capacity to induce B cells to differentiate into antibody-secreting cells. These observations indicate that CD4+ T cells primed by LCs or CD14+ dermal DCs functionally differ in their ability to help B cells.
CD4+ T cells associated with B cell help
The requirement of T cell help in the development of antibody responses was first described in 1960s24. CD4+ T cells were found to be necessary to develop germinal centers (GCs), a discrete structure in secondary lymphoid organs where selection of high-affinity B cells and development of B cell memory occur25, 26. In vitro studies in 80s, mostly using CD4+ T cell clones and recombinant cytokines, had concluded that Th2 is the major Th subset engaged in the help of B cells, through the secretion of IL-4 and IL-1027, 28. Recent extensive studies on CD4+ T cells present in GCs in mice and humans have established a novel Th subset, named T helper follicular (Tfh) cells, representing CD4+ T cells specialized for the help of humoral responses29-32. Tfh cells express the chemokine (C-X-C motif) receptor 5 (CXCR5) 29, 33, 34, which drives their migration into B cell follicle, as the ligand, CXCL13, is produced by follicular stromal cells including follicular DCs 35, 36. Several factors have been identified to be essential for Tfh cells to provide help to B cells. These include surface molecules such as CD40 ligand (CD40L) 27 and ICOS 37. In particular, Tfh cells secrete the cytokine IL-21 33, 38, which drives the growth, differentiation, and isotype switching of B cells 39, 40. Tfh cells secrete larger amounts of IL-21 than other conventional Th subsets, including Th1, Th2, and Th17 cells.
Human DCs induce IL-21-producing helper CD4+ T cells via IL-12
In mice, IL-21 itself is critically involved in the generation of Tfh cells41, 42. IL-21 provides a positive feedback loop to CD4+ T cells and induces human 43 and mouse 41, 42, 44-47 naïve CD4+ T cells to secrete more IL-21. However, as naïve CD4+ T cells or DCs do not secrete IL-21, IL-21-producing CD4+ T cells need to be induced through interaction with DCs. In mouse, IL-6 derived from DCs appears to be the major cytokine involved in the induction of mouse CD4+ T cells to secrete IL-21 20, 44, 48, 49. In contrast, in humans, we and others showed that IL-12 is the major cytokine by which DCs promote the development of IL-21-producing CD4+ T cells50, 51. Similar to Tfh cells52, CD4+ T cells induced by activated human DCs help B cell responses through IL-2150. IL-12 is the most potent DC-derived cytokine at inducing naïve human CD4+ T cells to become IL-21+ CD4+ T cells50. Consistently, activated DCs induce IL-21-producing helper CD4+ T cells via IL-12, as blocking IL-12 during DC-T coculture potently inhibit their development50. The induction of IL-21+CD4+ T cells by DCs might represent the first key step in the development of human Tfh cells. Alternatively, the induced IL-21-producing helper CD4+ T cells might be also associated with plasma cell differentiation at extrafollicular sites.
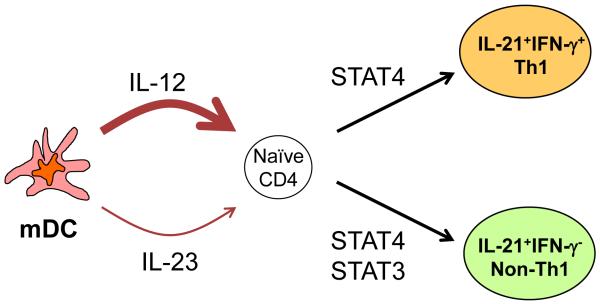
Notably, human naïve CD4+ T cells stimulated with activated DCs differentiate into two different types of IL-21+CD4+ T cells: IFN-γ+IL-21+ Th1 cells expressing T-bet, and IFN-γ−IL-21+ non-Th1 cells. Both IL-21+CD4+ T cells are dependent on signal transducers and activator of transcription (STAT)4 for their development by IL-12. IL-21 and IL-23 also contribute to the development of IL-21+CD4+ T cells, but at a much lesser extent. IL-21 and IL-23 induce the development of IFN-γ−IL-21+ non-Th1 cells in a manner dependent on STAT350 (Fig. 2). Thus, in humans, both STAT4 and STAT3 pathways contribute to the development of IL-21+CD4+ T cells. DCs regulate the balance of IFN-γ+IL-21+ Th1 cells and IFN-γ−IL-21+ non-Th1 cells through multiple pathways. For example, naïve CD4+ T cells primed in the presence of IL-12 and IL-23 promote the development of IFN-γ−IL-21+ non-Th1 cells (Schmitt et al. Unpublished observation). Whether which types of IL-21+CD4+ T cells, IFN-γ+IL-21+ Th1 cells or IFN-γ−IL-21+ non-Th1 cells, display more potent capacity to help B cells is under investigation.
Figure 2. Human activated DCs induce IL-21-producing T follicular helper (Tfh)-like cells via IL-12.
IL-12 induces human naïve CD4+ T cells to differentiate into two different types of IL-21-producing CD4+ T cells: IFN-γ+IL-21+ Th1 cells expressing T-bet, and IFN-γ−IL-21+ non-Th1 cells. Both IL-21-producing CD4+ T cells develop in a manner dependent on STAT4. IL-23 induces only IFN-γ−IL-21+ non-Th1 cells through STAT3. The induction of IFN-γ−IL-21+ non-Th1 cells by IL-12 appears to be partially dependent on STAT3 as well.
Distinct immune-modulatory capacity of IL-12 between mice and humans
Of note, the IL-12-IL-21 axis for helper T cell development does not appear to be operate in the mouse immune system, as IL-12 does not induce mouse naïve CD4+ T cells to secrete IL-21. Conversely, IL-6, a potent inducer of IL-21 in mouse CD4+ T cells, does not induce IL-21 expression in human naïve CD4+ T cells50. Thus, DC subsets and their activation paths involved in the development of IL-21+CD4+ T cells might differ between mice and humans. Accordingly, the in vivo biological effect of IL-12 might also differ between mice and humans. Mouse studies have demonstrated that IL-12, when administered as vaccine adjuvant, enhances the development of tumor-specific CTL and Th1 responses in vivo53, 54. In humans, the systemic administration of IL-12 has thus far shown only very modest clinical efficacy55, 56. The injection of IL-12 into tumor sites of head and neck cancer patients resulted in the activation of B cells in the draining lymph nodes57. Thus, IL-12 and adjuvants that promote the secretion of IL-12 might improve vaccines aimed at induction of neutralizing antibodies in humans.
Taken together, IL-12, a cytokine traditionally viewed as a potent inducer of Type 1 response, also contributes to humoral responses in humans. It acts through two independent paths: a direct path in DC-B cell interaction, and an indirect path through DC-T cell interaction by developing IL-21+ helper CD4+ T cells (Fig. 3). These two paths might act simultaneously in vivo, through the “ménage à trois” formation of antigen-presenting DCs with antigen-specific T cells and B cells at extrafollicular sites, as recently illustrated through in vivo imaging in mice58, 59.
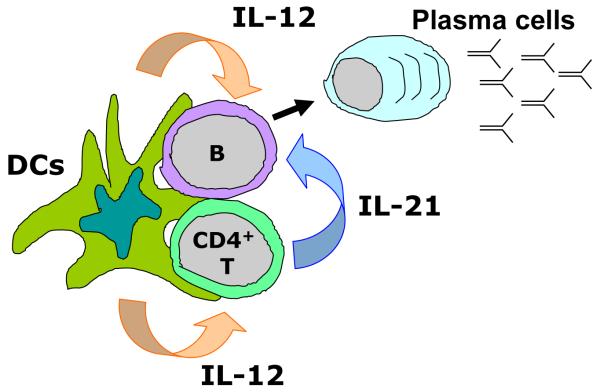
Figure 3. Possible involvement of IL-12 secreted from DCs in the development of antibody responses during DC-T cell-B cell “ménage à trois” complex.
When DCs form the complex with T cells and B cells at extrafollicular sites, IL-12 derived from activated DCs promotes B cells to differentiate into antibody-secreting cells (ASCs) by two different paths: a direct path via DC-B interaction, and an indirect path through induction of IL-21-producing helper cells.
Role of DCs in the development of Tfh cells
Tfh cells express the transcription factor, B cell lymphoma 6 (Bcl-6)60, which is essential for the development of germinal center B cells 61, 62. Recent mouse studies indicated that Bcl-6 is necessary and sufficient for the development of Tfh cells in vivo63-65. On the contrary, Blimp-1, a transcription factor repressing the function of Bcl-6, inhibits the generation of Tfh cells65. Furthermore, down regulation of Blimp-1 in CD4+ T cells promotes their differentiation into Tfh cells. Thus, development of Tfh cells is reciprocally regulated through two transcription factors, Bcl-6 and Blimp-1. However, Bcl-6 does not regulate IL-21 secretion in mouse CD4+ T cells64, 65, in contrast to other transcription factors engaged in the differentiation of other Th subsets, Th1, Th2, and Th17 cells, regulating the secretion of cytokines typical of each subset. How Bcl-6 expression leads to the generation of Tfh cells remains to be established.
Another key question is when and how naïve CD4+ T cells primed by DCs regulate the expression of Bcl-6 and Blimp-1, and commit to the Tfh developmental pathway. Differentiation of naïve CD4+ T cells towards other conventional Th subsets is mainly regulated by the signals that they receive from DCs during cognate interactions. If the generation of Tfh cells shares the same mechanism, which signals from DCs favor the upregulation of Bcl-6 in naïve CD4+ T cells rather than transcription factors associated with other Th subsets? Cytokines inducing IL-21 in CD4+ T cells, i.e., IL-6 in mice64 and IL-12 in humans51, 66, have been shown to upregulate Bcl-6 mRNA expression in activated naïve CD4+ T cells. However, as the induction of Bcl-6 mRNA by these cytokines appears to be transient, other factors derived from DCs might be involved in the regulation of Bcl-6 expression. It is also possible that acquisition of IL-21-producing ability and expression of Bcl-6 are regulated through independent processes, but not consequent events.
Alternatively, CD4+ T cells primed by DCs might regulate the expression of Bcl-6 and Blimp-1 after they encounter with B cells. Mouse studies showed that interaction of primed CD4+ T cells and B cells is essential for the development of Tfh cells in vivo65, 67. Furthermore, mouse CD4+ T cells deficient of the expression of SAP do not differentiate into Tfh cells in vivo, due to the lack of stable interaction with B cells68. This mechanism might also operate in humans, as SAP deficiency results in human X-linked lympho-proliferative disease, where GC formation and humoral immunity is profoundly impaired69, 70. Thus, the major role of DCs in the development of Tfh cells might be the induction of Tfh precursors capable of forming long-lasting interaction with antigen-presenting B cells. There, the location of migratory sites of DCs in secondary lymphoid organs will be critical. Notably, mouse studies showed that dermal DCs upon activation migrate into the outer paracortex just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex71 (Fig. 1). This observation further supports that dermal DCs, rather than LCs, are the major DC subset associated with the development of humoral immunity.
Future directions
Considerable progresses in the knowledge of human DC biology clearly open the avenues for development of novel strategies in clinical interventions. The capacity of LCs and CD14+ dermal DCs to preferentially prime cellular immunity and humoral immunity respectively has significant implications, most particularly in the context of novel human vaccines. Targeting LCs will be important for the design of vaccines that aim at eliciting strong CTL responses, while targeting CD14+ dermal DCs will be optimal to induce strong antibody responses.
Understanding the role of DCs in the regulation of humoral immunity is an underdeveloped research area. Further phenotypical and functional characterization of Tfh cells and their precursors will provide a fertile ground to understand how DCs regulate antibody responses through their development. Of note, Tfh cells appear to be composed of subsets secreting different sets of cytokines72-76, which differentially regulate isotype-switching of B cells. Subsets within Tfh cells are also present in humans (Morita et al. Unpublished observations). Accordingly, DCs stimulated through different paths might induce different Tfh subsets. Establishing the mechanisms whereby the DC system induces Tfh cells with different functions will facilitate the design of novel vaccines. In particular, establishing how DC system generates Tfh subsets associated with the induction of mucosal homing plasma cells will provide a significant insight in the development of novel mucosal vaccines.
Acknowledgments
We thank former and current members of the Institute for their contributions to our progresses. These studies have been supported by the NIH (P01 CA084514, U19 AI057234, U19 AI082715, R01 CA089440 and CA078846), the Dana Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 3.Yamamura M, Uyemura K, Deans RJ, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science (New York, N.Y. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 4.Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–25. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klechevsky E, Liu M, Morita R, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Human immunology. 2009;70:281–8. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, Collin MP, Bogunovic M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–46. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–31. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. The Journal of experimental medicine. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–90. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 11.Flacher V, Bouschbacher M, Verronese E, et al. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–67. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 12.Furio L, Billard H, Valladeau J, Peguet-Navarro J, Berthier-Vergnes O. Poly(I:C)-Treated human langerhans cells promote the differentiation of CD4+ T cells producing IFN-gamma and IL-10. The Journal of investigative dermatology. 2009;129:1963–71. doi: 10.1038/jid.2009.21. [DOI] [PubMed] [Google Scholar]
- 13.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB. TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. The Journal of clinical investigation. 2008;118:3440–52. doi: 10.1172/JCI34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–91. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 15.Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG. Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proceedings of the National Academy of Sciences of the United States of America. 2009 doi: 10.1073/pnas.0911472106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–34. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 17.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 18.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nature immunology. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Seminars in immunology. 2006;18:305–17. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31:491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Caux C, Massacrier C, Vanbervliet B, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–70. [PubMed] [Google Scholar]
- 22.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–39. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 23.Dubois B, Massacrier C, Vanbervliet B, et al. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–31. [PubMed] [Google Scholar]
- 24.Miller JF, Mitchell GF. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. The Journal of experimental medicine. 1968;128:801–20. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLennan IC. Germinal centers. Annual review of immunology. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 26.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annual review of immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 29.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. The Journal of experimental medicine. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. The Journal of experimental medicine. 2001;193:1373–81. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell DJ, Kim CH, Butcher EC. Separable effector T cell populations specialized for B cell help or tissue inflammation. Nature immunology. 2001;2:876–81. doi: 10.1038/ni0901-876. [DOI] [PubMed] [Google Scholar]
- 33.King C, Tangye SG, Mackay CR. T Follicular Helper (T(FH)) Cells in Normal and Dysregulated Immune Responses. Annu Rev Immunol. 2008 doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 34.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–65. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 35.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 36.Cyster JG, Ansel KM, Reif K, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunological reviews. 2000;176:181–93. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 37.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 38.Bryant VL, Ma CS, Avery DT, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–90. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 39.Spolski R, Leonard WJ. Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 40.Kuchen S, Robbins R, Sims GP, et al. Essential role of IL-21 in B cell activation, expansion, and plasma cell generation during CD4+ T cell-B cell collaboration. J Immunol. 2007;179:5886–96. doi: 10.4049/jimmunol.179.9.5886. [DOI] [PubMed] [Google Scholar]
- 41.Nurieva RI, Chung Y, Hwang D, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–49. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–37. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Caprioli F, Sarra M, Caruso R, et al. Autocrine regulation of IL-21 production in human T lymphocytes. J Immunol. 2008;180:1800–7. doi: 10.4049/jimmunol.180.3.1800. [DOI] [PubMed] [Google Scholar]
- 44.Suto A, Kashiwakuma D, Kagami SI, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008 doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 46.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. The Journal of biological chemistry. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 49.Dienz O, Eaton SM, Bond JP, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. The Journal of experimental medicine. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmitt N, Morita R, Bourdery L, et al. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–69. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma CS, Suryani S, Avery DT, et al. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009 doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 52.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi Y, Richards EC, Chen YT, Old LJ. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2219–23. doi: 10.1073/pnas.92.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tahara H, Zitvogel L, Storkus WJ, et al. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol. 1995;154:6466–74. [PubMed] [Google Scholar]
- 55.Motzer RJ, Rakhit A, Thompson JA, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–63. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 56.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–68. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 57.van Herpen CM, van der Voort R, van der Laak JA, et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. International journal of cancer. 2008;123:2354–61. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 58.Germain RN, Bajenoff M, Castellino F, et al. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunological reviews. 2008;221:163–81. doi: 10.1111/j.1600-065X.2008.00591.x. [DOI] [PubMed] [Google Scholar]
- 59.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science (New York, N.Y. 2006;312:1672–6. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 60.Chtanova T, Tangye SG, Newton R, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 61.Ye BH, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–70. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 62.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science (New York, N.Y. 1997;276:589–92. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 63.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–68. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Nurieva RI, Chung Y, Martinez GJ, et al. Bcl6 mediates the development of T follicular helper cells. Science (New York, N.Y. 2009;325:1001–5. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science (New York, N.Y. 2009;325:1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lund R, Ahlfors H, Kainonen E, Lahesmaa AM, Dixon C, Lahesmaa R. Identification of genes involved in the initiation of human Th1 or Th2 cell commitment. European journal of immunology. 2005;35:3307–19. doi: 10.1002/eji.200526079. [DOI] [PubMed] [Google Scholar]
- 67.Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J Immunol. 2007;179:5099–108. doi: 10.4049/jimmunol.179.8.5099. [DOI] [PubMed] [Google Scholar]
- 68.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–9. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purtilo DT, Cassel CK, Yang JP, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan’s disease) Lancet. 1975;1:935–40. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 70.Ma CS, Pittaluga S, Avery DT, et al. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. The Journal of clinical investigation. 2006;116:322–33. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–93. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–7. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206:991–9. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauquet AT, Jin H, Paterson AM, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]