Abstract
Altering the pattern of activation of the ventricle causes remodeling of the mechanical and electrical properties of the myocardium. The electrical remodeling is evident on the surface ECG as significant change in T-wave polarity following altered activation, this phenomenon is ascribed to as “T-wave memory” or “cardiac memory.” The electrophysiological remodeling following altered activation is characterized by distinct changes in regions proximal (early-activated) vs. distal (late-activated) to the site of altered activation. The early-activated region exhibits marked attenuation of epicardial phase 1 notch due to reduced expression of the transient outward potassium current (Ito). This is attributed to electrotonic changes during altered activation, and angiotensin mediated regulation of Kv4.3 (the pore-forming α subunit responsible for Ito). The late-activated region exhibits the most significant action potential prolongation due to markedly increased mechanical strain through a mechano-electrical feedback mechanism. Consequently, regionally heterogeneous action potential remodeling occurs following altered activation. This enhances regional repolarization gradients that underlie the electrophysiological basis for T-wave memory. Further, recent clinical studies highlight detrimental consequences of altered activation including worsening mechanical function and increased susceptibility to arrhythmias. Future studies to identify molecular mechanisms that link electrotonic and mechanical strain induced changes to cellular electrophysiolgy will provide important insights into the role of altered activation in regulating cardiac repolarization and arrhythmogenesis.
Keywords: ECG, T-wave, T-wave memory, electrical remodeling
Altered electrical activation secondary to dysfunction of the cardiac conduction system is a common manifestation of a wide variety of disease processes including ischemia, hypertrophy and heart failure. Ventricular pacing, the therapy for major disorders of the conduction system is also an important cause of altered activation. Altering the activation pattern of the heart due to impaired conduction or pacing remodels myocardial repolarization. This is evident on the surface ECG as marked inversion of T-wave polarity termed “T-wave memory” or “cardiac memory.” The pathological consequences of altered activation include deleterious mechanical and electrical remodeling. The potential adverse clinical consequences of pacing or shock induced mechanical remodeling was first noted in the MADITII trial when patients with defibrillator had increased frequency of hospitalization for CHF despite better survival.1 Electrical remodeling increases mortality and morbidity as noted in patients with atrial fibrillation following AV node ablation2, 3 and in the DAVID trial.4 In contrast, recent clinical studies aimed to synchronize electrical activation in patients with dyssynchrony improves survival.5 These observations highlight the importance of identifying mechanisms that cause electrophysiological remodeling triggered by altered activation. In this review we aim to provide a brief overview of electrophysiological changes that occur due to altered activation and potential mechanisms that underlie these changes.
What is T-wave memory?
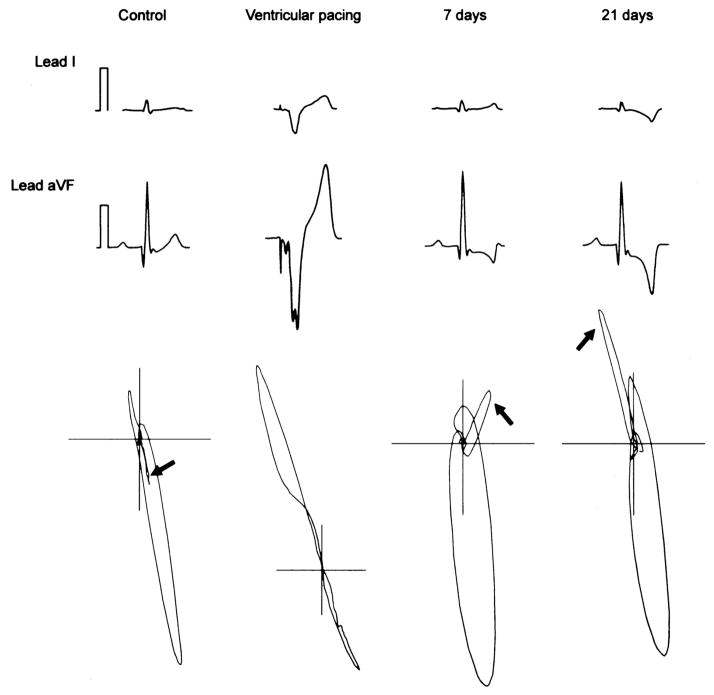
There are several case reports since the 1940’s of marked T-wave polarity changes in patients who present after transient episodes of tachyarrhythmias.6, 7 Since these changes commonly occurred following episodes of tachyarrhythmias they were termed “post-tachycardia T-wave changes.” Mauricio Rosenbaum conducted seminal experiments to explore the electrophysiological basis for T-wave changes and identified several key components involved in this process.8 He first noted that alteration in activation pattern of the ventricle was a more important trigger than change in heart rate in inducing T-wave changes. This is illustrated in figure 1, persistent and progressive chance in T-wave vector and polarity occurs following altered activation in a dog model of ventricular pacing.9, 10 Second, he coined the term “memory,” because the polarity of the T-wave following cessation of altered activation was in the same vector as the QRS during altered activation (figure 1), i.e. the T-wave vector following altered activation remembers the vector of the QRS during altered activation.8 “T-wave memory” or “cardiac memory” is the current clinical nomenclature to describe changes in T-wave vector following altered activation. Finally, the duration and frequency of altered activation also predicted how long the T-wave changes persisted after cessation of altered activation, this phenomenon is also referred to as “accumulation.” A recent clinical study quantitatively demonstrated accumulation in patients who developed T-wave memory following DDD-R pacing.11 T-wave changes lasting minutes to hours are described as short-term memory, commonly observed after brief episodes of tachyarrhythmias. In contrast, patients with temporary cessation of permanent pacing or after ablation of accessory pathway for WPW syndrome exhibit T-wave changes lasting hours to days referred to as long-term memory.12, 13
Figure 1. Time course of cardiac memory.
Surface ECG leads I and avF from a canine model of ventricular pacing to induce memory is illustrated. ECG’s and VCG’s recorded before onset of pacing, during pacing and at 7, 21 days after ventricular pacing are illustrated. The arrows indicate the change in T-wave vector which follows the vector of the paced QRS indicative of memory (adapted from Yu et al.)
What causes the T-wave and how does it change in memory?
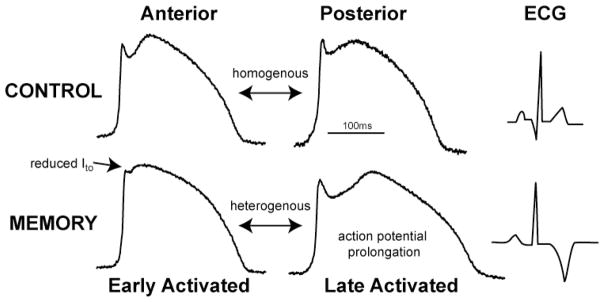
The origin of the normal upright T-wave has been controversial.14 The myocytes that span the transmural surface of the left ventricle exhibit the largest ventricular repolarization gradient in the canine left ventricle.15, 16 This is due to significantly longer action potential in endocardial and M cells compared to the shorter epicardial cells. Therefore, the direction of repolarization which begins in epicardial cells is opposite to the direction of depolarization. These opposing gradients provide the most likely current electrophysiological basis for the normal upright T-wave. Recently, we demonstrated that similar transmural gradients existed in myocardium obtained from multiple (anterior, lateral and posterior) regions of the canine LV.17 In contrast, the difference in action potential duration between the different regions of LV was minimal(figure 2). These data indicate that the most prominent repolarization gradients that cause the T-wave arise from the transmural surface of the left ventricle. The transmural basis for the T-wave was disputed in a recent study that examined in-vivo activation recovery interval (ARI) measurements from several regions of the canine heart. 18 In contrast to previous studies that utilized ex-vivo electrophysiological measurements, 15, 16 in vivo ARI measurements reveal greater regional dispersion compared to transmural dispersion.18 However, we believe these differences are likely related to current limitations in measuring whole heart activation and repolarization under physiological conditions. Further studies that provide high resolution action potential measurements from several regions of the heart are needed to account for all ventricular repolarization gradients that could modulate the T-wave.
Figure 2. Action potential remodeling in cardiac memory.
The top panel illustrates action potentials recorded using optical imaging from unpaced dogs. The action potentials from anterior and posterior walls are similar with minimal regional action potential gradients. In contrast, following memory there is marked action potential prolongation in late-activated region. Also note the significant attenuation of epicardial phase1 notch limited to the early-activated region (arrow). This heterogeneous action potential remodeling causes regional repolarization gradients which underlies the electrophysiological basis for T-wave memory.
To determine the electrophysiological basis for T-wave memory, we developed a pacing-induced model of memory.17 Specifically, we utilized atrial sensing with epicardial LV pacing to study the isolated effects of altered activation without changing the intrinsic heart rate. After 4 weeks of pacing significant change in T-wave polarity was noted indicative of cardiac memory. We examined transmural repolarization gradients in anterior, lateral and posterior regions of the left ventricle. Interestingly, minimal changes were observed in transmural gradients of repolarization after cardiac memory. Next we examined if regional repolarization differences, i.e. repolarization differences between different regions of the LV could account for the T-wave changes in memory. In control hearts, action potential duration was homogenous across multiple LV regions (figure 2), indicating that regional repolarization differences had minimal contribution to the normal T-wave. In contrast, marked regional action potential gradients occurred following cardiac memory (figure 2). Specifically, there was mild action potential prolongation in myocardial region that was close to site of pacing (early-activated). Interestingly, significant action potential prolongation was noted in the myocardial region that was farthest from site of pacing (late-activated). This focal action potential prolongation enhanced regional repolarization gradients in cardiac memory.
We next examined if the heterogeneous action potential remodeling follow in galtered activation could account for the marked T-wave changes in cardiac memory. ECG’s were calculated from action potentials recorded across the transmural surface as well as from different regions of the left ventricle. The transmural changes in repolarization were minimal in memory and did not account for the marked T-wave polarity changes. In contrast, ECG calculated from action potentials recorded from different regions of the left ventricle had marked T-wave polarity change identical to the surface ECG (figure 2). This strongly argues for the significant role of regional action potential gradients following altered activation in modulating T-wave polarity. In contrast to our data, a recent study that measured ARI and monophasic action potentials(MAP) in vivo illustrated changes in transmural action potential gradients and minimal changes in regional gradients after induction of long-term cardiac memory.19 In contrast to our study, ventricular pacing was performed at a rate of ~120/min to induce memory for a period of three weeks from the lateral wall of the LV (early-activated). Consequently, the inter-ventricular septum would be the late-activated region and predicted to exhibit the most action potential prolongation. However, ARI’s were not measured in the septum leading to a possible underestimation of the regional repolarization gradients after memory. Further, the onset and time course of remodeling in the late-activated segment remains to be fully characterized. Despite these differences, a common observation from the above studies is enhanced regional repolarization gradients as the electrophysiological basis for memory.
What are mechanisms that regulate cardiac memory?
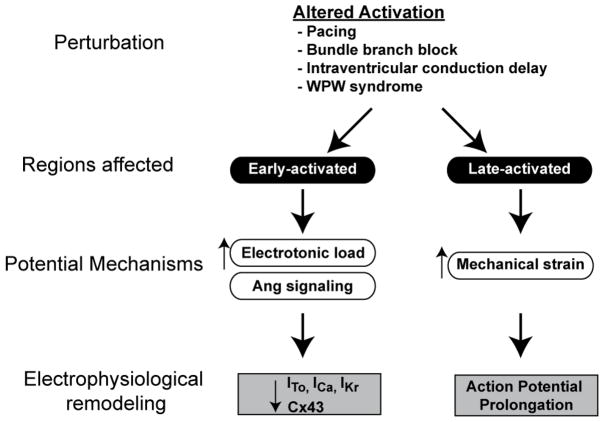
Several mechanisms have been proposed to underlie development of cardiac memory (summarized in figure 3). The first described mechanism was based on electrotonic flow dependent changes in membrane potential in the isolated guinea pig heart.20 This is caused by fully depolarized myocytes downstream of the site of altered activation that maintain and extend the action potential plateau of upstream cells. In accordance with this effect, action potential prolongation was noted in myocytes proximal to site of pacing, the action potential duration gradually shortened in the direction of propagation. 20 Further, electrotonic load changes were shown to modulate expression of transient outward current in short-term memory.21 One of the earliest electrophysiological events that occurs within minutes of altered activation is reduced density of transient outward potassium current.22 It is therefore plausible that remodeling of this current plays an important role in short-term memory. This phenomenon is also evident in hearts isolated from dogs subjected to 3 weeks of ventricular pacing; however, other mechanisms are operative in long-term memory discussed below.10, 17 The molecular mechanisms that link altered electrotonic load to reduced expression of sarcolemmal ion channels remains to be explored.
Figure 3. Summary of current understanding of pathophysiology and mechanisms that regulate cardiac memory.
The common sources of altered activation include diseases that affect the cardiac conduction system, accessory pathways and ventricular pacing. Two distinct anatomical regions are affected based on proximity to origin of altered activation. Early-activated region, i.e. region proximal to the site of altered activation exhibits attenuation of epicardial phase1 notch due to reduced Ito, reduced ICa/IKr and reduced connexin43 expression. This is attributed to electronic changes and Angiotensin II medicated signaling. The late-activated region exhibits marked action potential prolongation due to enhanced mechanical strain.
Angiotensin II receptor mediated signaling has been proposed to regulate development of cardiac memory.23, 24 Angiotensin II type 1 receptor blockade attenuates development of short-term memory in dog, however, minimal effects were noted in progression of long-term memory.24 Angiotensin II mediated signaling regulates Ito by regulating cell-surface expression of Kv4 channels25 and stability of the Kv4.3 mRNA.26 Since Ito is a major ionic remodeling in short-term memory, angiotensin II signaling is likely to have a major role in short-term memory. The role of Angiotensin II dependent mechanisms in long-term memory remains to be fully evaluated.
The most recent mechanism attributed to cardiac memory is mechanical-strain induced changes in action potential.17 We recently measured action potential remodeling following altered activation in several ventricular regions, the most prominent action potential remolding occurred in late-activated myocardial region. This paradoxical prolongation could not be accounted for by traditional biophysical principles that govern impulse conduction and repolarization in the heart. Prior studies have implicated an important role for altered activation in changing the myocardial strain pattern.27 Therefore, we examined using tagged MR imaging if marked action potential remodeling in late-activated region could be caused by mechanical strain. Indeed, we noted that late-activated myocardial regions experienced markedly increased mechanical strain. Further, mechanical stretch mediated electrophysiological remodeling and memory was also illustrated in a rabbit model of short-term memory.28 In summary, altered activation causes heterogeneous myocardial strain due to slow cell-to-cell conduction, this induces heterogeneous action potential remodeling through electrotonic and mechano-electric feedback mechanisms.
What are cellular electrophysiological changes that occur in cardiac memory?
The ionic current that has been extensively studied in cardiac memory is the transient outward potassium current(Ito).22 Seminal work from Dr. Rosen’s laboratory has demonstrated the time-course of Ito changes and molecular mechanisms that regulate Ito remodeling in memory.22 Pharmacological blockade of Ito attenuates development of short-term memory. Further, transient outward current is reduced in myocardial region close to site of altered activation within minutes of altered activation.29 This is due to altered gating of Kv4.3, as well as reduced expression of Kv4.3 and it’s regulatory β subunit KChIP2.9, 30 Recently, CREB dependent modulation of Kv4 expression was proposed as a molecular mechanism for reduced Ito proximal to site of pacing. Importantly, the phase1 action potential notch caused by Ito in epicardial cells is unchanged in myocardial regions that are distal to site of altered activation (Figure 2).17 Therefore, transient outward current remodeling is likely represents a local electrophysiological remodeling in memory.
Changes in L-type calcium current (ICa) and rapidly activated delayed rectifying potassium current (IKr) are also described in myocytes isolated from early-activated region in cardiac memory.24, 31 Interestingly, pharmacological blockade of L-type calcium current was shown to attenuate development of both short and long-term cardiac memory.24 Reduced epicardial expression of rapidly activated delayed rectifying potassium current with reduction in transmural gradient in IKr was also observed in cardiac memory.31 Further studies to explore changes in other ionic currents are needed to account for remodeling in both early and late-activated regions in cardiac memory.
Altered connexin expression was first described in the early-activated region with local reduction in conduction velocity.32 Connexin43(Cx43), the main gap junction protein in the ventricular myocardium plays an important role in cell-to-cell coupling. Altered Cx43 expression causes conduction slowing, enhances repolarization gradients and increases susceptibility to arrhythmia in heart failure.33 Cx43 expression was examined in the late-activated region of the canine dyssynchronous pacing induced heart failure model.34 In this model, experimental left bundle branch block was used to examine remodeling in early-activated(septum) and late-activated ventricular regions (lateral wall). In late-activated regions there was no reduction in expression of Cx43, however, redistribution of connexins to the lateral wall of the myocytes termed “lateralization” was observed with reduced conduction velocity.34 Therefore, altered connexin expression with regional changes in conduction likely plays a role in enhancing regional repolarization gradients observed in memory. To summarize, the pattern of sarcolemmal ion channel and connexin remodeling are dependent on the anatomical location of the myocardium remodeled with respect to the origin of altered activation.
What are clinical implications of altered activation?
The deleterious consequences of altered activation are related to adverse electrical and mechanical remodeling of the ventricle. The detrimental electrical remodeling was first evident in patients who had increased susceptibility to ventricular arrhythmias following AV-node ablation for atrial fibrillation.2, 3 These patients had a long period of irregular tachycardia preceding AV-node ablation, following ablation they were programmed to a slow ventricular paced rhythm. The adverse electrical remodeling during atrial fibrillation followed by persistent bradycardia created a milieu for development of ventricular arrhythmias. Further, epicardial pacing was also demonstrated to increase dispersion of repolarization and increase susceptibility to arrhythmias.35 In a recent clinical study which examined occurrence of cardiac memory patients with ventricular pacing for sick-sinus syndrome, increased repolarization heterogeneity was noted indicative of adverse electrical remodeling.36 The adverse mechanical consequences of altered activation were noted in the MADITII trial, despite improved survival in patients who received defibrillator therapy there was increased incidence of heart failure hospitalization.1 The DAVID trial was designed to test the hypothesis that therapeutic use of beta-blockade by maintaining physiological heart rates with ventricular pacing would improve survival in patients with heart failure.4 However, contrary to traditional wisdom patients with ventricular pacing had higher incidence of heart failure and increased cardiac mortality.
The adverse mechanical remodeling of altered activation is thought to arise from dyssynchronous activation of the ventricle. Dyssynchronous activation reduces the mechanical efficiency of the ventricle and activates a cascade of signaling pathways that cause adverse structural remodeling.37, 38 Recent therapeutic maneuvers using bi-ventricular pacing are aimed to synchronize electrical activation and reverse adverse structural remodeling.37, 38 This was initially reported in the MIRACLE trial in 200239 and the Companion trial in 200440 which showed significant improvement in functional capacity, quality of life, exercise tolerance as well as improved echo markers in patients with synchronous LV activation with Bi V pacing. Similar observations were made in the Care-HF trial when synchronous activation of the LV improved not only symptoms but also survival in patients with heart failure.5 More recent clinical evidence suggests that specifically implanting the left ventricular lead in the late-activated region produces the most clinical benefit in patients who receive a Bi-V pacemaker.41
Finally, it is currently assumed that electrical remodeling follows occurrence of structural remodeling in various forms of cardiac pathology. In our recent study we observed marked electrical remodeling one month after altered activation in the absence of overt structural remodeling.17 This illustrates that electrical remodeling precedes onset of structural remodeling and could play an important role in triggering cellular events that cause adverse structural remodeling. Identification of pathways that regulate this event will provide insights into mechanisms that regulate electrical remodeling and novel ways to prevent it.
Acknowledgments
This study was supported by National Institutes of Health grants RO1-HL54807 (David S. Rosenbaum), and Heart Rhythm Fellowship in pacing and cardiac electrophysiology (Darwin Jeyaraj).
References
- 1.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Geelen P, Brugada J, Andries E, Brugada P. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol. 1997;20(2 Pt 1):343–8. doi: 10.1111/j.1540-8159.1997.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 3.Darpo B, Walfridsson H, Aunes M, Bergfeldt L, Edvardsson N, Linde C, Lurje L, et al. Incidence of sudden death after radiofrequency ablation of the atrioventricular junction for atrial fibrillation. Am J Cardiol. 1997;80(9):1174–7. doi: 10.1016/s0002-9149(97)00635-8. [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Jama. 2002;288(24):3115–23. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 6.Currie GM. Transient Inverted T Waves after Paroxysmal Tachycardia. Br Heart J. 1942;4(4):149–52. doi: 10.1136/hrt.4.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell M. Inversion of T Waves after Long Paroxysms of Tachycardia. Br Heart J. 1942;4(1–2):49–56. doi: 10.1136/hrt.4.1-2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenbaum MB, Blanco HH, Elizari MV, Lazzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50(2):213–22. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 9.Yu H, McKinnon D, Dixon JE, Gao J, Wymore R, Cohen IS, Danilo P, Jr, et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation. 1999;99(14):1898–905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 10.Shvilkin A, Danilo P, Jr, Wang J, Burkhoff D, Anyukhovsky EP, Sosunov EA, Hara M, et al. Evolution and resolution of long-term cardiac memory. Circulation. 1998;97(18):1810–7. doi: 10.1161/01.cir.97.18.1810. [DOI] [PubMed] [Google Scholar]
- 11.Wecke L, Gadler F, Linde C, Lundahl G, Rosen MR, Bergfeldt L. Temporal characteristics of cardiac memory in humans: vectorcardiographic quantification in a model of cardiac pacing. Heart Rhythm. 2005;2(1):28–34. doi: 10.1016/j.hrthm.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Takada Y, Inden Y, Akahoshi M, Shibata Y, Shimizu A, Yoshida Y, Yamada T, et al. Changes in repolarization properties with long-term cardiac memory modify dispersion of repolarization in patients with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2002;13(4):324–30. doi: 10.1046/j.1540-8167.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 13.Helguera ME, Pinski SL, Sterba R, Trohman RG. Memory T waves after radiofrequency catheter ablation of accessory atrioventricular connections in Wolff-Parkinson-White syndrome. J Electrocardiol. 1994;27(3):243–9. doi: 10.1016/s0022-0736(94)80008-1. [DOI] [PubMed] [Google Scholar]
- 14.Opthof T, Coronel R, Wilms-Schopman FJ, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Rosen MR, et al. Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4(3):341–8. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol. 2001;96(6):517–27. doi: 10.1007/s003950170002. [DOI] [PubMed] [Google Scholar]
- 16.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation. 1998;98(18):1921–7. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 17.Jeyaraj D, Wilson LD, Zhong J, Flask C, Saffitz JE, Deschenes I, Yu X, et al. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115(25):3145–55. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 18.Janse MJ, Sosunov EA, Coronel R, Opthof T, Anyukhovsky EP, de Bakker JM, Plotnikov AN, et al. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation. 2005;112(12):1711–8. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]
- 19.Coronel R, Opthof T, Plotnikov AN, Wilms-Schopman FJ, Shlapakova IN, Danilo P, Jr, Sosunov EA, et al. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74(3):416–25. doi: 10.1016/j.cardiores.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Costard-Jackle A, Goetsch B, Antz M, Franz MR. Slow and long-lasting modulation of myocardial repolarization produced by ectopic activation in isolated rabbit hearts. Evidence for cardiac “memory”. Circulation. 1989;80(5):1412–20. doi: 10.1161/01.cir.80.5.1412. [DOI] [PubMed] [Google Scholar]
- 21.Libbus I, Wan X, Rosenbaum DS. Electrotonic load triggers remodeling of repolarizing current Ito in ventricle. Am J Physiol Heart Circ Physiol. 2004;286(5):H1901–9. doi: 10.1152/ajpheart.00581.2003. [DOI] [PubMed] [Google Scholar]
- 22.del Balzo U, Rosen MR. T wave changes persisting after ventricular pacing in canine heart are altered by 4-aminopyridine but not by lidocaine. Implications with respect to phenomenon of cardiac ‘memory’. Circulation. 1992;85(4):1464–72. doi: 10.1161/01.cir.85.4.1464. [DOI] [PubMed] [Google Scholar]
- 23.Ricard P, Danilo P, Jr, Cohen IS, Burkhoff D, Rosen MR. A role for the renin-angiotensin system in the evolution of cardiac memory. J Cardiovasc Electrophysiol. 1999;10(4):545–51. doi: 10.1111/j.1540-8167.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 24.Plotnikov AN, Yu H, Geller JC, Gainullin RZ, Chandra P, Patberg KW, Friezema S, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107(22):2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86(10):1062–8. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 26.Zhou C, Ziegler C, Birder LA, Stewart AF, Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98(8):1040–7. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33(6):1735–42. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2008;5(1):106–13. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Libbus I, Rosenbaum DS. Transmural action potential changes underlying ventricular electrical remodeling. J Cardiovasc Electrophysiol. 2003;14(4):394–402. doi: 10.1046/j.1540-8167.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- 30.Patberg KW, Obreztchikova MN, Giardina SF, Symes AJ, Plotnikov AN, Qu J, Chandra P, et al. The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovasc Res. 2005;68(2):259–67. doi: 10.1016/j.cardiores.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Obreztchikova MN, Patberg KW, Plotnikov AN, Ozgen N, Shlapakova IN, Rybin AV, Sosunov EA, et al. I(Kr) contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71(1):88–96. doi: 10.1016/j.cardiores.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Patel PM, Plotnikov A, Kanagaratnam P, Shvilkin A, Sheehan CT, Xiong W, Danilo P, Jr, et al. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12(5):570–7. doi: 10.1046/j.1540-8167.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 33.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287(4):H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 34.Spragg DD, Akar FG, Helm RH, Tunin RS, Tomaselli GF, Kass DA. Abnormal conduction and repolarization in late-activated myocardium of dyssynchronously contracting hearts. Cardiovasc Res. 2005;67(1):77–86. doi: 10.1016/j.cardiores.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Medina-Ravell VA, Lankipalli RS, Yan GX, Antzelevitch C, Medina-Malpica NA, Medina-Malpica OA, Droogan C, et al. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107(5):740–6. doi: 10.1161/01.cir.0000048126.07819.37. [DOI] [PubMed] [Google Scholar]
- 36.Wecke L, Rubulis A, Lundahl G, Rosen MR, Bergfeldt L. Right ventricular pacing-induced electrophysiological remodeling in the human heart and its relationship to cardiac memory. Heart Rhythm. 2007;4(12):1477–86. doi: 10.1016/j.hrthm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49(1):26–41. doi: 10.1016/j.pcad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dimaano VL, Lardo AC, et al. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation. 2008;117(11):1369–77. doi: 10.1161/CIRCULATIONAHA.107.706291. [DOI] [PubMed] [Google Scholar]
- 39.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 40.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Cardon P, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 41.Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, et al. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52(17):1402–9. doi: 10.1016/j.jacc.2008.06.046. [DOI] [PubMed] [Google Scholar]