Abstract
Purpose
We tested the hypothesis that modulation of the microenvironment (using antioxidants) will increase stem cell survival in hypoxia and after transplantation to the myocardium.
Procedures
Rat cardiomyoblasts were stably transfected with a reporter gene (firefly luciferase) for bioluminescence imaging (BLI). First, we examined the role of oxidative stress in cells under hypoxic conditions. Subsequently, stem cells were transplanted to the myocardium of rats using high-resolution ultrasound, and their survival was monitored daily using BLI.
Results
Under hypoxia, oxidative stress was increased together with decreased cell survival compared to control cells, both of which were preserved by antioxidants. In living subjects, oxidative stress blockade increased early cell survival after transplantation to the myocardium, compared to untreated cells/animals.
Conclusion
Modulation of the local microenvironment (with antioxidants) improves stem cell survival. Increased understanding of the interaction between stem cells and their microenvironment will be critical to advance the field of regenerative medicine.
Keywords: Bioluminescence, Firefly luciferase, Myoblasts, Molecular imaging, Stem cell, Oxidative stress, Myocardium, Antioxidants
Introduction
Coronary artery disease (CAD) is the leading cause of death in the United States [1]. Despite advances in medical (e.g., beta blockers, angiotensin-converting enzyme inhibitors, and statins) and interventional therapies (e.g., stents), it continues to be a cause of significant morbidity and mortality [2]. Thus, novel therapies may be needed to reinstitute coronary flow and cardiac function in certain situations.
Over the last few years, stem cell therapy has emerged as a therapeutic potential alternative for treatment of CAD [3]. However, significant questions remain regarding the biology of transplanted cells [4, 5]. Almost invariably, transplanted cells undergo a significant rate of cell death shortly after transplantation, approaching 90% within the first 24 h after transplantation [6–8]. Thus, significant efforts have been placed in identifying the biological factors that may play a role in the substantial cell death observed after transplantation [9, 10]. A more clear understanding of the biological pathways that regulated stem cell survival may likely lead to novel and improved cell therapies.
After they are transplanted to the myocardium, stem cells face an unfamiliar and likely noxious microenvironment, which may contribute to the increased cell death observed after transplantation [8]. Previous studies have shown that modulation of the myocardial microenvironment leads to increased cell survival [11, 12]. One of mechanisms that can modulate the local microenvironment is that of increased oxidative stress. Increased oxidative stress, an imbalance between pro-oxidants and antioxidants substances, has been demonstrated in hypoxic/ischemic states and can be deleterious to the local microenvironment [13–16], potentially affecting cell homing and survival. Using a model of hindlimb ischemia, Napoli et al. showed that bone marrow stem cell survival was improved when mice received an antioxidant therapeutic regimen [17]. These data suggest that modulation of the microenvironment, in particular maintaining oxidative status balance, may play a role in stem cell survival after transplantation to the living subject.
Until recently, stem cell studies have been limited in the capacity to assess cell survival, either in cell culture or in living subjects. Most studies on stem cell biology relied on traditional ex vivo assays and invasive molecular techniques (e.g., histology, western blotting, etc.), which are limited by their invasiveness and the number of time points that can be studied in any given subject [6]. This, in turn, might limit the ability to identify interim/temporal changes in stem cell biology in living subjects. Novel developments in non-invasive imaging have allowed us to study transgene expression and kinetics of cell therapy in living subjects, using imaging modalities such as bioluminescence imaging (BLI) [18–21]. Our laboratory has previously demonstrated that cell survival can be monitored longitudinally after transplantation to both the myocardium [7, 22] and peripheral muscle [23]. Use of these novel imaging modalities provides a unique opportunity to better understand the biology of stem cells in living subjects, which may lead to better and more optimized therapy.
Thus, we tested the hypothesis that modulation of the microenvironment, through the use of antioxidants, will increase cell survival in hypoxia and after transplantation to the myocardium, and that this effect can be detectable noninvasively using BLI.
Material and Methods
Experimental Approach
H9c2 rat cardiomyoblasts were stably transfected to express firefly luciferase, a bioluminescence reporter gene. Studies were divided into: cell culture studies and studies in living subjects. For cell culture studies, cells were divided into three groups: A control, B hypoxia, and C hypoxia+antioxidant-treated cells (AO). For the studies in living subjects, cells were delivered to untreated animals (control) or treated animals (AO).
Cell Culture Studies
Development of a Stable Cell Line Expressing Firefly Luciferase
Embryonic rat H9c2 cardiomyoblasts (American Type Culture Collection, Manassas, VA, USA) were grown in culture using Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA) complemented with 10% fetal bovine serum and 1% penicillin and streptomycin, as previously described [22, 23]. Cells were stably transfected with plasmids carrying the fluc gene driven by the cytomegalovirus (CMV) promoter (H9c2-fluc) and the antibiotic resistance gene G418. After antibiotic selection, high-expressor clones, assessed by firefly luciferase protein activity and detected by CCD camera, were isolated, grown, and used for the study [23]. To determine if stable transfection of H9C2 cells altered the proliferation of these cells, both H9c2 and H9c2-fluc cells were simultaneously plated in replicate 10-cm culture plates (150,000 cells per plate), and all plates were incubated together. One plate of each cell type was trypsinized and counted each day for 4 days. Plates were trypsinized in 2 ml of trypsin (0.05%) and counted a minimum of four times on a hemacytometer slide. The average total number of cells per plate was plotted versus time for each day of counting.
Role of Hypoxia on Cell Viability
Embryonic rat H9c2 cardiomyoblasts were plated in 96-well plates (1.5×104 cells per well, n=8 per group) and incubated in a hypoxic chamber (1%O2, 4%CO2, 95% N2) for 24 h. To block oxidative stress during this period, cells were simultaneously incubated with increasing doses of the superoxide dismutase mimetic Tempol [24] (AO group: 1, 2.5, 5, and 10 mm/L for 4 h) [25]. Untreated cells under hypoxic conditions served as controls. After 24 h of hypoxia, cell viability was tested with the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenylte-trazolium Bromide (MTT) assay [26]. Briefly, cells were incubated with the MTT reagent for 3 h, after which a solvent (0.4 N HCl isopropanol) was added, and plates were placed on a shaker at room temperature for 4 h. Lastly, absorbance was read in a spectrophotometer at a wavelength of 590 nm.
To assess if apoptosis played a role in hypoxia, Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using the Roche tetramethylrhodamine (TMR) red in situ cell death detection kit according to manufacturer’s directions (Roche, Indianapolis, IN). Briefly, cells were plated on chamber slides at 55,000 cells per well, fixed in 4% paraformaldehyde, and permeabilized for 2 min with 0.1% Triton X-100 in 0.1% sodium citrate. Positive controls were incubated with DNase I for 10 min at room temperature. Cells from the different groups were then incubated with the TUNEL solution (except for negative control, which was incubated with the labeling solution except for the enzyme) for 1 h in a dark, humidified chamber. Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and then visualized on an LSM 510 confocal microscope (DAPI: excitation of 364 and emission of 385–470 nm, TUNEL: excitation 563 and emission of 560–615 nm) at ×10 magnification.
Assessment of Oxidative Stress
Production of endogenous oxidative stress by-product hydrogen peroxide (H2O2) was assessed using the conversion of 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA, Molecular Probes, Eugene, OR) [27]. Briefly, cells were plated in 12-well plates (70,000 per well) in DMEM. Twenty-four hours later, experimental plates were placed in hypoxia chambers (1% O2, 4% CO2,, 95%N2) and incubated for 24 h. Increasing doses of tempol were added to the experimental plates before hypoxia. Experiments were performed in tetraplicates. Plated cells were washed with phosphate-buffered saline (PBS) and exposed to DCHFDA (5 μM/L) in PBS for 30 min at 37°C. Surface fluorescence was minimized by washing the slides with DMEM. Control plates were kept in 37°C incubators under normoxic conditions. Visual assessment of DCHFDA was performed using an inverted fluorescence microscope (Nikon EFD-3, Nikon Inc., Melville, NY, USA); excitation: 480 nm, emission: 510 nm. For quantification of H2O2 production, cells from the different groups were lysed using passive lysis buffer (Promega, Madison, WI, USA). The cell lysate was removed and centrifuged at 4°C at 13,000 rpm, and the DCHFDA fluorescence in the supernatant was read with an excitation wavelength of 480 nm and emission at 510 nm on a Spectramax Gemini EM (Molecular Devices, Sunnyvale, CA, USA) plate reader. To normalize fluorescence per cell, protein was determined by Coomassie Plus Assay (Pierce, ThermoFisher Inc. Rockford, IL, USA) following manufacturer’s directions. Fluorescence was then normalized by dividing the optical signal by the protein content and expressed as fluorescence per microgram of protein.
To assess the production of reactive oxygen species (ROS), we measured the conversion of dihydroethidium (DHE) [28–30]. Cells from the different groups were plated 15,000 per well on eight-well chamber slides in DMEM containing 10% fetal bovine serum and 1% antibiotics (penicillin and streptomycin) and no phenol red. Twenty four hours after platting, cells from the experimental groups (hypoxia and hypoxia + tempol) were incubated under hypoxic conditions (1% O2, 4% CO2, 95% N2) and compared to controls. After 24 h of hypoxia, production of ROS was tested. Briefly, cells were rinsed with PBS, and a solution of 5 μM DHE (Invitrogen, Carlsbad, CA) in PBS was then added to each well. Slides were placed in the dark in a 37°C incubator. After 30 min, cells were rinsed three times with PBS and then returned to the incubator (at 21% O2) in DMEM for a 1-h period to allow for the conversion of the DHE, a process that is mediated by ROS [31–33]. After 1 h, cells were fixed with 4% paraformaldehyde and counterstained for 5 min with DAPI, coverslips were mounted with ProLong Gold (Invitrogen, Carlsbad, CA) and visualized on a Zeiss Axiovert LSM 510 inverted confocal fluorescent microscope (Carl Zeiss, Inc., Oberkochen, Germany, excitation: 480–530 nm and emission: 567–610 nm). DHE fluorescence was analyzed with ImageJ (NIH, Bethesda, MD) using a minimum of four representative images from each well, four wells per group (n=16 in each group). The threshold for each individual image was obtained (to minimize noise and increase signal to noise ratio), and percentage area of field showing red fluorescence (after threshold correction) was calculated for each image. DAPI-stained nuclei were counted manually for each image, and the percentage area was normalized by dividing by the number of nuclei in each field (to reflect the amount of staining per cell).
Western Blotting
Western blotting was performed following standard protocols [34, 35]. Equal amounts of cell lysates (25 μg protein) were loaded onto 10% polyacrylamide gel electrophoresis gels which were electrophoresed for 90 min at 90 V in Tris–glycine–sodium dodecyl sulfate (SDS) buffer, then transferred to Polyvinylidene fluoride (PVDF) membranes in Tris–glycine–SDS–20% methanol at 100 V for 1 h in ice bath. Membranes were blocked for 1 h in Tris-Buffered Saline Tween-20 (TBST) containing 5% milk (TBST: milk) followed by overnight incubation on rocker at 4°C with primary Ab diluted in TBST:milk. Antibodies used included NAD(P)H p22phox, NAD(P)H p47phox, and NAD(P)H gp91phox (Santa Cruz Biotechnology, Santa Cruz, CA, dilution 1:200), MN-superoxide dismutase (SOD), and Cu-Zn–SOD (Santa Cruz Biotech., Santa Cruz, CA, dilution 1:200), and β-actin (Abcam, Cambridge, MA, 1:2000). Following incubation with primary antibody, membranes were washed once for 5 min, then three times for 10 min each in TBST and then incubated on rocker for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody diluted 1:5000 in TBST:milk. Wash steps were repeated as above, and then membranes were incubated for 5 min. with SuperSignal West Pico chemiluminescent substrate per manufacturer’s instructions and imaged by 5 min (or 15 s for β-actin) film exposure. Band densities were analyzed by ImageJ and normalized to corresponding β-actin band densities.
Assessment of Cell Survival Using Reporter Genes in Cell Culture
To further characterize the effect of tempol on cell survival in a state of increased oxidative stress and to corroborate that reporter genes can be used to evaluate the effect of modulation of the microenvironment on cell survival, we assessed cell viability with BLI using a charge-cooled coupled device (CCD) camera. We have previously shown that light emission correlates with the number of cells [22], so it can be used as a measure of number of viable cells and thus cell survival.
Briefly, H9c2-fluc cells were plated in 12-well plates (7×104 per well), and 24 h later, cells from the experimental groups were placed in hypoxia following the protocol described above and compared to control. After 24 h of either hypoxia or control conditions, medium was removed from all wells, wells were washed with 1 mL of PBS, and 1 μg/mL of D-luciferin was added to each well. Experiments performed in tetraplicates. Plates were imaged with a CCD camera in a 1 min high-sensitivity acquisition format. For analysis of detected light, a grid was constructed that measures total flux (in photons per second).
To rule out any effect of tempol on the light emission of the stably transfected H9c2-fluc, cells were plated in six-well plates (1×105 cells per well). Twenty-four hours after platting, cells were exposed to tempol and imaged using BLI (as described above).
Studies in Living Subjects
Ultrasound-Guided Intramyocardial Transplantation of Embryonic H9c2 Cardiomyoblasts
Protocols were approved by the Stanford Animal Research Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (publication no. 85-23, revised 1996). Stem cell transplantation was performed as previously described from our laboratory [22]. Briefly, female rats (Charles River Laboratories, Wilmington, MA, USA) weighing 140–150 g were divided into two groups (n=7 each): control and AO. In preparation for these studies, control animals received regular tap water, while animals in the AO group received tempol in the drinking water (10 mm/L) starting 24 h before cell transplantation. Cells were plated Twenty four hours before transplantation cells were incubated with either regular medium (control) or regular medium + tempol (10 mM/L, AO group). On the day of the study, cells were harvested and resuspended in 40 μl of either PBS or PBS + tempol (10 mM/L), corresponding to control and AO groups, respectively.
On the day of the study, animals were anesthetized with 2% isoflurane, their anterior chest was shaved, and the animals were positioned on the ultrasound scanning table [22]. EKG and temperature were monitored throughout the experiment. Using a dedicated small animal high resolution ultrasound system (VeVo 770; Visualsonics, Inc., Toronto, ON, Canada), parasternal long axis images were obtained. A high resolution ultrasound probe (frequency of 30 MHz) was used for cardiac imaging and guided cell delivery. Global left ventricular ejection fraction (before and after stem cell transplantation) was estimated using the parasternal long axis view of the left ventricle (LV). After identification of a proper ultrasound (US)-scanning window (most commonly the parasternal long axis view), cell transplantation was performed to the anterior cardiac wall (via manual injection using a 28-gauge needle). Animals were then observed and monitored until recovery for approximately 10 min after cell transplantation.
In a separate set of animals, cells from both groups (control and AO, n=2 each) were labeled ex vivo with the fluorescent dye DiI (5 μl/mL medium for 30 min) and transplanted to the myocardium. Six hours after transplantation, animals were euthanized, and hearts were harvested. Myocardial frozen sections were mounted in optimum cutting temperature (OCT, Sakura Tissue-Tek), and 5-μm sections were obtained and visualized under the microscope (Nikon EFD-3, Nikon Inc. Melville, NY, USA) using a Texas Red filter.
Optical Bioluminescence Imaging of Cardiomyoblast Transplantation
Animals were imaged immediately after cell transplantation (day 0), 6 h after transplantation, and then daily until no BLI signal was detected. BLI was performed using a cooled CCD camera (Xenogen, Alameda, CA, USA) [7, 22]. After intraperitoneal injection of the reporter substrate D-luciferin (250 mg/kg of body weight), rats were imaged for 30 min using 5-min acquisition scans. Bioluminescence was quantified as maximal radiance in photons/s/cm2/sr.
Statistical Analysis
Data are given as means±standard error of the mean(SEM). Comparisons were performed using unpaired Student’s t test of unequal variance. Statistical significance was accepted for p<0.05.
Results
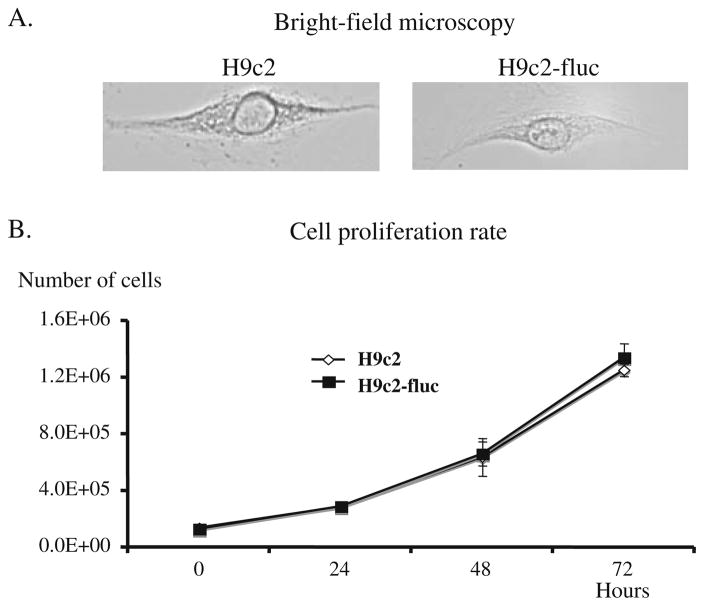
Stable clones of h9c2 rat cardiomyoblasts carrying the fluc gene, driven by the CMV promoter, were created using an antibiotic selection strategy [22]. Over the period examined (5 days), there was no difference in the cell gross morphology or proliferation rate (as measured by cell counting in a hemacytometer) of labeled and unlabeled cells (H9c2-fluc and H9c2, respectively, Fig. 1), suggesting that introduction of reporter genes did not significantly affect the phenotype or dividing capacity of cardiomyoblasts in our study.
Fig. 1.
Cell proliferation rate of rat cardiomyoblasts (H9c2) and H9c2 carrying the firefly luciferase gene driven by the cytomegalovirus promoter (H9c2-CMV-fluc). There were no significant changes in gross morphology (a) or proliferation capacity (b) of rat cardiomyoblasts between the groups.
Effect of Hypoxia on Cell Viability and Survival
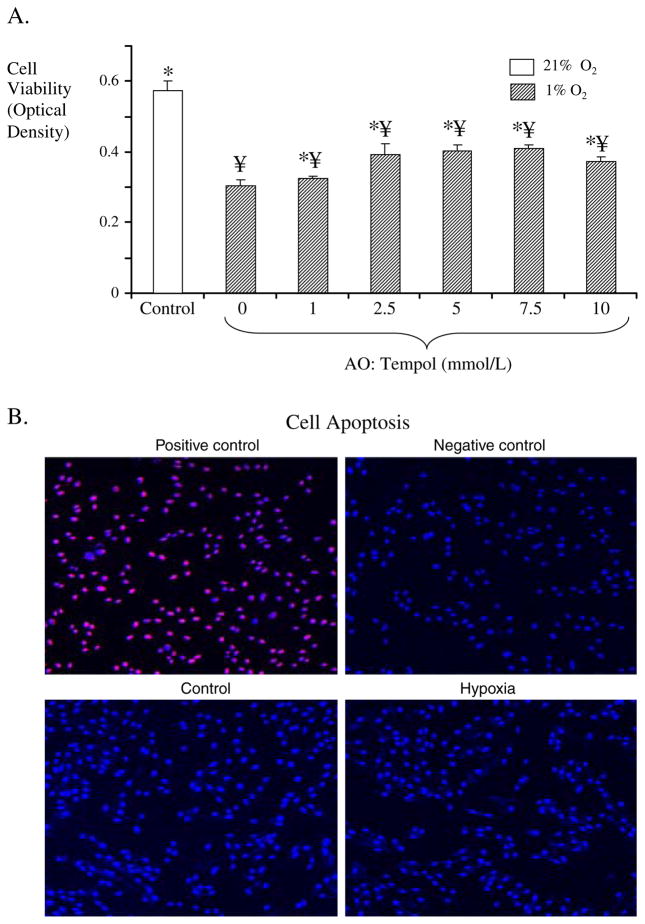
We examined whether hypoxic conditions (condition that stem cells may encounter in vivo) affect cell survival. When exposed to hypoxic conditions (1% O2), cells had a decrease in survival compared to cells that were maintained under standard cell culture conditions (Fig. 2). As expected, a hypoxic microenvironment resulted in decreased cell viability (as assessed by the MTT assay, Fig. 2a). However, when cells (under hypoxic conditions) were coincubated with the superoxide dismutase mimetic Tempol, cell viability was improved in a dose-dependent manner (Fig. 2a). The changes in cell viability were not related to increased apoptosis, as no differences in TUNEL staining were observed between the groups (Fig. 2b).
Fig. 2.
a Cell survival (assessed using the MTT assay) under hypoxic conditions (1% O2, 4% CO2, 95% N2), in the absence and presence of oxidative stress blockade (Tempol, 0–10 mm/L). Cells in which oxidative stress was blocked had increased survival compared to untreated cells. ¥p<0.05 compared to control, *p<0.05 compared to hypoxia. b TUNEL staining for assessment of apoptosis in control cells and cells after hypoxia. Positive and negative controls are also shown. As shown in the representative slides, this level of hypoxia did not result in an increase in apoptosis.
Assessment of Oxidative Stress
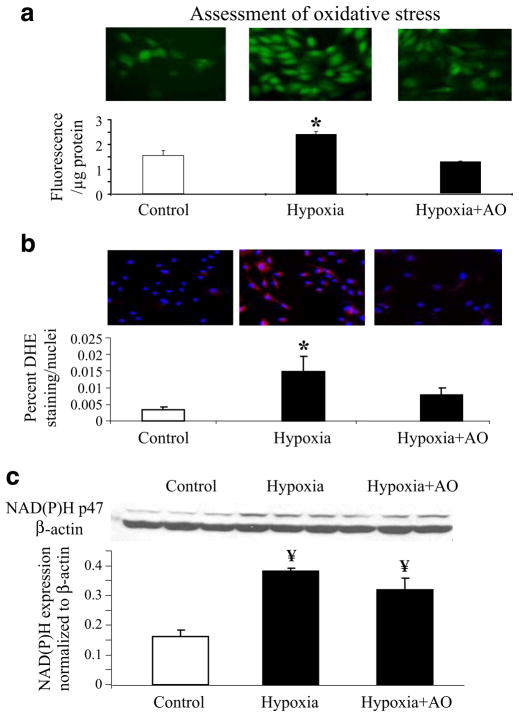
Then, we assessed whether hypoxic conditions led to increases in oxidative stress, compared to control conditions. Cells under control conditions had minimal expression of the oxidative stress markers DCHFDA and DHE (Fig. 3a and b, respectively). In response to hypoxia, however, there was an increase in fluorescence staining to DCHFDA and DHE, suggesting that hypoxia leads to a pro-oxidant status with increase in the production of H2O2 and the amount of ROS (Fig. 3a and b). Furthermore, in cells pretreated with antioxidants, the staining for oxidative stress markers was not different from cells under control conditions (Fig. 3a and b), again suggesting the critical role that oxidative stress plays in hypoxic states.
Fig. 3.
Assessment of oxidative stress. a Top Representative fluorescence staining of the oxidative stress conversion of 2′,7′-dichlorodihydrofluorescein diacetate (DCHFDA). Bottom Fluorescence quantification of the conversion of DCF (expressed as fluorescence per microgram protein). b Top Representative fluorescence staining of the oxidative stress conversion of dihydroethidium (DHE). Bottom Quantification of the percent area where DHE staining was present, normalized by the number of nuclei in each microscopic field analyzed. c Protein expression bands and densitometric analysis of NAD(P)H oxidase. Under hypoxic conditions, there was increased protein expression of NAD(P)H p47phox, together with an increase in the amount of reactive oxygen species suggesting a pro-oxidant state. In summary, hypoxia led to a pro-oxidant state that was improved when cells were treated with antioxidants, mainly through the metabolism of the increased reactive oxygen species. *p<0.05 compared to control and hypoxia + AO, ¥p<0.05 compared to control. These data (Figs. 2 and 3) suggest that increased oxidative stress plays a role in the decreased stem cell viability under hypoxic conditions.
To understand whether the increase in oxidative stress was related to a decrease in ROS metabolism or an increase in oxidative stress production, we assessed the protein expression of one of the main ROS producers (NAD(P)H oxidase) and found that, under hypoxia, cells had an increase in NAD(P)H expression. This increase was mainly of the NAD(P)H subfraction p47phox (Fig. 3c), while other sub-fractions (i.e., p22phox, p91phox) remain unchanged compared to controls (data not shown). Importantly, in cells that were treated with antioxidants, the level of NAD(P)H p47phox expression was not significantly different compared to that seen in cells under hypoxic conditions. Furthermore, hypoxia did not alter the expression of the endogenous scavenger enzyme SOD, in either of its two major forms (Mn-SOD or CuZn-SOD, data not shown).
Imaging of Cell Survival Noninvasively in Cell Culture
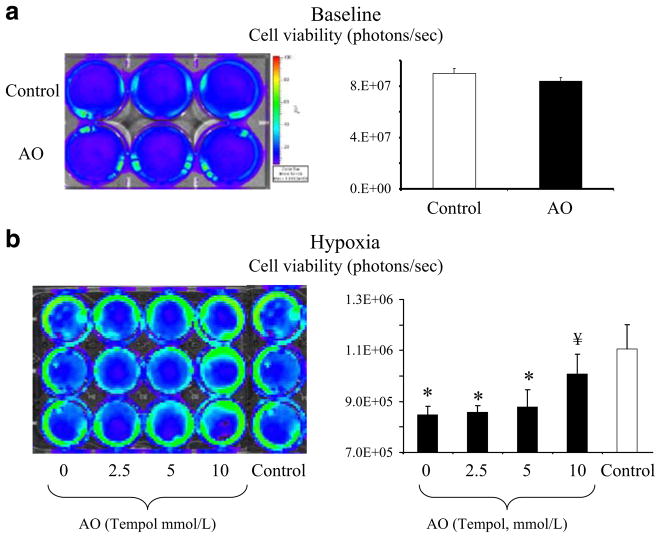
To determine the feasibility of detecting differences in cell survival using BLI (the imaging modality that will be used in living subjects), we performed similar studies in H9c2-fluc cells and used BLI and a cooled CCD camera system to detect light emission (a surrogate of cell viability) [22, 36]. At baseline, detected light between treated and untreated cells was similar (Fig. 4, top), showing that the addition of Tempol did not induce any changes in baseline light emission. However, when H9c2-fluc cells were exposed to hypoxia, they had a decrease in cell survival which was improved when cells were cotreated with tempol (AO, Fig. 4, bottom) and that effect could be assessed noninvasively with BLI.
Fig. 4.
Cell survival assessment was based on reporter gene-light emission and detection using bioluminescence (BLI) and a charge-coupled device camera. Top Untreated (control) and tempol-treated (AO) cells were imaged under baseline conditions. No difference in light detection was observed between control and AO cells, at baseline, suggesting that the addition of tempol does not result in differences in detected light. Bottom Cell survival assessment after hypoxia in the absence and presence of oxidative stress blockade (tempol, 0–10 mm/L). These data demonstrates that a hypoxia leads to a decrease in cell survival and b cells in which oxidative stress was blocked had increase survival compared to untreated cells. These studies corroborated the results obtained using the MTT assay (more traditional method for the assessment of cell viability) but most importantly, demonstrate that reporter genes can be used to assess cell survival in vivo, supporting the translation of these monitoring strategies to the living subject. *p<0.05 compared to control, ¥p<0.05 compared to hypoxia.
Studies of Living Subjects
In these studies, we examined whether modulation of the local microenvironment (using antioxidants) preserved cell survival after transplantation to the myocardium. Delivery of stem cells to the myocardium was guided by high resolution ultrasound, and longitudinal monitoring was performed using BLI.
US-Guided Intramyocardial Transplantation of Embryonic H9c2-fluc Cardiomyoblasts
Fourteen Sprague Dawley rats (weight: 125±10 g, heart rate: 337±14 bpm) were imaged with HiRes US (frequency 30 MHz, average procedure time was 12±2 min). There was no morbidity (as assessed by changes in respiratory rate or heart rate) or mortality associated with the procedure. In addition, no changes in the electrocardiogram (lead II) were observed during the procedure. Stem cells were delivered to the anterolateral wall of the myocardium, using HiRes US, and appropriate delivery was confirmed by post delivery monitoring, where no significant leakage of delivered material into the LV was observed. As expected, there were no changes in LV ejection fraction after stem cell transplantation (data not shown). After the delivery, animals were monitored until recovery, and no complications were observed.
Noninvasive Monitoring of Cardiomyoblast Survival Following Transplantation
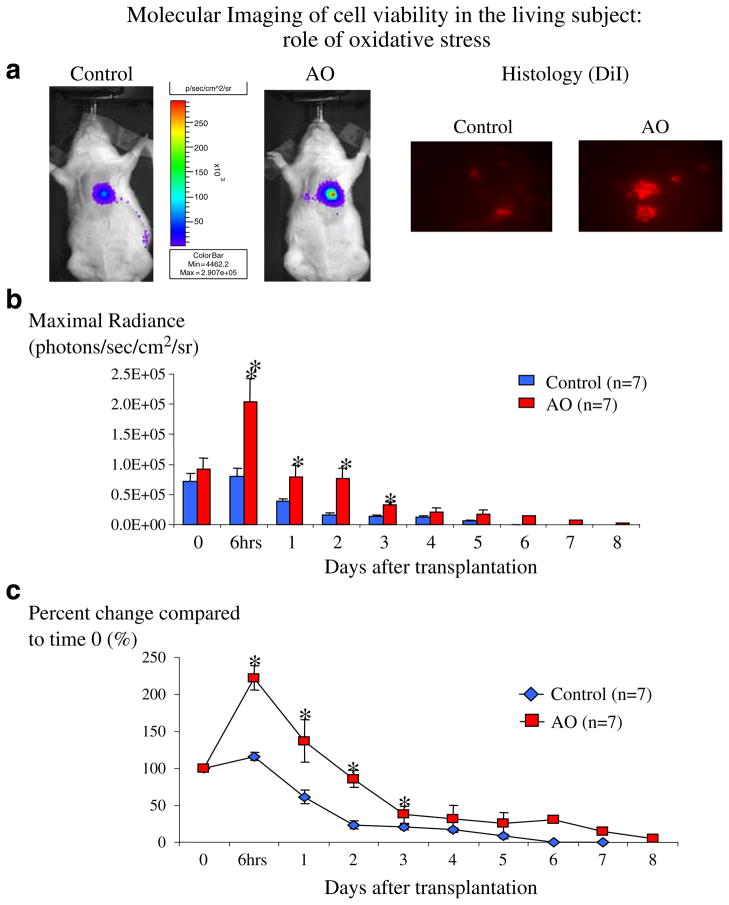
Animals from both groups were followed up to day 8 or until no BLI signal was detected in the myocardial area (Fig. 5). Baseline cell viability was similar in both groups (control: 6.07±1.25× 104, AO: 7.67±1.65×104 photons/sec/cm2/sr, p=0.22). Six hours after transplantation, cell viability of H9c2-fluc cells was higher in both groups, when compared to baseline (control: to 6.33±1.19×104, AO: to 1.68×105±3.55×104 photons/sec/cm2/sr; p=0.01 and 0.0002 compared to baseline, respectively, Fig. 5b), probably reflecting engraftment of cells. Importantly, compared to time 0, the increase in the detected signal in the AO was significantly higher compared to the control group (+104.1±19.9 vs +14.6±5.4%, p= 0.001). The increased cell survival in the AO group, compared to controls, was observed for the first 3 days (Fig. 5b). After that, cells in the AO group still had higher viability than cells in the control group, but this did not reach statistical significance. It is important to mention that for the remaining animals (after day 5), the detected myocardial signal was not different than background.
Fig. 5.
Noninvasive monitoring of cell engraftment and survival in living rats (n=7 in each group). a Representative images from control and AO animals 6 h after cell transplantation (left) and histologic confirmation (using the cell marker DiI, right) of the increased survival observed in AO animals compared to controls, showing the increase in early stem cell engraftment observed in AO animals, compared to controls. b, c Longitudinal monitoring and quantification of cell viability in living subjects. Data is expressed as maximal radiance (photons/sec/cm2/steridian, b) and as percent change (%) of maximal radiance compared to day 0 (c). Until day 3, cells that were treated with antioxidants (AO) had higher myocardial engraftment and survival compared to untreated cells (control). After day 3, cell survival was similar between the two groups. Error bars represent SEM. *p<0.05 compared to day 0.
Furthermore, to ensure that the results were not a result of interanimal variability, all results were also plotted as a percent change from baseline (day 0), and results showed similar results as when maximal radiance was used (Fig. 5c).
To have an independent ex vivo confirmation of the results observed with BLI, in a separate set of animals, stem cells from the same groups (control and AO) were labeled ex vivo with the fluorescent marker DiI and then transplanted to the myocardium. Hearts were harvested 6 h after stem cell delivery, and we found more transplanted cardiomyoblasts in the AO group, compared to control (Fig. 5 upper right), confirming the results obtained using BLI.
Discussion
In the current study, we demonstrated that modulation of the microenvironment, using antioxidants, results in an increase in stem cell survival early after transplantation to the myocardium in small animals. Furthermore, we show that noninvasive reporter gene imaging can be used to study stem cell biology in cardiac disease. Studies like the one described here will play a major role in the understanding of the interaction between stem cells and their microenvironment in the living subject.
In the current study, we provide evidence that increased oxidative stress is involved in the decreased stem cell viability observed under severe hypoxic conditions. Furthermore, our data suggest that the increase in oxidative stress seen in hypoxia is due to an increase in the production of ROS, and that the beneficial effect seen with antioxidants (as used in this study) is mainly through the metabolism of ROS, rather than the modulation of pro-oxidant enzymes. From the present study, we are not able to discern if other pro-oxidant enzymes could play a role in the increase of oxidative stress seen in hypoxia. Overall, the beneficial effect of oxidative stress blockade likely provides a more favorable microenvironment for stem cell engraftment and survival in the heart, as previously shown in the hindlimb [17]. Another factor to be taken into consideration is that the oxidative stress levels and how different cells adjust to different pathophysiological states may vary across different cell types (e.g., mesenchymal stem cells or rat cardiomyoblasts—used in this study). Thus, extrapolating these results to other cell populations or pathophysiological states should be done with caution. In the current study design, the benefit observed is not a long-term effect, as other mechanisms are likely responsible for the cell death in medium and long term (e.g., prolonged inflammation, immune response), which should be the target in future studies. Conceivably, strategies that target medium and late cell death may lead to novel therapeutic strategies for a more effective stem cell therapy approach. Due to the study design, from our study, we are not able to separate the effects of cell pretreatment from the potential beneficial effects provided by antioxidants in the drinking water. However, due to the early and relatively short-term duration of the beneficial effects of antioxidant blockade on cell survival, it is unlikely that the latter contributed significantly to the early increase in cell survival observed in our study.
In the present study, we chose to use the firefly luciferase reporter gene system, imaged using a cooled CCD camera, because it allows fast and high-throughput imaging of cell survival. This is the first report, to our knowledge, where reporter gene technology has been successfully used to report increased survival of transplanted cells in the myocardium, through exogenous and systemic modulation of the microenvironment. While cell survival in our study was similar to that observed in other studies [6, 12], some reports have reported higher rates of cell survival after transplantation [7], and there are many potential reasons for that. As mentioned before, different cell types may have different responses to comparable challenge and thus, different survival. One of the most likely explanations is that a combination of immune response, ischemia, and apoptosis account for the relatively short survival of the transplanted cells [8]. While use of antioxidants improved cell engraftment and survival, providing evidence to the deleterious role of increased oxidative stress in early stem cell death, it was not sufficient to counteract the deleterious stimuli faced by transplanted cells. In other words, it is unlikely that antioxidant intervention by itself will be sufficient to result in a meaningful increase in stem cell survival after transplantation. However, a combination of antioxidant blockade with other interventions that target stem cell survival at later stages may prove useful. Another potential explanation is that reporter genes might have experienced a certain degree of gene silencing (previously described with full-length CMV promoter) [37, 38]. However, this is unlikely as gene silencing has been shown to be more prominent at later stages after cell transplantation [37]. Nevertheless, use of a constitutive mammalian promoter (such as ubiquitin or α-actin) may help to address this issue. Furthermore, we have not observed any effect of tempol on the level of fluc expression under control conditions. While no gross morphological changes or alterations in proliferation rate were observed after stable cell transfection, we cannot completely exclude that introduction of reporter genes, or the CMV promoter might be associated with alterations in cell genotype. In fact, previous reports have suggested that introduction of reporter genes may result in upregulation and downregulation of certain genes and even changes in cell growth characteristics [39]. Because most, if not all, of these studies used a strategy of random DNA integration, it is possible that different studies may have different responses in terms of cell alterations, what may translate in differences in protein expression. In addition, in plasmid-mediated integration of DNA, variable number of copies of the exogenous DNA (i.e., reporter gene) may get integrated into the genome, what may result in changes of the cell’s phenotype. Thus, further studies are needed to clarify this issue, as molecular imaging strategies come closer to clinical applications. In addition, it is possible that the reporter genes were relatively weak, and that after the first week, the amount of light emission was below the detection limit for the cooled CCD camera.
In the current study, we delivered cells using an epicardial approach. This route of delivery is the most commonly used route in small animal models [6, 7, 40] and has also been used in clinical studies. Furthermore, we have shown that the use of US guidance for cell delivery is reproducible and consistent [22] and likely results in less perturbation of the microenvironment (compared to open chest injections) as well as less morbidity and mortality. However, it may not be the cell delivery route of choice when the target is a certain coronary distribution (and a large area of myocardium), rather than a specific area of the myocardium (e.g., intracoronary delivery in large animals or patients after myocardial infarction). In the present study, we used intact unperturbed rats, so results from this study should not be extrapolated to other models of disease (e.g., myocardial ischemia/infarction). Nevertheless, the results reported here open novel avenues to investigate the effect of metabolic intervention in ischemic myocardium. Furthermore, while the use of ischemic or infarcted models may be closer to clinical practice, even in intact subjects (like the ones used in this study), stem cells will face a noxious microenvironment after transplantation to the myocardium and have been shown to have short cell survival [6, 7]. Furthermore, the potential noxious environment that stem cells will face after transplanted to diseased tissue (e.g., infarcted myocardium) is likely more significant than that observed in healthy tissue (as used in this study). Thus, modulation of the microenvironment in that setting may translate into a more pronounced beneficial effect on stem cell survival.
In summary, in the current study, we demonstrated that modulation of the cell and myocardial microenvironment can have an impact on stem cell survival. We showed that antioxidants preserved cell survival after transplantation to the myocardium of intact animals. Furthermore, we showed that reporter gene imaging can be used to monitor stem cell biology of transplanted cells to the myocardium. Increased understanding of the interaction between stem cells and their local microenvironment will likely leads to more successful therapeutic strategies for CAD.
Acknowledgments
Funding. This work was supported in part by National Health Lung Blood Institute R01 HL078632 (SSG), National Cancer Institute ICMIC CA114747 P50 (SSG), National Institutes of Health K99-R00 HL88048 (MR-P), and the Mayo Clinical Scholarship Program, Mayo Clinic College of Medicine, Rochester, Minnesota (MR-P).
References
- 1.American Heart Association. [accessed January 2009];Heart disease and stroke statistics—2009 update. www.americanheart.org.
- 2.Roger VL, Jacobsen SJ, Weston SA, et al. Trends in the incidence and survival of patients with hospitalized myocardial infarction, Olmsted County, Minnesota, 1979 to 1994. Ann Intern Med. 2002;136:341–348. doi: 10.7326/0003-4819-136-5-200203050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Welt FG, Losordo DW. Cell therapy for acute myocardial infarction: curb your enthusiasm? Circulation. 2006;113:1272–1274. doi: 10.1161/CIRCULATIONAHA.105.613034. [DOI] [PubMed] [Google Scholar]
- 5.Engelmann MG, Franz WM. Stem cell therapy after myocardial infarction: ready for clinical application? Curr Opin Mol Ther. 2006;8:396–414. [PubMed] [Google Scholar]
- 6.Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. Faseb J. 2004;18:1153–1155. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 7.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 9.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 10.Urbinati F, Lotti F, Facchini G, et al. Competitive engraftment of hematopoietic stem cells genetically modified with a truncated erythropoietin receptor. Hum Gene Ther. 2005;16:594–608. doi: 10.1089/hum.2005.16.594. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Yasuda T, Weisel RD, Li RK. Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol. 2006;291:H939–H947. doi: 10.1152/ajpheart.00155.2006. [DOI] [PubMed] [Google Scholar]
- 12.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Porcel M, Herrman J, Chade AR, et al. Long-term antioxidant intervention improves myocardial microvascular function in experimental hypertension. Hypertension. 2004;43:493–498. doi: 10.1161/01.HYP.0000111834.03000.e4. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Porcel M, Lerman A, Best PJ, et al. Hypercholesterolemia impairs myocardial perfusion and permeability: role of oxidative stress and endogenous scavenging activity. J Am Coll Cardiol. 2001;37:806–815. doi: 10.1016/s0735-1097(00)01139-6. [DOI] [PubMed] [Google Scholar]
- 15.Griendling KK, Alexander RW. Oxidative stress and cardiovascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- 16.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 17.Napoli C, Williams-Ignarro S, de Nigris F, et al. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci U S A. 2005;102:17202–17206. doi: 10.1073/pnas.0508534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contag CH, Ross BD. It’s not just about anatomy: in vivo bioluminescence imaging as an eyepiece into biology. J Magn Reson Imaging. 2002;16:378–387. doi: 10.1002/jmri.10178. [DOI] [PubMed] [Google Scholar]
- 19.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev. 2006;6:484–90. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 20.Shah K, Jacobs A, Breakefield XO, Weissleder R. Molecular imaging of gene therapy for cancer. Gene Ther. 2004;11:1175–1187. doi: 10.1038/sj.gt.3302278. [DOI] [PubMed] [Google Scholar]
- 21.Wu JC, Tseng JR, Gambhir SS. Molecular imaging of cardiovascular gene products. J Nucl Cardiol. 2004;11:491–505. doi: 10.1016/j.nuclcard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Porcel M, Gheysens O, Chen IY, Wu JC, Gambhir SS. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12:1142–1147. doi: 10.1016/j.ymthe.2005.07.532. [DOI] [PubMed] [Google Scholar]
- 23.Gheysens O, Lin S, Cao F, et al. Noninvasive evaluation of immunosuppressive drug efficacy on acute donor cell survival. Mol Imaging Biol. 2006;8:163–170. doi: 10.1007/s11307-006-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marwaha A, Lokhandwala MF. Tempol reduces oxidative stress and restores renal dopamine D1-like receptor- G protein coupling and function in hyperglycemic rats. Am J Physiol. 2006;291:F58–66. doi: 10.1152/ajprenal.00362.2005. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Pearlman A, Luo Z, Wilcox CS. Hydrogen peroxide mediates a transient vasorelaxation with tempol during oxidative stress. Am J Physiol Heart Circ Physiol. 2007;293:H2085–H2092. doi: 10.1152/ajpheart.00968.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Ksiazek K, Piwocka K, Brzezinska A, et al. Early loss of proliferative potential of human peritoneal mesothelial cells in culture: the role of p16INK4a-mediated premature senescence. J Appl Physiol. 2006;100:988–995. doi: 10.1152/japplphysiol.01086.2005. [DOI] [PubMed] [Google Scholar]
- 28.Herrmann J, Saguner AM, Versari D, et al. Chronic proteasome inhibition contributes to coronary atherosclerosis. Circ Res. 2007;101:865–874. doi: 10.1161/CIRCRESAHA.107.152959. [DOI] [PubMed] [Google Scholar]
- 29.Lim SD, Sun C, Lambeth JD, et al. Increased Nox1 and hydrogen peroxide in prostate cancer. The Prostate. 2005;62:200–207. doi: 10.1002/pros.20137. [DOI] [PubMed] [Google Scholar]
- 30.Yang CS, Lee HM, Lee JY, et al. Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. Journal of Neuroinflammation. 2007;4:27. doi: 10.1186/1742-2094-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esterhazy D, King MS, Yakovlev G, Hirst J. Production of reactive oxygen species by complex I (NADH:ubiquinone oxidoreductase) from Escherichia coli and comparison to the enzyme from mitochondria. Biochemistry. 2008;47:3964–3971. doi: 10.1021/bi702243b. [DOI] [PubMed] [Google Scholar]
- 32.Honjo T, Otsui K, Shiraki R, et al. Essential role of NOXA1 in generation of reactive oxygen species induced by oxidized low-density lipoprotein in human vascular endothelial cells. Endothelium. 2008;15:137–141. doi: 10.1080/10623320802125433. [DOI] [PubMed] [Google Scholar]
- 33.Morten KJ, Ackrell BA, Melov S. Mitochondrial reactive oxygen species in mice lacking superoxide dismutase 2: attenuation via antioxidant treatment. J Biol Chem. 2006;281:3354–3359. doi: 10.1074/jbc.M509261200. [DOI] [PubMed] [Google Scholar]
- 34.Chade AR, Mushin OP, Zhu X, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 35.Chade AR, Rodriguez-Porcel M, Herrmann J, et al. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 36.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan M, Park JM, Cao F, et al. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. Faseb J. 2006;20:106–108. doi: 10.1096/fj.05-4551fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12:3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- 39.Wu JC, Spin JM, Cao F, et al. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kutschka I, Kofidis T, Chen IY, et al. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–I180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]