Abstract
We have shown altered expression of gamma-aminobutyric acid A (GABAA) and gamma-aminobutyric acid B (GABAB) receptors in the brains of subjects with autism. In the current study, we sought to verify our western blotting data for GABBR1 via qRT-PCR and to expand our previous work to measure mRNA and protein levels of 3 GABAA subunits previously associated with autism (GABRα4; GABRα5; GABRβ1). Three GABA receptor subunits demonstrated mRNA and protein level concordance in superior frontal cortex (GABRα4, GABRα5, GABRβ1) and one demonstrated concordance in cerebellum (GABBR1). These results provide further evidence of impairment of GABAergic signaling in autism.
Keywords: GABBR1, GABRα4, GABRα5, GABRβ1, autism, brain
Autism is a debilitating disorder that is characterized by a number of behavioral deficits, including ritualized or stereotyped behaviors, impairment of social interactions, and deficiencies in communication (APA, 1994). Gamma-aminobutyric acid (GABA) is responsible for the majority of inhibitory neurotransmission in the brain and there are few, if any, areas in the brain that are not affected by GABA. However, it has only been relatively recently that postmortem studies have begun to suggest a role for the GABAergic system in autism. Changes in levels of glutamic acid decarboxylase (GAD), the rate limiting enzyme that is responsible for conversion of glutamate to GABA, and GABAA and GABAB receptor subunits in autistic brain and blood have been observed (Blatt et al., 2001; Dhossche et al., 2002; Fatemi et al., 2002, 2009a,b; Yip et al., 2007).
Binding of GABA to its receptors - GABAA, GABAB, and GABAC - transduces signals underlying various inhibitory transmissions in the brain. There are over 19 individual GABAA receptor subunits (Brandon et al., 2000; Ma et al., 2005; Olsen et al., 2008), two GABAB subunits (Jones et al., 1998), and two GABAC subunits (Qian and Ripps, 2009). GABA receptor subunits have been localized to multiple chromosomes (Table 1), and there are several GABA receptor gene clusters at various loci (Table 1). Duplications, deletions, and inversions at the 15q11-q13 locus, which includes genes for three GABAA receptors (Table 1; GABRα5, GABRβ3 and GABRγ3), occur in 1-4% of autistic patients (Schroer et al., 1998), and single nucleotide polymorphisms (SNPs) for these genes have been associated with autism (McCauley et al., 2004; Samaco et al., 2005; Ashley Koch et al., 2006; Kim et al., 2006; Hogart et al., 2007, 2009), suggesting a role for these genes in the pathology of autism.
Table 1.
Chromosomal Locations of GABA Receptor Subunit Genes and Clusters
| Chromosomal location | GABA receptor subunit(s) |
|---|---|
| 1p36.3 | GABRδ |
| 4q13-p12 | GABRα2, GABRβ1 |
| 4p14-q12 | GABRα4 |
| 5q31.1-q35 | GABRα6 |
| 5q32-q33 | GABRπ |
| 5q34-q35 | GABRα1, GABRβ2 |
| 6q14-q21 | GABRρ2 |
| 6p21.3 | GABBR1 |
| 9q22.1 | GABBR2 |
| 15q11.2-q13 | GABRα5, GABRβ3, GABRγ3 |
| 15q31.1-q33.1 | GABRγ2 |
| Xq28 | GABRα3, GABRε, GABRθ |
Chromosomal location obtained from Online Mendelian Inheritance in Man (OMIM) database.
Our laboratory has recently demonstrated significant reductions in protein levels for selected GABAA receptor subunits, and were the first group to show significant reductions of GABAB receptor subunits in brains of subjects with autism (Fatemi et al., 2009a,b). Here, we extend our previous investigations of the GABAergic system in autism to verify our western blotting results for GABBR1 via qRT-PCR and measure the expression of mRNA and protein for three GABAA receptor subunits that have been associated with autism (GABRα4, GABRα5, GABRβ1) in parietal cortex (Brodmann's area 40 (BA40)), superior frontal cortex (BA9), and cerebellum of subjects with autism and matched controls. Based on our previous work, we hypothesized that there would be a reduction in mRNA and protein expression of GABA receptor subunits in all three brain regions.
Methods
Tissue Preparation
All experimental procedures were approved by the Institutional Review Board of the University of Minnesota School of Medicine. Postmortem blocks of BA40, BA9, and cerebella (lobar origin unknown) were obtained from the Autism Research Foundation and various brain banks (NICHD Brain and Tissue Bank for Developmental Disorders; TARF; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program). The tissue samples (Table 2) were prepared as described previously (Fatemi et al., 2009a,b).
Table 2.
Demographic Data for Subjects with Autism and Controls
| Case | Dx | Sex | Age | PMI (Hrs.) | Ethnicity | Medication History | Cause of Death | MR* | Seizure* | Brain Areas |
|---|---|---|---|---|---|---|---|---|---|---|
| B1078 | Autistic | M | 22 | 14.3 | Caucasian | Dilantin, Tegretol, Phenobarbital, Theodure. | Asphyxia | Yes | Yes | BA40 |
| B1045 | Autistic | M | 28 | 16.3 | Caucasian | Cefobid, Urecholine, Duracef. | Cardiac arrest | Yes | Yes | Cer, BA40 |
| B5000 | Autistic | M | 27 | 8.3 | Caucasian | Synthroid | Drowning | Yes | No | Cer |
| B1401 | Autistic | F | 21 | 20.6 | Caucasian | Tetracycline | Pneumonia, sepsis | Yes | Yes | Cer, BA9, BA40 |
| B1664 | Autistic | M | 20 | 15 | Caucasian | Vitamins B, C | Perforation of ulcer; asphyxia | Yes | Yes | Cer, BA9, BA40 |
| B2825 | Autistic | M | 19 | 9.5 | Caucasian | None | Seizure | Yes | Yes | Cer, BA9, BA40 |
| B3511 | Autistic | M | 29 | 15 | Caucasian | None | Hit by train | Yes | Yes | Cer, BA9, BA40 |
| B3845 | Autistic | M | 30 | 28.4 | Caucasian | Mellaril, Phenobarbital, Dilantin. | Shock; acute pancreatitis | Yes | Yes | Cer, BA9, BA40 |
| B1484 | Autistic | M | 19 | 15 | Caucasian | None | Burns | Yes | No | BA9, BA40 |
| B3829 | Control | M | 22 | 24.3 | Caucasian | None | MVA | No | No | Cer |
| B4267 | Control | M | 26 | 20 | African-American | None | MVA | No | No | Cer |
| B4268* | Control | M | 30 | 22 | African-American | None | Cardiomyopathy | No | No | Cer, BA40 |
| B4269 | Control | M | 28 | 24 | Caucasian | Lidocaine 12.0 mg/L found in blood. | Areteriosclerotic cardiovascular disease | No | No | Cer, BA9, BA40 |
| B4272 | Control | M | 19 | 17 | Caucasian | None | Accident; chest injuries | No | No | Cer |
| B4275* | Control | M | 20 | 16 | Caucasian | None | Accident | No | No | Cer, BA9, BA40 |
| B4279 | Control | F | 20 | 21 | Caucasian | None | MVA | No | No | Cer |
| B4362 | Control | M | 30 | 20 | African-American | None | MVA | No | No | Cer, BA9 |
| B4101 | Control | M | 24 | 5 | Unknown | None | Gun shot wound | No | No | Cer, BA40 |
| B4271 | Control | M | 19 | 21 | African-American | EtOH, Advil, Amoxapine. | Epiglottitis | No | No | BA40 |
| B4756 | Control | M | 56 | 23 | Unknown | None | Myocardial infarction | No | No | Cer |
| B4363 | Control | M | 21 | 9 | Caucasian | None | MVA | No | No | Cer, BA40 |
| UMB1376** | Control | M | 37 | 12 | African-American | None | Areteriosclerotic cardiovascular disease | No | No | BA9 |
not included in qRT-PCR analysis;
not included in western blotting analysis;
Dx, diagnosis; Hrs, hours; PMI, postmortem interval; M, male; F, female; EtOH, alcohol; MVA, motor vehicle accident; MR, Mental retardation
Communication from Dr. M. Bauman.
qRT-PCR
For this gene expression study, a two-step RT-PCR method was used to quantify RNA, because we wanted to store cDNA for later use so as to obtain greater consistency in experimental parameters. Also, empirically, it has been shown that there is close to one-to-one conversion of RNA to cDNA using most commercial reverse transcription reagents, and hence, we could circumvent quantifying cDNA which requires a cumbersome preparatory/cleaning process with rather limited sample availability. The RNA was quality checked and quantified by UV spectrophotometry and capillary electrophoresis using the Agilent Bioanalyzer 2100. Approximately 2 μg of total RNA from each sample was used to generate high fidelity cDNA using random hexamer primers and the High Capacity cDNA Synthesis Kit (Applied Biosystems, Foster City CA) following the manufacturer's protocol. After 1:10 dilution, 20 ng of cDNA was used in a 20 μl reaction including TaqMan Universal PCR Master Mix (Applied Biosystems) and the indicated inventoried TaqMan Gene Expression Assays. Inventoried TaqMan assays contain optimized concentrations of oligonucleotide primers and fluorescently labeled TaqMan probes (FAM, MGB) with sequence compositions designed for consistent amplification efficiency across assays under universal thermal cycling conditions. Specific assays were also selected for consistency in amplicon size. Tissue from cerebellum (N=9 control; N=4 autistic), BA40 (N=5 control; N=5 autistic), and BA9 (N=5 control; N=4 autistic) were used. All reactions were performed in triplicate per experiment and each experiment was replicated three times with GAPDH and β-actin as endogenous controls. Values from beta-actin and GAPDH were averaged together as the endogenous controls. The real-time RT-PCR amplifications were run on a 7900HT Real Time PCR System (Applied Biosystems). Universal thermal cycling conditions were as follows: 10 min at 95°C, 40 cycles of denaturation at 95°C for 15 sec, and annealing and extension at 60°C for 1 min. Data were collected at every temperature phase during each cycle. Raw data were analyzed using the Sequence Detection Software RQ Manager (ABI, Foster City, CA) while relative quantitation using the comparative threshold cycle (CT method) was performed in Bioconductor using the ABqPCR package in Microsoft Excel (ABI Technote#2: Relative Gene Expression Quantitation). Calculations were done assuming that 1 delta Ct equals a 2-fold difference in expression. Significance values were determined using unpaired t-tests.
The probe IDs used were: 1) GABRα4: Hs00608034_m1; 2) GABRα5: Hs00181291-m1; 3) GABRβ1: Hs00181306_m1; 4) GABBR1: Hs00559488_m1; 5) β-actin: Hs99999903_m1; and 6) GAPDH: Hs99999905_m1.
SDS-PAGE and Western Blotting
Tissue samples from cerebellum (N=11 control, N=7 autistic), BA9 (N=3 control; N=4-5 autistic), and BA40 (N=5-6 control, N=4-8 autistic) were prepared and SDS PAGE and western blotting performed as described previously (Fatemi et al., 2009a,b). The primary antibodies used were: anti-GABAA receptor alpha 4 (GABRα4) (AB5459, Chemicon (Temecula, CA), 1:1,000), anti-GABAA receptor alpha 5 (GABRα5) (ab10098, Abcam Inc. (Cambridge, MA), 1:1,000), anti-GABAA receptor beta 1 (GABRβ1) (AB9680, Chemicon (Temecula, CA), 1:1,000), anti-β actin (A5441, Sigma Aldrich (St. Louis, MO), 1:5,000). Secondary antibodies used were A9169 (Sigma Aldrich, goat anti-rabbit IgG 1:80,000) and A9044 (Sigma Aldrich, rabbit anti-mouse IgG 1:80,000). The molecular weights of approximately 64 kDa (GABRα4), 58 kDa and 55 kDa (GABRβ1), 52 kDa (GABRα5), and 42 kDa (β actin) immunoreactive bands were quantified with background subtraction. Results obtained are based on at least two independent experiments.
Statistical Analysis
Statistical analysis of protein data was performed as previously described (Fatemi et al., 2009a,b). We investigated the potential confounding effect of postmortem interval by examining group differences with postmortem interval as a covariate. We similarly examined effects of ethnicity, age, seizure, and anti-convulsant use.
Results
GABA Receptor Protein Levels in BA9, BA40 and Cerebellum of Subjects with Autism
All western-blotting experiments were normalized against β-actin and are shown as ratios of the various GABA receptor subunits to β-actin. In BA9 from subjects with autism, there was a 31% reduction in GABRα4 protein (p<0.0086), a 50% reduction in GABRα5 protein (p<0.035), a 52% reduction in GABRβ1 58 kDa protein (p<0.012), and a 53% reduction in GABRβ1 55 kDa protein (p<0.014) (Figure 1, Table 3). We have previously found a significant 70% reduction in GABBR1 protein in BA9 (Fatemi et al., 2009b) (Figure 1, Table 3).
Figure 1.
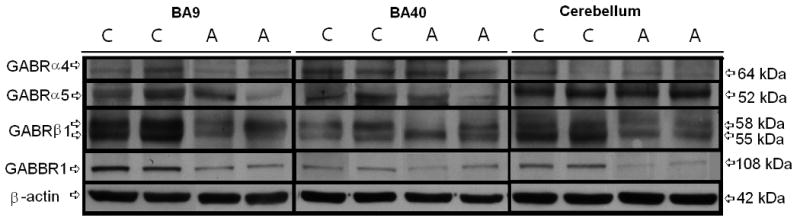
Representative samples of GABRα4 (64 kDa), GABRα5 (52 kDa), GABRβ1 (58 kDa and 55 kDa), GABBR1 (108 kDa), and β-Actin (42 kDa) in BA9, BA40, and cerebellum of subjects with autism (A) and matched controls (C). Note: Images for GABBR1 protein levels are reprinted with kind permission from Springer Science+Business Media: Cerebellum, Expression of GABA(B) Receptors Is Altered in Brains of Subjects with Autism, volume 8, 2009, page 67, Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD, Figure 1.
Table 3.
Western Blotting Results for Selected GABA Receptor Genes and β-Actin in BA9, BA40, and Cerebellum
| BA9 | Control | Autistic | Change | P |
|---|---|---|---|---|
| GABRα4 / β-Actin | 0.058 ± 0.007 | 0.040 ± 0.004 | ↓ 31% | 0.0086 |
| GABRα5 / β-Actin | 0.576 ± 0.040 | 0.288 ± 0.176 | ↓ 50% | 0.035 |
| GABRβ1 / β-Actin (58 kDa) | 0.198 ± 0.05 | 0.095 ± 0.021 | ↓ 52% | 0.012 |
| GABRβ1 / β-Actin (55 kDa) | 0.213 ± 0.058 | 0.100 ± 0.021 | ↓ 53% | 0.014 |
| GABBR1/ β-Actin1 | 0.076 ± 0.023 | 0.023 ± 0.024 | ↓ 70% | 0.021 |
| β-Actin | 30.4 ± 1.38 | 27.8 ± 2.85 | ↓ 8.5% | ns |
| Age ± SD (years) | 26.0 ± 5.29 | 23.8 ± 5.29 | ↓ 8.4% | ns |
| PMI ± SD (years) | 20 ± 4.0 | 17.7 ± 7.15 | ↓ 11% | ns |
| Gender | 3M:0F | 4M:1F | - | -- |
| BA40 | Control | Autistic | Change | P |
| GABRα4 / β-Actin | 0.195 ± 0.039 | 0.230 ± 0.045 | ↑ 18% | ns |
| GABRα5 / β-Actin | 0.439 ± 0.118 | 0.217 ± 0.024 | ↓ 51% | 0.01 |
| GABRβ1 / β-Actin (58 kDa) | 0.278 ± 0.213 | 0.213 ± 0.139 | ↓ 23% | ns |
| GABRβ1 / β-Actin (55 kDa) | 0.315 ± 0.112 | 0.270 ± 0.049 | ↓ 14% | ns |
| GABBR1/ β-Actin1 | 0.078 ± 0.049 | 0.023 ± 0.026 | ↓ 71% | 0.019 |
| β-Actin | 15.6 ± 1.16 | 13.9 ± 2.14 | ↓ 11% | ns |
| Age ± SD (years) | 23.6 ± 5.03 | 22.8 ± 3.59 | ↓ 3.4% | ns |
| PMI ± SD (years) | 18.4 ± 6.02 | 16.6 ± 2.82 | ↓ 9.8% | ns |
| Gender | 5M:0F | 3M:1F | - | -- |
| Cerebellum | Control | Autistic | Change | P |
| GABRα4 / β-Actin | 0.066 ± 0.021 | 0.054 ± 0.015 | ↓ 18% | ns |
| GABRα5 / β-Actin | 0.387 ± 0.194 | 0.436 ± 0.152 | ↑ 13% | ns |
| GABRβ1 / β-Actin (58 kDa) | 0.216 ± 0.038 | 0.195 ± 0.035 | ↓ 9.7% | ns |
| GABRβ1 / β-Actin (55 kDa) | 0.444 ± 0.106 | 0.425 ± 0.129 | ↓ 4.3% | ns |
| GABBR1/ β-Actin1 | 0.051 ± 0.019 | 0.017 ± 0.006 | ↓ 67% | 0.0049 |
| β-Actin | 20.0 ± 1.43 | 20.1 ± 2.21 | ↑ 0.5% | ns |
| Age ± SD (years) | 26.36 ± 10.63 | 24.86 ± 4.67 | ↓ 5.7% | ns |
| PMI ± SD (years) | 18.0 ± 5.98 | 16.16 ± 6.81 | ↓1.8% | ns |
| Gender | 10M:1F | 6M:1F | - | -- |
Values for GABBR1 are reprinted with kind permission from Springer Science+Business Media: Cerebellum, Expression of GABA(B) Receptors Is Altered in Brains of Subjects with Autism, volume 8, 2009, page 67, Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD, Table 2. ns, not significant
In BA40, Western blotting experiments revealed a significant 51% reduction in protein for GABRα5 (p<0.01; Figure 1, Table 3). Our laboratory has previously found a significant 71% reduction in GABBR1 protein in BA40 (Fatemi et al., 2009b) (Figure 1, Table 3). GABBR1 protein was also significantly reduced by 67% in cerebella of subjects with autism when compared with controls (Fatemi et al., 2009b) (Figure 1, Table 3). We measured protein levels of β-Actin in BA9, BA40, and cerebellum and found slight, non-significant changes in subjects with autism (8.5%, 11%, and 0.5%, respectively; Table 3).
Demographic Factors and Effects of Confounds
Mean age was measured and no significant differences were found between subjects with autism and matched controls in BA9, BA40, and cerebellum (Table 3). Gender ratios varied from 10 men to 1 woman in controls to 6 men to 1 woman in subjects with autism (Table 3). A comparison of subjects on race (African-American vs. Caucasian) found no significant difference on GABA receptors. We also examined the effect on anti-convulsant use and found no significant differences on GABA receptors. Seven out of nine subjects with autism were comorbid with seizure disorder (Table 2). A second analysis was performed comparing protein levels of GABBR1 (Fatemi et al., 2009b), GABRα4, GABRα5, GABRβ1 in subjects with autism comorbid with seizure disorder vs. controls. There was no confounding effect of seizure or postmortem interval on levels of GABA receptors of interest. We also conducted a series of ANCOVA's with postmortem interval as the covariate. There was no confounding effect of postmortem interval on levels of GABA receptors of interest.
mRNA Expression for GABRα4, GABRα5, GABRβ1, and GABBR1
All qRT-PCR experiments were normalized against both β-actin and GAPDH and these values were averaged. In BA9 of subjects with autism there were significant reductions in mRNA for GABRα4 (p<0.00062), GABRα5 (p<0.0024), and GABRβ1 (p<0.0099), verifying our western blotting results for these receptors, while there was no significant change for GABBR1 (Table 4). In BA40 there was a significant increase in mRNA for GABBR1 (p<0.016) (Table 4). In cerebella of subjects with autism there was a significant downregulation of mRNA for GABBR1 (p<0.0044), verifying our western blotting results (Table 4). In contrast, there were significant increases in mRNAs for GABRα4 (p<0.029), GABRα5 (p<0.002), and GABRβ1 (p<0.003) (Table 4).
Table 4.
mRNA Levels for GABRα4, GABRα5, GABRβ1, and GABBR1 in Brains of Subjects with Autism
| Area | Gene | GOI relative to averaged normalizersa | P |
|---|---|---|---|
| BA9 | GABRα4 | -2.12 | 0.00062 |
| GABRα5 | -3.36 | 0.0024 | |
| GABRβ1 | -1.11 | 0.0099 | |
| GABBR1 | -0.27 | ns | |
| BA40 | GABRα4 | -0.28 | ns |
| GABRα5 | 0.16 | ns | |
| GABRβ1 | -0.008 | ns | |
| GABBR1 | 0.378 | 0.016 | |
| Cerebellum | GABRα4 | 1.55 | 0.029 |
| GABRα5 | 4.48 | 0.002 | |
| GABRβ1 | 0.86 | 0.003 | |
| GABBR1 | -0.33 | 0.0044 | |
Genes of interest were normalized against both beta actin and GAPDH, and the values were averaged and expressed as Log2(FC). ns, not significant
Discussion
In the current study we have demonstrated reduced GABRα4, GABRα5, GABRβ1 proteins in BA9 and reduced GABRα5 protein in BA40 of subjects with autism when compared with age and PMI matched controls. We have previously demonstrated reduced GABBR1 protein in all three brain regions from subjects with autism (Fatemi et al., 2009b). These reductions were specific for the GABA subunits as there were no significant differences in β-actin in the three brain regions between subjects with autism and matched control subjects. Moreover, the reduction in GABRα4, GABRα5, GABRβ1 proteins in BA9 was accompanied by a similar reduction in their mRNA levels. Similarly, in cerebellum there was a similar reduction in mRNA for GABBR1.
The GABA receptors that show concordant results are of particular interest since altered expression has been demonstrated at two levels (i.e. reduction of mRNA and protein). The reduction is likely to affect GABA receptor kinetics resulting in dysfunctional GABAergic transmission. Discordant results between protein and mRNA (i.e. increased mRNA and reduced protein or vice versa) may result from multiple ways: 1) microRNA (miRNA) may regulate specific GABA receptor genes preventing translation, however, this does not appear to be the case here; 2) epigenetics – one or more regulatory genes may affect the chromatin structure of a given gene effectively silencing its expression; 3) post-translational modification through phosphorylation or glycosylation may lead to altered trafficking; 4) improper folding may lead to endoplasmic reticulum retention and ultimately degradation of the receptor intracellularly; 5) however, the most likely scenario at play here is that chronic receptor protein downregulation, as seen for GABBR1 in BA40 cerebellum, may lead to a compensatory upregulation of its respective mRNA. Further study is required to investigate these possibilities.
GABRα5, one of the three GABA receptors clustered at the 15q11.2-q13 locus displayed concordance for mRNA and protein data in BA9 (Tables 3 and 4). Individual single nucleotide polymorphisms (SNPs) across GABRα5 have been found to be nominally associated with autism (McCauley et al., 2004). However, a recent report found no association between GABRα5 and autism in a Japanese population sample (Tochigi et al., 2007). Our results establish further evidence of a connection between GABRα5 and autism.
Genetic evidence suggests an association of GABRα4 and GABRβ1 with autism and, moreover, these two genes may interact to increase risk of autism (Ma et al., 2005; Collins et al., 2006). Ma et al. (2005) identified SNPs for GABRα4 and GABRρ2 using a family-based study for allelic association with autism. Genotypic and haplotypic association analysis identified SNPs for GABRα2, GABRα4, and GABRβ2 associated with autism (Ma et al., 2005). Extended multifactor-dimensionality reduction analysis revealed a gene-gene effect involving GABRα4 and GABRβ1 which positively associated with autism (Ma et al., 2005). A further study provided further evidence of GABRα4 as a risk for autism in Caucasians and African Americans and the joint involvement of GABRα4 and GABRβ1 was again found for Caucasians (Collins et al., 2006). Further evidence of an association of GABRα4 and autism was reported recently in a case study of an autistic patient with a mosaic of chromosome 4p who had three signals for GABRα4, GABRγ1, and GABRα2, as measured by fluorescence in situ hybridization (Kakinuma et al., 2008). We observed that both mRNA and protein levels for both GABRα4 and GABRβ1 were reduced in BA9 of subjects with autism compared with controls further supporting the role of these genes for autism.
Our laboratory is the first to demonstrate changes in GABBR1 and GABBR2 in brains of subjects with autism (Fatemi et al., 2009b). GABBR1 has been linked to other disorders including temporal lobe epilepsy (Princivalle et al., 2003), obsessive compulsive disorder (Zai et al., 2005a), and schizophrenia (Zai et al., 2005b). We have now verified our western blotting data for GABBR1 in cerebellum via qRT-PCR. The reduction in GABBR1 may increase the liability to seizure disorder, which is more common in subjects with autism when compared to the general population (Tuchman and Rapin, 2002).
Aside from autism, changes in the GABA receptors examined in the current study may be associated with other neuropsychiatric and neurodevelopmental disorders. While there has been no established relationship between GABRα4 and schizophrenia, a polymorphism of GABRα4 has been associated with development of neuroleptic-induced tardive dyskinesia (Inada et al., 2008). GABRα5 levels in hippocampi of mice have been associated modulation of prepulse inhibition (Hauser et al., 2005), which is disturbed in subjects with schizophrenia and autism. A week positive association between a polymorphism of GABBR1 (A-7265G) and schizophrenia has been established (Zai et al., 2005a, b). This relationship was also observed using a convergent functional genomic approach (Le-Niculescu et al., 2007). However, there has not been any postmortem analysis of protein or mRNA levels for these receptors in schizophrenia. There hasn't been a connection established between any of the GABA receptors examined in the current study with Fragile X syndrome. Deletion of the 11.2-q13 region of chromosome 15, where the GABRα5 gene is located, has been associated with a more severe clinical picture of Angelman Syndrome (Borgatti et al., 2003). However, this is likely due to the presence of ubiquitin protein ligase E3A (UBE3A) in this region (Matsuura et al., 1997). Of these three disorders, further investigation of the GABAergic system in schizophrenia warrants further study. While autism and schizophrenia are two very different disorders, multiple proteins are similarly altered in both disorders including GABAergic proteins glutamic acid decarboxylase 65/67 (Fatemi et al., 2002, 2005). Postmortem studies of GABRα4, GABRα5, GABRβ1, and GABBR1 in subjects with schizophrenia could be a logical next step in examining GABAergic dysfunction in both disorders.
In conclusion, our results have demonstrated concordance between protein and mRNA for GABRα4, GABRα5, GABRβ1 in BA9 and GABBR1 in cerebella of subjects with autism, suggesting widespread GABAergic dysfunction. Altered expression of GABA receptor subunits and GAD65/67 may lead to the impairment of the excitatory/inhibitory balance in the brain, resulting in various cognitive impairments associated with autism and the presence of seizure disorder.
Acknowledgments
Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program and is gratefully acknowledged. Grant support by National Institute of Child Health and Human Development (#5R01HD052074-01A2; and 3R01HD052074-03S1) to SHF is gratefully acknowledged.
Footnotes
Portions of this paper have been presented at the following meetings: Collegium Internationale Neuro-Psychopharmacologicum, Munich, 2008; American College of Neuropsychopharmacology, Scottsdale AZ, 2008; University of Tehran, School of Medicine, 2008; Autism One Conference, Chicago, 2009.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: APA Press; 1994. [Google Scholar]
- Ashley-Koch AE, Mei H, Jaworski J, Ma DQ, Ritchie MD, Menold MM, Delong GR, Abramson RK, Wright HH, Hussman JP, Cuccaro ML, Gilbert JR, Martin ER, Pericak-Vance MA. An analysis paradigm for investigating multi-locus effects in complex disease: examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder. Annals of Human Genetics. 2006;70:281–292. doi: 10.1111/j.1469-1809.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. Journal of Autism and Developmental Disorders. 2001;31:537–544. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Borgatti R, Piccinelli P, Passoni D, Romeo A, Viri M, Musumeci SA, Elia M, Cogliati T, Valseriati D, Grasso R, Raggi ME, Ferrarese C. Peripheral markers of the gamma-aminobutyric acid (GABA)ergic system in Angelman's syndrome. Journal of Child Neurolology. 2003;18:21–25. doi: 10.1177/08830738030180010801. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Smart TG, Moss SJ. Regulation of GABAA Receptors by protein phosphorylation. In: Martin D, Olsen R, editors. GABA in the nervous system: the view at fifty years. Philadelphia, PA: Lippincott, Williams and Wilkins; 2000. pp. 191–206. [Google Scholar]
- Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, Hussman JP, Haines JL, Cuccaro ML, Gilbert JR, Pericak-Vance MA. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J. Elevated plasma gama-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for GABA hypothesis of autism. Medical Science Monitor. 2002;8:PR1–PR6. [PubMed] [Google Scholar]
- Fatemi SH, Halt A, Stary J, Kanodia R, Schulz SC, Realmuto G. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in parietal and cerebellar cortices of autistic subjects. Biological Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophrenia Research. 2005;72:109–122. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophrenia Research. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) Receptor Downregulation in Brains of Subjects with Autism. Journal of Autism and Developmental Disorders. 2009a;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) Receptors Is Altered in Brains of Subjects with Autism. Cerebellum. 2009b;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Molecular Psychiatry. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11-13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Human Molecular Genetics. 2007;16:691–703. doi: 10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogart A, Leung KN, Wang NJ, Wu DJ, Driscoll J, Vallero RO, Schanen NC, La Salle JM. Chromosome 15q11-13 duplication syndrome brain reveals epigenetic alterations in gene expression not predicted from copy number. Journal of Medical Genetics. 2009;46:86–93. doi: 10.1136/jmg.2008.061580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Koga M, Ishiguro H, Horiuchi Y, Syu A, Yoshio T, Takahashi N, Ozaki N, Arinami T. Pathway-based association analysis of genome-wide screening data suggest that genes associated with the gamma-aminobutyric acid receptor signaling pathway are involved in neuroleptic-induced, treatment-resistant tardive dyskinesia. Pharmacogenetics and Genomics. 2008;18:317–323. doi: 10.1097/FPC.0b013e3282f70492. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kakinuma H, Ozaki M, Sato H, Takahashi H. Variation in GABA-A subunit gene copy number in an autistic patient with mosaic 4p duplication (p12p16) American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147B:973–975. doi: 10.1002/ajmg.b.30663. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Association of GABRB3 polymorphisms with autism spectrum disorders in Korean trios. Neuropsychobiology. 2006;54:160–165. doi: 10.1159/000098651. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Balaraman Y, Patel S, Tan J, Sidhu K, Jerome RE, Edenberg HJ, Kuczenski R, Geyer MA, Nurnberger JI, Jr, Faraone SV, Tsuang MT, Niculescu AB. Towards understanding the schizophrenia code: an expanded convergent functional genomics approach. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2007;144B:129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, Ritchie MD, Delong GR, Abramson RK, Wright HH, Cuccaro ML, Hussman JP, Gilbert JR, Pericak-Vance MA. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. American Journal of Human Genetics. 2005;77:377–388. doi: 10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, Rommens JM, Beaudet AL. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nature Genetics. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, Jacobs MM, Folstein SE, Haines JL, Sutcliffe JS. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2004;131:51–59. doi: 10.1002/ajmg.b.30038. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid (A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacological Reviews. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princivalle AP, Duncan JS, Thom M, Bowery NG. GABA(B1a), GABA(B1b) AND GABA(B2) mRNA variants expression in hippocampus resected from patients with temporal lobe epilepsy. Neuroscience. 2003;122:975–984. doi: 10.1016/j.neuroscience.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Qian H, Ripps H. Focus on Molecules: The GABA(C) Receptor. Experimental Eye Research. 2009;88:1002–1003. doi: 10.1016/j.exer.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer RJ, Phelan MC, Michaelis RC, Crawford EC, Skinner SA, Cuccaro M, Simensen RJ, Bishop J, Skinner C, Fender D, Stevenson RE. Autism and maternally derived aberrations of chromosome 15q. American Journal of Medical Genetics. 1998;76:327–336. doi: 10.1002/(sici)1096-8628(19980401)76:4<327::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Kato C, Koishi S, Kawakubo Y, Yamamoto K, Matsumoto H, Hashimoto O, Kim SY, Watanabe K, Kano Y, Nanba E, Kato N, Sasaki T. No evidence for significant association between GABA receptor genes in chromosome. Journal of Human Genetics. 2007;52:985–989. doi: 10.1007/s10038-007-0207-5. [DOI] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Epilepsy in autism. Lancet Neurology. 2002;1:352–358. doi: 10.1016/s1474-4422(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- Zai G, Arnold P, Burroughs E, Barr CL, Richter MA, Kennedy JL. Evidence for the gamma-amino-butyric acid type B receptor 1 (GABBR1) gene as a susceptibility factor in obsessive-compulsive disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2005a;134:25–29. doi: 10.1002/ajmg.b.30152. [DOI] [PubMed] [Google Scholar]
- Zai G, King N, Wong GW, Barr CL, Kennedy JL. Possible association between the gamma-aminobutyric acid type B receptor 1 (GABBR1) gene and schizophrenia. European Neuropsychopharmacology. 2005b;15:347–352. doi: 10.1016/j.euroneuro.2004.12.006. [DOI] [PubMed] [Google Scholar]


