Abstract
Background
Concerns have been raised regarding minimally invasive surgery (MIS) and its possible effect on postoperative functional recovery, complications, and survival rate after TKA.
Questions/purposes
We specifically asked whether MIS TKA would be associated with (1) increased operative time, (2) reduced blood loss, (3) shortened hospital stay, (4) faster recovery of ROM, (5) higher knee scores, (6) inferior component positioning, and (7) increased complications.
Methods
We performed a systematic literature search of randomized controlled trials between minimally invasive and standard approaches in TKA that compared operative time, blood loss, ROM, knee scores, component positioning, and complications. We conducted a systematic review and meta-analysis of 13 trials published from 2007 to 2009 of MIS versus standard TKA.
Results
Patients in the MIS group had longer operating times (10–19 minutes). Mean Knee Society scores were better after MIS than after the standard procedure at 6 and 12 weeks postoperatively, but not after 6 months. Improvement in ROM occurred more rapidly in the MIS group 6 days after TKA but later improvements are not clearly documented. We identified no differences between minimally invasive and standard approaches regarding the short-term overall complications and alignment of femoral and tibial components. However, wound healing problems and infections occurred more frequently in the MIS group.
Conclusions
MIS leads to faster recovery than conventional surgery with similar rates of component malalignment but is associated with more frequent delayed wound healing and infections. Potential benefits in long-term survival rate and functional improvement require additional investigation.
Level of Evidence Level II, therapeutic study (systematic review). See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
A TKA alleviates pain, restores joint function, and allows patients with severe arthritis to return to varied activities of daily living [29, 33, 35, 49]. However, prolonged postoperative pain and delayed return of function that may contribute to patient dissatisfaction or lengthy recuperative periods are the major concerns with the standard surgical approaches [20]. The concept of MIS TKA, introduced more than a decade ago, has created debate as a result of interest from orthopaedic surgeons and patients [46].
There generally is no accepted definition of an MIS approach in TKA. Defining MIS based on only the incision size is inappropriate; the aim of minimally invasive arthroplasty is to minimize damage to soft tissues and consequently to quadriceps function and knee stability. One study defined MIS TKA as an approach that was less damaging to the extensor mechanism and avoided eversion of the patella [20]. Several MIS TKA techniques have been described: traditional mini (mini-medial parapatellar [15], mini-midvastus [20, 21], mini-subvastus [22, 43]) and fully innovative techniques (quad-sparing) [25, 39, 48]. Advocates of MIS TKA commonly suggest it allows patients faster recovery time [9, 15], less blood loss [20], decreased soft tissue trauma [30, 37], more pain relief [18], better cosmetic appearance [15, 18, 26], and measurable improvement of function [9, 15, 48]. Critics of MIS TKA emphasize possible complications associated with long-term outcomes (eg, lower survival rates) and the length of the learning curve [31, 38]. For example, the reduced exposure and visibility associated with MIS has generated unique risks with respect to component alignment [2, 10].
Despite the rapid emergence and promotion of MIS TKA, some surgeons are uncertain if MIS TKA is a reasonable option because of these conflicting observations. Also, the promise of a faster recovery time with less pain and less unsightly scarring through MIS has motivated patients to explore the possibility of undergoing these MIS techniques for their TKA [24, 46]. It therefore is important to clarify the role and benefits of MIS TKA for surgeons who want to offer the option of MIS TKA to patients. Several randomized, controlled trials (RCTs) or quasi-randomized, controlled trials (qRCTs) have compared the outcomes of conventional and minimally invasive techniques for TKA [8, 9, 15, 19–22, 25, 27, 32, 39, 40, 47]. RCTs are widely accepted as the most reliable method of determining the effectiveness of specific therapies. However, these data have not yet been pooled for evaluation of overall outcomes.
Therefore, we performed our meta-analysis in line with the Quality of Reporting of Meta-analyses guidelines. We specifically asked whether MIS TKAs would be associated with (1) increased operative time, (2) reduced blood loss, (3) shortened hospital stay, (4) faster recovery of ROM, (5) higher knee scores, (6) inferior component positioning, and (7) increased complications.
Materials and Methods
We performed a literature search to identify all published RCTs and qRCTs comparing the MIS approach versus standard approach in patients undergoing TKA. The most common databases of published medical literature included Medline, EMBASE, Web of Science, CBMdisc, and Cochrane Library, and these were searched for articles published in English and other languages. We used the following key words: “knee arthroplasty”, “knee replacement”, “minimally invasive”, “mini-incision”. The latest date for this search was April 1, 2009. We conducted our electronic search in three stages. In the first step, two authors (TC, TL) identified titles that discussed any type of comparison between MIS and the standard approach for TKA. In the second stage, two of us (TC, GZ) reviewed the abstracts and chose potentially eligible studies for retrieval of the full text. In the third stage, two of the authors (TC, TL) checked the full-text articles for eligibility criteria. Bibliographies and review articles were examined manually for additional suitable references. If relevant information was needed, we contacted the corresponding authors for additional data.
To be included in the analysis, studies had to (1) compare MIS and standard approaches in patients undergoing TKA for symptomatic knee disease; and (2) be a published RCT or qRCT. Exclusion criteria were if (1) the outcomes of interest were not reported for the two techniques; (2) it was impossible to extrapolate or calculate the necessary data from the published results; and (3) studies contained previously published data.
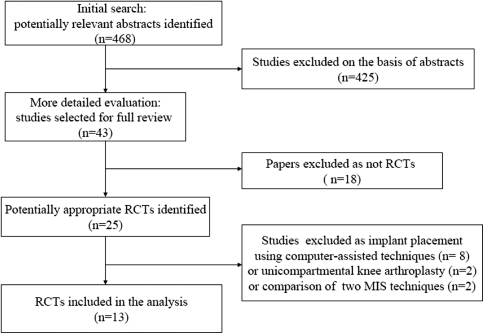
After the computerized search was performed and reference lists were crosschecked extensively, approximately 468 abstracts were identified; of these, 30 studies did not meet the inclusion criteria and were excluded for the following reasons: (1) the study was not a controlled clinical trial (n = 18); or (2) the aim of the article was not to compare the clinical outcome between MIS and standard approaches (n = 12). In the end, 13 articles fulfilled all inclusion criteria and these were selected for data extraction and data analysis (Table 1; Fig. 1). Together, the studies included 827 patients with individual sample sizes ranging from 30 to 120 patients. Most of the studies were from Europe, Asia, and North America, primarily single centers, and from teaching or urban institutions. The choice of implants and cementing techniques, when reported, varied across studies. All studies described patient baseline characteristics adequately and patient samples were well balanced in the available basic demographic items. In most trials, TKA was performed for primary osteoarthritis. However, there may be meaningful differences of baseline patient characteristics between the included studies.
Table 1.
Characteristics of included trials
| Study (number, author, year, country) | Methods | Participants | Interventions | Outcome |
|---|---|---|---|---|
| 1. Chin et al., 2007 [8] Singapore |
RCT, Table generated Sealed envelope Patient and observer blinded |
Group MS: 60; Group SI: 30 Similar DC; Inclusion: OA Exclusion: genu valgus, previous knee surgery, an active infection, or malignancy, and those deemed medically unfit for surgery |
Brand: Zimmer DePuy Patella: resurfaced Cement: yes MI: MMV/QS SI: MPP Two senior surgeons |
Total operative time Total blood loss Length of hospital stay Radiographic results Postoperative complications |
| 2. Kim et al., 2007 [25] Korea |
RCT Computer-generated Observer blinded |
Group MS: 120; Group SI:120 Similar DC Inclusion: OA, ON, RA |
Brand: Zimmer Cement: yes MI: QS 10.5 cm SI: MPP 10.8 cm A senior surgeon Bilateral TKA Followup 21.5 months |
Operative time/tourniquet time Intraoperative blood loss VAS KSS ROM Radiographic results Complications |
| 3. Kolisek et al., 2007 [27] USA |
RCT Multicenter, Stratified by six investigators Sealed envelope Blinding not described |
Group MS: 40; Group SI: 40 Similar DC; Inclusion: OA, Exclusion: varus or valgus > 10° |
Brand: Stryker Patella: resurfaced Cement: yes MI: MMV 9 cm SI: midvastus, medial, parapatellar 16 cm Followup 3 months |
Tourniquet time Intraoperative blood loss Length of hospital stay KSS Quadriceps strength Radiographic results Early complications |
| 4. Shen et al., 2007 [39] China |
RCT Method of randomization, allocation concealment or blinding not described |
Group MS: 26; Group SI: 33 Similar DC Inclusion: OA RA valgus < 10° varus < 20° flexion contracture < 15° Exclusion: BMI > 30 kg/m2, severe valgus or varus deformation, life-threatening medical conditions |
Brand: Zimmer Patella: resurfaced Cement: yes MI: QS 9.5 cm SI: MPP 14.0 cm The same surgeon Unilateral TKA Followup 17 months |
Tourniquet time VAS Knee flexion SLR KSS Radiographic results Complications |
| 5. Tashiro et al., 2007 [40] Japan |
qRCT Method of randomization, allocation concealment or blinding not described |
Group MS: 20; Group SI: 21 Similar DC Inclusion: OA Exclusion: osteotomy or severe osteoporosis |
Brand: Zimmer MI: MMV SI: MPP Unilateral TKA Followup 16 or 14 months |
Operative time Extensor and flexor torque VAS SLR KSS Radiographic results Perioperative complications |
| 6. Chotanaphuti et al., 2008 [9] Thailand |
RCT Method of randomization, allocation concealment or blinding not described |
Group MS: 20; Group SI: 20 Similar DC; Inclusion: OA knee Ahlbach stage 5, flexion contracture < 20° Exclusion: inflammatory joint disease, severe joint laxity > Grade 2, bone loss, life-threatening medical condition |
MI: 2 cm Quad SI: MPP The same experienced surgeon Unilateral TKA |
Operative time/tourniquet time Length of hospital stay ROM Quadriceps function |
| 7. Han et al., 2008 [15] Korea |
RCT Table generated Observer blinded |
Group MS: 15; Group SI: 15 Similar DC; Inclusion: age < 70 years old; no cardiopulmonary comorbidities; flexion contracture ≤ 30°; BMI ≤ 33 kg/m2; OA |
Brand: Zimmer Patella: resurfaced Cement: yes MI: MMPP 10.6 cm SI: MPP 13.4 cm A senior surgeon Bilateral TKA Followup 28 months |
Blood loss Tourniquet time ROM Radiographic results Complications Pain medication |
| 8. Lüring et al., 2008 [32] Germany |
RCT Lot envelope in a box Sealed envelope Exclusion: > 75 years old, previous operation, or trauma varus/valgus/extension > 20° BMI > 35 kg/m2 |
Group MS: 30; Group SI: 30 Similar DC |
Brand: DePuy Cement: yes MI: MMPP 13.2 cm SI: MPP 17.3 cm Two experienced surgeons Unilateral TKA Followup 12 weeks |
Operative time Blood loss KSS WOMAC VAS Radiographic results Complications |
| 9. Karachalios et al., 2008 [20] Greece |
RCT Computer-generated Sealed envelope Patient and observer blinded |
Group MS: 50; Group SI: 50 Similar DC; Inclusion: OA 50–80 years old good mental health, varus or valgus < 15°, fixed flexion < 20°, flexion > 90°, BMI < 35 kg/m2 Exclusion: RA, previous surgery, arthritis of the ipsilateral hip contralateral hip and knees |
Brand: Smith & Nephew Patella: no resurfacing Cement: yes MI: MMV 11 cm SI: MPP 18 cm A surgeon Unilateral TKA Followup 23 months |
Operative time Blood loss Length of hospital stay VAS KSS Oxford score ROM Radiographic results Complications |
| 10. Kashyap & van Ommeren, 2008 [22] UK |
qRCT Choosing alternately Binding not described |
Group MS: 25; Group SI: 25 Similar DC |
Brand: Biomet Patella: no resurfacing MI: MSV 10.5 cm SI: MPP 18.5 cm The same surgeon Unilateral TKA Followup 2 years |
Tourniquet time Blood loss Length of hospital stay ROM Knee flexion KSS, Oxford score Radiographic results Complications |
| 11. Wohlrab et al., 2008 [47] Germany |
RCT Method of randomization, allocation concealment or blinding not described |
Group MS: 30; Group SI: 30 Similar DC; Exclusion: life-threatening medical condition, osteoporosis, fracture or osteotomy |
Brand: Zimmer MI: MMV SI: MPP Unilateral TKA Followup 12 weeks |
Blood loss VAS KSS Radiographic results |
| 12. Juosponis et al., 2009 [19] Lithuania |
RCT Sealed envelope Observer blinded |
Group MS: 35; Group SI: 35 Inclusion: OA Exclusion: BMI > 35 kg/m2, valgus > 10°, varus > 20°, obese and extremely deformed, active flexion < 70°, previous knee surgery |
Brand: DePuy Patella: not resurfacing Cement: yes MI: MMV 9 cm SI: MPP Two experienced surgeons Unilateral TKA Followup 3 months |
Operative time ROM KSS Radiographic results Complications |
| 13. Karpman & Smith, 2009 [21] USA |
RCT Computer-generated Patient and observer blinded |
Group MS: 40; Group SI: 19 Similar DC; Inclusion: OA Exclusion: age > 90 years old, BMI > 38 kg/m2, instrumentation unavailable |
Brand: Zimmer Patella: resurfaced Cement: yes MI: MMV 9.0 cm QS 8.4 cm SI: MPP 10.7 cm One surgeon Unilateral TKA Followup 6 months |
Operative time Blood loss Length of hospital stay VAS ROM Quadriceps strength KSS WOMAC Radiographic results Complications |
RCT = randomized, controlled trial; MI = mini-incision; SI = standard incision; ON = osteonecrosis; RA = rheumatoid arthritis; DC = demographic characteristic; MMV = mini-midvastus; MMPP = mini-medial parapatellar; MPP = medial parapatellar; MSV = mini-subvastus; QS = quadriceps-sparing; VAS = visual analog scale; BMI = body mass index; SLR = straight leg raising.
Fig. 1.
The flow diagram shows identification of randomized, controlled trials (RCTs) in the systematic review.
Two of us (TC, TL) independently assessed methodologic quality of the included trials without masking of authors or source. All disagreements were resolved by discussion. RCTs were judged according to the checklists [16]. The methodologic quality of individual studies was variable (Table 2). All studies were RCTs or qRCTs. However, some of the included randomized trials did not adequately describe quality items (eg, mode of randomization, blinding, allocation concealment) that often are used for assessment of overall trial quality. The method of random sequence generation in five studies included computers and tables [8, 15, 20, 21, 25]. The method of concealment reportedly was sealed envelopes in five studies [8, 19, 20, 27, 31]. None of the studies used blinding of the surgeon. Because in surgical trials the surgeon cannot be blinded, it is possible that surgeons were biased in treatment of patients with MIS, such as more intensive postoperative care pathways and postoperative surveillance and monitoring pain management, that might alter the overall comparative effect of the treatment group. Two studies used patient blinding and seven studies used observer blinding.
Table 2.
Quality assessment of randomized controlled trials
| Item | Chin et al. 2007 [8] | Kim et al. 2007 [25] | Kolisek et al. 2007 [27] | Shen et al. 2007 [39] | Tashiro et al. 2007 [40] | Chotanaphuti et al. 2008 [9] | Han et al. 2008 [15] | Lüring et al. 2008 [32] | Karachalios et al. 2008 [20] | Kashyap & van Ommeren 2008 [22] | Wohlrab et al. 2009 [47] | Juosponis et al. 2009 [19] | Karpman & Smith [21] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was there clear concealment of allocation? | 3 | 1 | 3 | 1 | 1 | 1 | 0 | 3 | 3 | 0 | 1 | 3 | 1 |
| 2. Were the inclusion and exclusion criteria clearly defined? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 3. Were the outcomes of trial participants who withdrew or excluded after allocation described and included in an intention-to-treat analysis? | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 4. Were the treatment and control groups adequately described at entry, and if so were the groups well matched or appropriate covariate adjustment made? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5.Were the healthcare workers/ surgeons experienced in both interventions prior to the start of the trials? | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| 6. Were the care programs other than trial options identical? | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 7.Were the outcome measures clearly defined in the text with a definition of any ambiguous terms encountered? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8. Were the outcome assessors blind to assignment status? | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| 9. Was the timing of outcome measures appropriate? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 10.Was followup active with the trial participants being called back or approached for assessment at set times or passive? | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 11.Was loss to followup reported, and if so were less than 5% of trial participants lost to followup? | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 12.Were the authors able to provide supplementary details of the trial in addition to published data? | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 11 | 9 | 8 | 10 | 7 | 7 | 11 | 12 | 11 | 7 | 5 | 10 | 10 |
Two investigators (TC, XZ) independently extracted the name of the first author, year of publication, study design, participant demographics, type and manufacturer of prosthesis, inclusion and exclusion criteria, surgical approach, total number of patients, duration of followup, and outcome measure used. The following outcomes parameters were used to compare the MIS approach with the standard approach: (1) perioperative outcomes included blood loss, operative time, length of hospital stay, and ROM; (2) composite knee scores such as Knee Society score (KSS); (3) postoperative radiographs included prosthesis alignment and limb alignment; and (4) overall and individual complications included delayed wound healing, infection.
We tested for the presence of heterogeneity with the chi square-based Q statistic (with a level of significance of p = 0.1) and quantified its extent with the I2 statistic. The possible value of I2 ranges from 0% to 100% and values 75% or greater imply very high heterogeneity [17]. Subgroup analyses were attempted to determine the effects of the surgical approach and duration of flowup. We conducted a sensitivity analysis based on the quality of the study for each outcome to study the causes of potential heterogeneity between these studies. Publication bias was assessed with funnel plots, which showed the precision in estimating the treatment effect increases as the sample size of the trials increases. Results were combined using a weighted mean difference (WMD) for continuous outcomes. For categorical outcomes the odds ratio or risk difference was calculated as the summary statistics. To pool data, we used a random effects model to control for increased study heterogeneity. The more conservative random effects model involves an assumption that the effects being estimated in the different studies follow some distribution, but results in wider confidence intervals [11, 44, 45].
Results
Of the 13 studies, 10 recorded operative time or tourniquet time. Using a random effects model, the overall results showed a longer operating time and tourniquet time for the MIS groups by 10.49 minutes and 12.08 minutes, respectively (Table 3). Subgroup analysis for studies with the mini-midvastus (MMV) approach showed there was a longer operating time for the MIS group by 18.95 minutes. Subgroup analysis for studies with the quadriceps-sparing (QS) approach showed there was a longer tourniquet time for the MIS group by 13.24 minutes. These studies reported the MIS approach averaged a longer operative time or tourniquet time than the standard approach. However, Karpman and Smith [21] found operations performed with the standard medial parapatellar incision and MMV mini-incision took a shorter total time as compared with the QS incision. Chotanaphuti et al. [9] found no difference between the conventional TKA group and the 2-cm limited quadriceps exposure MIS TKA group.
Table 3.
Results of overall meta-analysis
| Outcome | Number of studies | Number | Point estimate WMD/OR | Tests for heterogeneity |
|---|---|---|---|---|
| (sample size) | of patients | (95% CI) (p value) | (p value, I2) | |
| Perioperative outcome | ||||
| Operative time | 7 | 648 | 10.49 (4.18, 16.79) (p = 0.001) | p < 0.00001,88% |
| Tourniquet time | 6 | 499 | 12.08 (5.08, 19.07) (p = 0.0007) | p < 0.00001, 86% |
| Total blood loss | 4 | 198 | −31.05 (−99.40, 37.31) (p = 0.37) | p = 0.60, 0% |
| Intraoperative blood loss | 2 | 320 | 3.60 (−23.71, 30.91) (p = 0.80) | p = 0.46, 0% |
| Postoperative blood loss | 3 | 370 | −183.89 (−464.10,96.31) (p = 0.20) | p = 0.002 84% |
| Length of stay | 5 | 299 | −4.10 (−10.67, 2.48) (p = 0.22) | p = 0.008, 65% |
| ROM | ||||
| At 6 days | 3 | 200 | 10.80 (8.00, 13.59) (p < 0.00001) | p = 0.23, 32% |
| KSS (knee scoring) | ||||
| At 6 weeks | 4 | 309 | 9.71 (2.11, 17.32) (p = 0.01) | p < 0.00001, 92% |
| At 12 weeks | 7 | 658 | 2.84 (1.58, 4.10) (p < 0.0001) | p = 0.37, 7% |
| At 6 months | 2 | 149 | 2.49 (−2.41, 7.39) (p = 0.32) | p = 0.10, 62% |
| At 12 months | 4 | 448 | 0.18 (−2.12, 2.48) (p = 0.88) | p = 0.33, 13% |
| KSS (functional scoring) | ||||
| At 6 weeks | 5 | 388 | 3.79 (−4.36, 11.95) (p = 0.36) | p < 0.00001, 93% |
| At 12 weeks | 8 | 677 | 4.27 (−1.15, 9.69) (p = 0.12) | p < 0.00001, 87% |
| At 6 months | 2 | 227 | 0.58 (−12.75, 13.90) (p = 0.93) | p < 0.00001, 96% |
| At 12 months | 4 | 389 | 4.06 (−5.31, 13.43) (p = 0.40) | p < 0.00001, 96% |
| Radiographic outcomes | ||||
| α angle | 3 | 349 | 0.83 (−0.84, 2.50) (p = 0.33) | p = 0.008, 79% |
| β angle | 3 | 349 | 1.08 (−0.16, 2.31) (p = 0.09) | p = 0.001, 85% |
| γ angle | 3 | 349 | 0.35 (−1.13, 0.43) (p = 0.38) | p = 0.17, 43% |
| δ angle | 3 | 349 | 1.05 (−0.21, 2.31) (p = 0.10) | p = 0.010, 78% |
| Femorotibial angle | 4 | 408 | 0.00 (−0.68, 0.68) (p = 1.00) | p = 0.10, 51% |
| Femoral alignment outlier | 3 | 229 | 1.45 (0.10, 21.66) (p = 0.79) | p = 0.12, 60% |
| Tibial alignment outlier | 3 | 229 | 0.31 (0.08, 1.05) (p = 0.06) | p = 0.94, 0% |
| Complication | 8 | 747 | 1.53 (0.90, 2.59) (p = 0.11) | p = 0.53, 0% |
OR = odds ratio; WMD = weighed mean difference; CI = confidence interval; KSS = Knee Society scoring; ROM = range of motion.
Six studies (n = 398 patients) evaluated total, intraoperative, or postoperative blood loss. Using a random effects model, we found no differences in blood loss between the two groups (Table 3). Subgroup analysis for studies with the MMV approach showed there also was no difference in blood loss between the two groups (Table 4). Most studies reported no difference between the two groups regarding blood loss. However, three studies found blood loss was less in the MIS study arms as compared with the standard technique study arms [15, 20, 23].
Table 4.
Results of subgroup meta-analysis
| Outcome | Number of studies | Number | WMD/OR | Tests for HG |
|---|---|---|---|---|
| (sample size) | of patients | (95% CI) (p value) | (p value, I2) | |
| Studies with score of 10 or greater | ||||
| Operative time | 5 | 697 | 12.52 (6.29, 18.74), < 0.0001 | < 0.00001, 88% |
| Complications | 6 | 747 | 1.31 (0.67, 1.2.57), 0.43 | 0.35, 11% |
| Studies with MMV approach | ||||
| Operative time | 5 | 318 | 18.95 (7.36, 30.55), 0.001 | < 0.00001,94% |
| Complications | 6 | 398 | 1.17 (0.51, 2.67), 0.71 | 0.34, 11% |
| Studies with a followup of at least 12 months | ||||
| Complications | 5 | 747 | 1.73 (0.89, 3.36), 0.11 | 0.64, 0% |
HG = heterogeneity; WMD = weighted mean difference; OR = odds ratio; CI = confidence interval; MMV = mini-midvastus.
Five studies provided details regarding the length of hospital stay. Using a random effects model, we found no difference between the MIS and standard approach. Subgroup analysis for studies with the MMV approach showed no difference between the two groups.
The progression of ROM was faster in the MIS group 6 days after TKA. These advantages of MIS TKA may be beneficial to patients during the early postoperative period after TKAs. However, middle and long-term ranges of motion for patients having TKA are unavailable in most studies, therefore, we did not pool middle and long-term followup data.
The KSS, Oxford knee score, WOMAC score, and Hospital for Special Surgery knee score were used for clinical assessment of patients (in eight trials, two trials, two trials, and one trial, respectively). However, the times at which the outcomes were measured differed among these trials, therefore, it was not possible to pool all the knee score data across all of the included studies. After pooling those data at different times, the KSS was better after MIS than after the standard procedure at 6 and 12 weeks postoperatively, but this difference no longer was significant after 6 months (Table 3).
Four trials reported radiographic measurements or alignment of the femoral and tibial components by Knee Society radiographic evaluation. Using random effects modeling, meta-analysis showed no difference between the two groups with respect to assessment of the femoral and tibial components (Table 3).
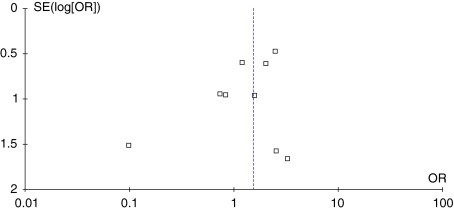
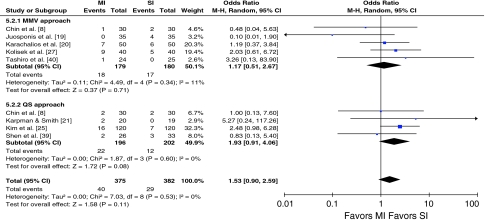
The funnel plot for the outcome of complications shows some mild asymmetry (Fig. 2). In this situation, the effect calculated in a meta-analysis will tend to overestimate the treatment effect. The pooled odds ratio for overall postoperative complications showed no difference between the two groups without heterogeneity (Table 3). Although tests for heterogeneity were nonsignificant, we conducted sensitivity analyses to test our hypotheses. Subgroup analysis for studies with the MMV or QS approach showed no difference between the two groups without heterogeneity (I2 = 0%) (Fig. 3). When studies with the quality score of 10 or greater and followup of at least 12 months were analyzed separately, there also was no difference without heterogeneity (I2 = 0%) (Table 4). Eight studies presented data regarding the incidence of superficial wound healing problems (such as superficial infection, and delayed healing) and three presented data for deep infections. Pooling of the results showed there was no difference between the two groups regarding superficial wound healing problems and deep infection, respectively (Table 5). Pooling of the data for the prevalence of deep venous thrombosis (DVT) in four studies also showed no difference between the two groups (Table 5).
Fig. 2.
The funnel plot asymmetry for the outcome of postoperative complications shows the evidence of publication bias.
Fig. 3.
The forest plot for postoperative complications shows there were no differences between minimally invasive and standard techniques. CI = confidence interval.
Table 5.
Results of postoperative complications
| Outcome | Number of studies | Number | MI | SI | Risk Difference | Tests for HG |
|---|---|---|---|---|---|---|
| (sample size) | of patients | (95% CI) (p value) | (p value, I2) | |||
| Surgical adverse events | ||||||
| Superficial wound healing | 8 | 727 | 15 | 5 | 2.25 (0.90, 5.61), 0.08 | 1.00, 0% |
| Deep infections | 3 | 349 | 4 | 1 | 2.09 (0.40, 11.01), 0.39 | 0.95, 0% |
| Deep venous thrombosis | 4 | 449 | 3 | 7 | −0.02 (−0.07, 0.03), 0.45 | 0.10, 52% |
| Knee stiffness | 3 | 240 | 3 | 3 | ||
| Periprosthetic fracture | 2 | 200 | 3 | 4 | ||
| Notching of the anterior femoral condyle | 1 | 120 | 5 | 2 | ||
| Tibial component subside | 1 | 80 | 1 | 1 | ||
| Sciatica | 1 | 80 | 1 | 0 | ||
| Palsy of the deep peroneal nerve | 1 | 120 | 2 | 0 | ||
| Anterior knee pain | 1 | 100 | 1 | 1 | ||
| Patella displacement | 1 | 70 | 0 | 4 | ||
| Laceration of the tendon | 1 | 120 | 3 | 0 | ||
| Medial complications | ||||||
| Myocardial infarction | 1 | 80 | 1 | 0 | ||
| Mental changes | 1 | 80 | 1 | 0 | ||
| Congestive heart failure | 1 | 80 | 0 | 1 | ||
| Total | 9 | 43 | 29 | |||
MI = mini-incision; SI = standard incision; HG = heterogeneity.
Discussion
Although the literature suggests high survival rates for conventional TKAs [36], patients often have major concerns regarding postoperative pain and length of recovery [7, 24]. Surgeons have attempted to achieve additional improvement in postoperative outcomes by reducing the size and number of incisions [4, 28, 41]. To provide more comprehensive information regarding the advantages and disadvantages of MIS, we systematically reviewed RCTs or qRCTs (Level I or II) comparing MIS techniques with conventional TKA. Our study reviewed these articles published during the last few years to clarify whether patients having MIS TKA were prone to less blood loss, more operative time, shorter length of hospital stay, better knee scores, inferior component positioning, and increased complications.
Although we attempted a well-designed study, we encountered numerous limitations with the literature. First, for some end points in our meta-analysis (eg, operative time), there was strong statistical heterogeneity. Therefore the corresponding syntheses should be interpreted with caution. This clinical heterogeneity was evident in the nonuniform definitions, end points, types of implants, and care programs, and also in the possible differences in baseline demographics, such as severity of the disease. This underscores the need for uniformity in outcomes measurement methods and better controlled cohorts in clinical orthopaedic research. A random effects model can compensate for statistical heterogeneity, but not for real clinical heterogeneity. We tried to control for this heterogeneity by clearly defining a priori inclusion and exclusion criteria and the outcomes to be studied. Our study is a systematic analysis of the best our research has offered on this topic in numbers that make our conclusions useful. Furthermore, potential sources of heterogeneity should be explored with sensitivity analyses and subgroup analyses. Second, there is an obvious lack of long-term prospective RCTs. The followup periods were short but covered the critical time when the benefits of the MIS approach to THA are maximal. We believe these parameters from short-term followup may reflect the long-term results to some extent. However, the short followups do not allow one to determine any potential adverse longer-term effects such as higher revision rates. Third, we did not distinguish major from minor complications in our research. Clearly, all complications are not the same, as some are trivial for the patient and do not affect function or survival in the long term, and others are major and may lead to permanent disability or death. In trials with small numbers of subjects, the absence of significant differences in individual complications must be interpreted with caution, because these studies often are inadequately powered to detect such differences. Considering postoperative complication rates were low, we pooled all types of postoperative complications. Fourth, because we did not search abstracts of meetings, publication bias may have affected the results. Although publication bias has long been associated with funnel plot asymmetry, the result from such an analysis should be interpreted with caution because funnel plots have limited power to detect bias if the number of small trials is limited. Incomplete reporting or nonreporting of outcomes may be related to their level of significance in the pertinent trials. This phenomenon has been termed “outcome reporting bias” and it may influence the results of the quantitative synthesis. In contrast to prior systematic reviews on this topic, our study was limited to RCTs and therefore was substantially less prone to selection bias. In addition, we made no restrictions for language. The trials included in our meta-analysis are from authors from different countries and institutions.
The overall and subgroup meta-analyses showed operative and tourniquet times were longer with the MIS approach. Although it is uncertain if 10.49 to 18.95 minutes is clinically important, the theoretical disadvantages of prolonged operative and tourniquet time include increased blood loss and tourniquet-associated ischemia, increased incidence of infection, delayed wound healing, and added cost. Actually, we found there were more frequent wound problems and infections in the MIS group. There was some evidence suggesting the operative time of MIS could be shortened by increased experience and familiarity with the new technique and instruments in some high-volume centers [1, 31]. A learning phase was identified as approximately 10 months or 21 knee arthroplasties using the MIS technique. In one study, it took 50 operations before the surgical time equaled that of the open technique [23]. It is possible there would be a gradual reduction of operative time for patients undergoing MIS during the learning phase, but even later it takes slightly longer than standard surgery. Although the senior surgeon performed MIS TKA to eliminate bias resulting from the learning curve in most of the included RCTs, one cannot presume the ability of each arthroplasty surgeon to perform MIS TKA would be at the same level. This may explain why heterogeneity was very high in our meta-analysis.
MIS techniques require more surgical steps to achieve sufficient observation of anatomic landmarks to place the instrument and implant. Moreover, with the small incision, intuitive optimization of the moving surgical window was required in which the knee was ranged more frequently. All of these steps are time-consuming and directly related to the technique. A ubiquitous problem that comes with MIS is conversion to standard incision surgery. Two RCTs provide some evidence regarding intraoperative conversion of the mini-incision to a standard approach as a result of surgical exposure and technical difficulty [20, 39]. We believe additional modifications of instruments and prostheses are needed to make the minimally invasive technique more accurate and easier.
A key consideration when a new surgical technique is introduced is the rate of complications. Total and subgroup analyses indicated there was no difference between the two groups with no significant heterogeneity. In all included studies, there were no intraoperative major complications. However, the clinically important difference in postoperative complications may depend on the underlying risk of events. It may be possible that a risk difference of 2% has little practical value, but the trends of our data may represent a proportionally much larger and potentially important change in infection and DVT rates. Postoperative DVT was more common in the standard approach group. MIS allows patients to have early postoperative mobilization, which may improve venous blood flow, thereby decreasing the risk of having a DVT postoperatively after orthopaedic surgery. However, delayed wound healing and superficial wound infections were more common with the MIS techniques. The increased incidence of delayed wound healing in the MIS group may be related to forceful skin stretching and retracting as a result of limited observation. In addition, the time the tissue is exposed to the outside environment is prolonged in the MIS group, which may lead to potential wound contamination and perioperative hypoxia. The knee is a superficial joint, being easy to expose surgically, and yet superficial wound-healing problems after primary TKA are at increased risk for additional complications, including deep infection and/or major subsequent surgery, specifically, resection arthroplasty, amputation, or muscle flap coverage [13]. The much higher infection rates and wound problems in the MIS group should not be ignored.
Although we cannot definitively conclude that the ranges of component alignment in these studies directly translates to inferior or improved implant longevity for TKA, the literature and clinical experience tell us technical errors of component positioning may adversely affect the long-term performance and low revision rates. A varus/valgus malalignment greater than 3° influences the longevity of the prosthesis [32]. We found no differences between the groups in terms of the analyses of component angles. The incidence of patients who have malignment exceeding 3° is also a valuable outcome measure. However, the lack and inconsistency of reporting of the end point result is the difficulty of quantitative summary. Barrack et al. [2] reported MIS TKA accounted for a substantial percentage of revision TKA in recent years at three referral centers that had been performing revision surgery predominantly by area community surgeons. Huang et al. [18] found that although the QS group was faster in regaining quadriceps strength and knee flexion and had less pain during the first 2 postoperative weeks, there were more outliers and bone injuries during surgery. However, these studies were not RCTs. Our inferences based on the results of all available evidence from RCTs and qRCTs may be reliable, but we should note that decreased observation of the operative field associated with MIS might lead to increased prosthetic malalignment. Repeated prudent examination of the position of instruments and cut surface is important to avoid outliers. Computer-assisted navigation systems are promising for increasing the precision of implanting prostheses during TKAs, but the navigation procedure further extends surgery time [3, 5, 6, 12, 42].
The reliability of a TKA performed through MIS will depend on some factors, including appropriate patient selection, surgeon experience, and surgical environment [1, 26]. In our meta-analysis, most trials had defined strict inclusion and exclusion criteria, limiting the generalizability of these results. A high-volume arthroplasty surgeon should perform MIS TKA in selected cases only after appropriate counseling of patients [20]. Although the rates of outliers and tourniquet time may improve with time and experience, surgeons must be careful in their learning curve [26]. The absolute length of the incision is not important, but the goal is to restrict the surgical trauma to only what is needed for safe exposure. In a comparative study, one interesting finding was worse responses from patients undergoing MIS regarding sinking and curling of scar edges [14]. Niki et al. [34] reported the degree of muscle damage was equivalent between MIS TKA and conventional TKA by measuring postoperative serum levels of various muscle-related enzymes. Surgeons should reconsider what is meant by the term minimally invasive to the knees during TKA.
Our meta-analysis of RCTs suggests no differences in short-term total complication rate and radiographic implant alignment between minimally invasive and standard approaches. In comparison with the standard approach, MIS TKA was associated with faster recovery of knee function and longer operating time. However, MIS TKA also was associated with greater risk of delayed wound healing and infections. It is difficult to recruit patients in RCTs if the differences in incision lengths are marked. We collected as many RCTs as possible to perform the systematic review to obtain some key information regarding MIS TKA. More larger, well-conducted, and well-reported RCTs with long-term followups are required to evaluate the clinical results and cost benefits of MIS TKA.
Acknowledgments
We thank all corresponding authors from the studies we used in this meta-analysis for their assistance in obtaining additional data that contributed to our study.
Footnotes
One of more of the authors (TC and XZ) received funding from the Doctoral Students Innovation Fund of Shanghai Jiao Tong University School of Medicine (BXJ0930) and New Medical Technology Development Program of Shanghai Shenkang Hospital Development Center (SHDC12006103).
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Aglietti P, Baldini A, Giron F, Sensi L. Minimally invasive total knee arthroplasty: is it for everybody? HSS J. 2006;2:22–26. doi: 10.1007/s11420-005-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrack RL, Barnes CL, Burnett RS, Miller D, Clohisy JC, Maloney WJ. Minimal incision surgery as a risk factor for early failure of total knee arthroplasty. J Arthroplasty. 2009;24:489–498. doi: 10.1016/j.arth.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Biasca N, Wirth S, Bungartz M. Mechanical accuracy of navigated minimally invasive total knee arthroplasty (MIS TKA) Knee. 2009;16:22–29. doi: 10.1016/j.knee.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Boerger TO, Aglietti P, Mondanelli N, Sensi L. Mini-subvastus versus medial parapatellar approach in total knee arthroplasty. Clin Orthop Relat Res. 2005;440:82–87. doi: 10.1097/01.blo.0000185755.09777.2d. [DOI] [PubMed] [Google Scholar]
- 5.Bonutti PM, Dethmers D, Ulrich SD, Seyler TM, Mont MA. Computer navigation-assisted versus minimally invasive TKA: benefits and drawbacks. Clin Orthop Relat Res. 2008;466:2756–2762. doi: 10.1007/s11999-008-0429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonutti PM, Dethmers DA, McGrath MS, Ulrich SD, Mont MA. Navigation did not improve the precision of minimally invasive knee arthroplasty. Clin Orthop Relat Res. 2008;466:2730–2735. doi: 10.1007/s11999-008-0359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullens PH, Loon CJ, Waal Malefijt MC, Laan RF, Veth RP. Patient satisfaction after total knee arthroplasty: a comparison between subjective and objective outcome assessments. J Arthroplasty. 2001;16:740–747. doi: 10.1054/arth.2001.23922. [DOI] [PubMed] [Google Scholar]
- 8.Chin PL, Foo LS, Yang KY, Yeo SJ, Lo NN. Randomized controlled trial comparing the radiologic outcomes of conventional and minimally invasive techniques for total knee arthroplasty. J Arthroplasty. 2007;22:800–806. doi: 10.1016/j.arth.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Chotanaphuti T, Ongnamthip P, Karnchanalerk K, Udombuathong P. Comparative study between 2 cm limited quadriceps exposure minimal invasive surgery and conventional total knee arthroplasty in quadriceps function: prospective randomized controlled trial. J Med Assoc Thai. 2008;91:203–207. [PubMed] [Google Scholar]
- 10.Dalury DF, Dennis DA. Mini-incision total knee arthroplasty can increase risk of component malalignment. Clin Orthop Relat Res. 2005;440:77–81. doi: 10.1097/01.blo.0000185757.17401.7b. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Dutton AQ, Yeo SJ, Yang KY, Lo NN, Chia KU, Chong HC. Computer-assisted minimally invasive total knee arthroplasty compared with standard total knee arthroplasty: a prospective, randomized study. J Bone Joint Surg Am. 2008;90:2–9. doi: 10.2106/JBJS.F.01148. [DOI] [PubMed] [Google Scholar]
- 13.Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91:48–54. doi: 10.2106/JBJS.G.01371. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein WM, Ali R, Branson JJ, Berland KA. Comparison of patient satisfaction with incision cosmesis after standard and minimally invasive total hip arthroplasty. Orthopedics. 2008;31:368. doi: 10.3928/01477447-20080401-11. [DOI] [PubMed] [Google Scholar]
- 15.Han I, Seong SC, Lee S, Yoo JH, Lee MC. Simultaneous bilateral MIS-TKA results in faster functional recovery. Clin Orthop Relat Res. 2008;466:1449–1453. doi: 10.1007/s11999-008-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handoll HH, Parker MJ. Conservative versus operative treatment for hip fractures in adults. Cochrane Database Syst Rev. 2008;3:CD000337. doi: 10.1002/14651858.CD000337.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Huang HT, Su JY, Chang JK, Chen CH, Wang GJ. The early clinical outcome of minimally invasive quadriceps-sparing total knee arthroplasty: report of a 2-year follow-up. J Arthroplasty. 2007;22:1007–1012. doi: 10.1016/j.arth.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Juosponis R, Tarasevicius S, Smailys A, Kalesinskas RJ. Functional and radiological outcome after total knee replacement performed with mini-midvastus or conventional arthrotomy: controlled randomised trial. Int Orthop. 2009;33:1233–1237. doi: 10.1007/s00264-008-0630-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karachalios T, Giotikas D, Roidis N, Poultsides L, Bargiotas K, Malizos KN. Total knee replacement performed with either a mini-midvastus or a standard approach: a prospective randomised clinical and radiological trial. J Bone Joint Surg Br. 2008;90:584–591. doi: 10.1302/0301-620X.90B5.20122. [DOI] [PubMed] [Google Scholar]
- 21.Karpman RR, Smith HL. Comparison of the early results of minimally invasive vs standard approaches to total knee arthroplasty: a prospective, randomized study. J Arthroplasty. 2008;24:681–688. doi: 10.1016/j.arth.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Kashyap SN, Ommeren JW. Clinical experience with less invasive surgery techniques in total knee arthroplasty: a comparative study. Knee Surg Sports Traumatol Arthrosc. 2008;16:544–548. doi: 10.1007/s00167-008-0523-0. [DOI] [PubMed] [Google Scholar]
- 23.Kashyap SN, Ommeren JW, Shankar S. Minimally invasive surgical technique in total knee arthroplasty: a learning curve. Surg Innov. 2009;16:55–62. doi: 10.1177/1553350609331396. [DOI] [PubMed] [Google Scholar]
- 24.Kim TK, Choi J, Shin KS, Chang CB, Seong SC. Patients’ perspective on controversial issues in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2008;16:297–304. doi: 10.1007/s00167-007-0468-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Kim JS, Kim DY. Clinical outcome and rate of complications after primary total knee replacement performed with quadriceps-sparing or standard arthrotomy. J Bone Joint Surg Br. 2007;89:467–470. doi: 10.1302/0301-620X.89B4.18663. [DOI] [PubMed] [Google Scholar]
- 26.King J, Stamper DL, Schaad DC, Leopold SS. Minimally invasive total knee arthroplasty compared with traditional total knee arthroplasty: assessment of the learning curve and the postoperative recuperative period. J Bone Joint Surg Am. 2007;89:1497–1503. doi: 10.2106/JBJS.F.00867. [DOI] [PubMed] [Google Scholar]
- 27.Kolisek FR, Bonutti PM, Hozack WJ, Purtill J, Sharkey PF, Zelicof SB, Ragland PS, Kester M, Mont MA, Rothman RH. Clinical experience using a minimally invasive surgical approach for total knee arthroplasty: early results of a prospective randomized study compared to a standard approach. J Arthroplasty. 2007;22:8–13. doi: 10.1016/j.arth.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Laskin RS. Minimally invasive total knee arthroplasty: the results justify its use. Clin Orthop Relat Res. 2005;440:54–59. doi: 10.1097/01.blo.0000186562.08685.a2. [DOI] [PubMed] [Google Scholar]
- 29.Laskin RS. Surgical exposure for total knee arthroplasty: for everything there is a season. J Arthroplasty. 2007;22(4 suppl 1):12–14. doi: 10.1016/j.arth.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi AV, Jr, Viacava AJ, Berend KR. Rapid recovery protocols and minimally invasive surgery help achieve high knee flexion. Clin Orthop Relat Res. 2006;452:117–122. doi: 10.1097/01.blo.0000238824.56024.7a. [DOI] [PubMed] [Google Scholar]
- 31.Lubowitz JH, Sahasrabudhe A, Appleby D. Minimally invasive surgery in total knee arthroplasty: the learning curve. Orthopedics. 2007;30(8 suppl):80–82. [PubMed] [Google Scholar]
- 32.Lüring C, Beckmann J, Haiböck P, Perlick L, Grifka J, Tingart M. Minimal invasive and computer assisted total knee replacement compared with the conventional technique: a prospective, randomised trial. Knee Surg Sports Traumatol Arthrosc. 2008;16:928–934. doi: 10.1007/s00167-008-0582-2. [DOI] [PubMed] [Google Scholar]
- 33.Morgan SS, Bonshahi A, Pradhan N, Gregory A, Gambhir A, Porter ML. The influence of postoperative coronal alignment on revision surgery in total knee arthroplasty. Int Orthop. 2008;32:639–642. doi: 10.1007/s00264-007-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niki Y, Mochizuki T, Momohara S, Saito S, Toyama Y, Matsumoto H. Is minimally invasive surgery in total knee arthroplasty really minimally invasive surgery? J Arthroplasty. 2009;24:499–504. doi: 10.1016/j.arth.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Rousseau MA, Lazennec JY, Catonné Y. Early mechanical failure in total knee arthroplasty. Int Orthop. 2008;32:53–56. doi: 10.1007/s00264-006-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santini AJ, Raut V. Ten-year survival analysis of the PFC total knee arthroplasty: a surgeon’s first 99 replacements. Int Orthop. 2008;32:459–465. doi: 10.1007/s00264-007-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroer WC, Diesfeld PJ, Reedy ME, Lemarr AR. Isokinetic strength testing of minimally invasive total knee arthroplasty recovery. J Arthroplasty. 2010;25(2):274–279. doi: 10.1016/j.arth.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Shankar NS. Minimally invasive technique in total knee arthroplasty: history, tips, tricks and pitfalls. Injury. 2006;37(suppl 5):S25–S30. doi: 10.1016/S0020-1383(07)70008-6. [DOI] [PubMed] [Google Scholar]
- 39.Shen H, Zhang XL, Wang Q, Shao JJ, Jiang Y. [Minimally invasive total knee arthroplasty through a quadriceps sparing approach: a comparative study] [in Chinese] Zhonghua Wai Ke Za Zhi. 2007;45:1083–1086. [PubMed] [Google Scholar]
- 40.Tashiro Y, Miura H, Matsuda S, Okazaki K, Iwamoto Y. Minimally invasive versus standard approach in total knee arthroplasty. Clin Orthop Relat Res. 2007;463:144–150. [PubMed] [Google Scholar]
- 41.Tria AJ, Jr, Coon TM. Minimal incision total knee arthroplasty: early experience. Clin Orthop Relat Res. 2003;416:185–190. doi: 10.1097/01.blo.0000093030.56370.d9. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich SD, Mont MA, Bonutti PM, Seyler TM, Marker DR, Jones LC. Scientific evidence supporting computer-assisted surgery and minimally invasive surgery for total knee arthroplasty. Exp Rev Med Devices. 2007;4:497–505. doi: 10.1586/17434440.4.4.497. [DOI] [PubMed] [Google Scholar]
- 43.Varela-Egocheaga JR, Suárez-Suárez MA, Fernández-Villán M, González-Sastre V, Varela-Gómez JR, Rodríguez-Merchán C. Minimally invasive subvastus approach: improving the results of total knee arthroplasty: a prospective, randomized trial. Clin Orthop Relat Res. 2009 Nov 13 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 44.Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip: systematic review and meta-analysis. Clin Orthop Relat Res. 2009;467:3283–3289. doi: 10.1007/s11999-009-0924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vavken P, Dorotka R. A systematic review of conflicting meta-analyses in orthopaedic surgery. Clin Orthop Relat Res. 2009;467:2723–2735. doi: 10.1007/s11999-009-0765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warth LC, Callaghan JJ, Liu SS, Klein GR, Hozack WJ. Internet promotion of minimally invasive surgery and computer-assisted orthopedic surgery in total knee arthroplasty by members of American Association of Hip and Knee Surgeons. J Arthroplasty. 2007;22(6 suppl 2):13–16. doi: 10.1016/j.arth.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 47.Wohlrab D, Gutteck N, Hildebrand M, Zeh A, Hein W. Influence of the surgical approach on postoperative rehabilitation after TKA [in German] Z Orthop Unfall. 2008;146:200–205. doi: 10.1055/s-2008-1038398. [DOI] [PubMed] [Google Scholar]
- 48.Yu JK, Yu CL, Ao YF, Gong X, Wang YJ, Wang S, Xing X, Chen LX, Ju XD. Comparative study on early period of recovery between minimally invasive surgery total knee arthroplasty and minimally invasive surgery-quadriceps sparing total knee arthroplasty in Chinese patients. Chin Med J (Engl). 2008;121:1353–1357. [PubMed] [Google Scholar]
- 49.Zhang XL, Shen H, Qin XL, Wang Q. Anterolateral muscle sparing approach total hip arthroplasty: an anatomic and clinical study. Chin Med J (Engl). 2008;121:1358–1363. [PubMed] [Google Scholar]