Abstract
Background
Minimally invasive approaches such as sclerotherapy have been introduced to treat aneurysmal bone cysts. Sclerotherapy has been associated with reasonable healing rates during the past two decades. However, it is unclear whether sclerotherapy compares with the more traditional extended curettage and bone grafting.
Questions/purposes
We therefore compared the healing rates and functional scores in patients having percutaneous repetitive sclerotherapy using polidocanol (Group 1) with those with intralesional excision (extended curettage with a high-speed burr) and bone grafting (Group 2) for treatment of aneurysmal bone cyst.
Patients and Methods
We randomly divided 94 patients into two treatment groups. We assessed healing rates (primary outcome measure), pain relief, time to healing and recurrence, hospital stay, and the Enneking functional score. Forty-five patients from Group 1 and 46 from Group 2 were available for study. The minimum followup was 3.2 years (mean, 4.4 years; range, 3.2–6.1 years).
Results
At last followup, 93.3% in Group 1 and 84.8% in Group 2 had achieved healing. Complications in Group 1 were minor and resolved. In Group 2, three patients had deep infections and five had superficial infections, and two had growth disturbances. Although the healing rates were similar, we found higher rates of clinically important complications, worse functional outcomes, and higher hospital burden associated with intralesional excision.
Conclusions
Repetitive sclerotherapy using polidocanol is a minimally invasive, safer method of treatment for aneurysmal bone cysts compared with intralesional excision and bone grafting. In this preliminary study, we found similar recurrence rates for the two treatment methods, however, this will require confirmation in larger studies.
Level of Evidence
Level II, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
An aneurysmal bone cyst (ABC) is an uncommon tumorlike lesion often presenting during the second decade of life with an incidence of approximately 0.14 per 100,000 population [25]. Understanding of the pathogenesis is still evolving; however, the original description by Lichtenstein [26] favoring a hemodynamic disturbance is the most popular [1, 3, 9, 10, 33, 42]. Clonal chromosomal translocation t(16:17)(q22:p13) leading to CDH11 (promoter region of osteoblast cadherin 11 gene) and USP6 (ubiquitin specific peptidase 6) rearrangements may represent the basic alteration leading to altered regulation of actin remodeling and development of primary ABC, but detailed pathophysiologic mechanisms relating to hemodynamic disturbance need elaboration [2, 33]. The translocation characteristically is seen in the mild to moderately mitotic spindle cells while absent from inflammatory and giant cells. The promoter fusion gene rearrangement for USP6 is absent in secondary ABCs [2, 33], which suggests primary ABC is a de novo lesion.
Intralesional excision with bone grafting is considered the standard among several treatment methods practiced sporadically or circumstantially [3, 9, 10, 29, 30, 46]. Curettage of the lesion with or without bone grafting has been associated with high and sometimes unacceptable recurrence rates of 18% to 59% [8, 10, 13, 27, 29, 30, 46]. Various adjuvants have been in vogue to reduce the recurrence rates of the curettage-only method [3, 9, 10, 13, 20, 30, 42, 46]. Curettage using a high-speed burr (extended curettage) is considered adequate and more feasible but still has an approximate 15% recurrence rate [9, 13, 20]. Some authors suggest younger age, juxtaphyseal location of cyst, and female gender are associated with increased recurrence rates [3, 8, 20, 27].
Sclerotherapy [1, 6, 9, 10, 12, 14, 19, 22, 45] is a newer treatment modality [1] introduced in the 1990s, although the comparative safety (complications) and efficacy (recurrences) have not been as clearly established as for surgical methods. Sclerotherapy is based primarily on the premises that ABC is a vascular malformation predisposed on a hemodynamic disturbance and would heal if the vascular lesion is controlled. An alcoholic solution of zein (Ethibloc®; Ethicon, Inc, Somerville, NJ) has been used with healing rates of as much as 92% [1, 12, 14, 16, 19, 22] and no recurrences but requires a secondary procedure in 0% [14, 16, 19] to 25% [12] of patients. Topouchian et al. [45] raised concerns regarding the higher treatment failure (27%) and unacceptable complication rates (five of 15 patients). Recently Shisha et al. [44] also raised concerns regarding the fatal complications of Ethibloc® injection in rabbits. Since 1997, we have used polidocanol as the sclerosing agent, which is popular for treatment of various vascular malformations among surgeons and dermatologists [18]. In a preliminary report of the effectiveness of polidocanol in the treatment of ABC in 72 patients studied over 6.5 years with a mean followup of 34 months [38], we observed two recurrences (3%) that were treated successfully by repeat sclerotherapy. However, whether this form of sclerotherapy produces healing rates similar to those of curettage and bone grafting is unknown.
We asked whether (1) primary healing rates obtained with repetitive sclerotherapy would be similar to those obtained with intralesional curettage supplemented with high-speed burring and bone grafting (null hypothesis); (2) hospital stay, time to healing, improvement in pain, functional scores at the end of treatment and followup, and complications would differ between the two treatment methods; and (3) age or gender or juxtaphyseal location of cyst would influence recurrence rates.
Patients and Methods
Between January 2002 to December 2005 (patient selection and treatment period), 107 patients presented (or were referred) for the first time without being treated elsewhere for treatment of primary ABCs (lesions not associated with any other identifiable disorder). The patients were briefed comprehensively regarding the procedures and consent was obtained for inclusion in the study. We obtained radiographs and histology for all patients to confirm suitability for inclusion. Radiographs typically showed subperiosteal, often metaphyseal, eccentric, expansile lesions. Additional MRI was performed in eight patients with axially distributed lesions (four in the pelvis, three in the clavicle, and one in the spine) to identify the characteristic double-density fluid levels and septations. Three of the 107 patients were excluded for identifiable secondary lesions and 10 others refused to take part in the study. The remaining 94 patients with primary ABCs were randomized using a random number table (by SR) into two treatment groups: one receiving repetitive sclerotherapy (Group 1, n = 47) and the other treated with curettage supplemented with high-speed burring (extended curettage) and autologous bone grafting (Group 2, n = 47). Taking into account the reported healing rates of intralesional curettage with high-speed burring of 88% [20] and 95% with sclerotherapy [16, 19, 22], and assuming an alpha of 0.05 and an expected power of study of 80%, the required sample size in each group was 192 patients. Two patients from Group 1 and one patient from Group 2 were lost to followup, resulting in 45 in Group 1 and 46 in Group 2 (Table 1). There were 29 males and 16 females in Group 1 and 32 males and 14 females in Group 2. The mean age of the patients was 21.3 years (median, 19 years) in Group 1 and 26.7 years (median, 24 years) in Group 2. The groups were comparable with respect to tumor size (p = 0.227) and gender distribution (p = 0.608), but differed with respect to age distribution (p = 0.025). The site distribution of lesions was similar in the two groups, with the humerus being the most common site for lesions in both groups (Table 2). The minimum followup in each group was 3.2 years (mean, 4.4 years; range, 3.2–6.1 years). The study was reviewed and approved by the institutional review board committee.
Table 1.
Patient characteristics and results
| Parameter | Group 1 | Group 2 | p Value |
|---|---|---|---|
| Number of patients | 45 | 46 | |
| Gender ratio (male:female) | 29:16 | 32:14 | 0.608 |
| Mean age (years) | 21.3 (median, 19) | 26.7 (median, 24) | 0.025 |
| Number of patients younger than 15 years | 23 | 16 | 0.031 |
| Number of juxtaphyseal lesions | 17 | 14 | 0.093 |
| Mean volume of lesions (cm3) | 37.8 (range, 12.7–119.1) | 41.5 (range,14.9–113.5) | 0.227 |
| Mean number of injections/procedures | 2.3 | 1.2 | 0.014 |
| Mean hospital stay (days) | < 1 | 7.5 | 0.002 |
| Mean time to radiographic healing (months) | 5.7 | 5.1 | 0.118 |
| Number of recurrences (during followup) | 2 (both males; 1 patient younger than 15 years) | 7 (4 males; 3 females; 2 patients younger than 15 years) | 0.194 |
| Number of treatment failures | 3 | 7 | 0.315 |
| Primary healing rate | 93.3% | 84.8% | 0.315 |
| Mean pain relief (time taken for VAS to reduce to 3/10) (days) | 7 | 41 | 0.008 |
| Mean functional score at treatment completion | 72 | 57 | < 0.0001 |
| Mean functional score at 3 years followup | 91 | 69 | < 0.0001 |
VAS = visual analog scale.
Table 2.
Distribution of lesions in the two groups
| Site | Frequency | |
|---|---|---|
| Group 1 | Group 2 | |
| Humerus | 19 | 21 |
| Forearm | 3 | 1 |
| Femur | 8 | 11 |
| Tibia | 6 | 4 |
| Fibula | 2 | 3 |
| Pelvis | 3 | 1 |
| Clavicle | 2 | 1 |
| Hand | 1 | 2 |
| Foot | 0 | 2 |
| Vertebra | 1 | 0 |
Tissue for histologic examination was obtained with a trephine biopsy. Owing to initially indeterminate histology, we repeated the biopsy in 33 patients to confirm the diagnosis, whereas one patient required an open biopsy for confirmation. Histologically, the presence of blood-filled cystic spaces separated by fibrous septa (membranes) of mononuclear stromal cells containing scattered multinucleated giant cells, and less commonly, reactive bone was suggestive of diagnosis. Atypical mitoses and chondroid matrix were notably absent.
A treatment holiday of 3 months was given to all patients after histopathologic confirmation based on reports of spontaneous healing of the lesion after bone puncture or trauma [28, 41]. During this 3-month period, patients in both groups were instructed specifically to note any increase in pain or sudden appearance of pain and were followed up every 3 weeks for clinical assessment for change in size of swelling; radiographs were obtained every 6 weeks to assess spontaneous healing or rapid progression of the lesion.
Patients in Group 1 were treated as outpatients by two of us (MKV, VT) with intralesional injection of 3% polidocanol (hydroxypolyethoxydodecan; Kreussler Pharma, Wiesbaden, Germany). The approximate volume of the lesion was calculated using plain radiographs by multiplying the maximum length and breadth in AP projection and the depth in lateral projection. The calculation is suitable for small and spherical lesions less than 3 cm in diameter [38]. More accurate measurement could be performed using ultrasonography or CT; however, considering the smallest of the lesions (as small as 2 cm in one dimension) would exceed the maximum permissible volume of drug to be injected (5 mL) and patients were distributed in two groups randomly and not on the basis of the size of lesion, approximate calculation was considered adequate. We injected 1 mL for each 1 cm3 of lesion to a maximum of 5 mL in one sitting. C-arm fluoroscopy was used to guide the site of injection and identify specific loculi to be injected in patients requiring multiple sittings. Percutaneous bone puncture was performed using a 16-gauge needle and contents of the cyst were aspirated to ensure position. After injection of sclerosant, 1 mL saline flush was used and the needle end was plugged for 1 minute.
Patients were followed up every 6 weeks with a new radiograph after injection. With each radiograph, two of us (MKV, VT) jointly assessed the opacification of the lesion (or all loculi) reflected by a uniform increase in density of the lesion radiographically on comparative qualitative analysis with initial radiographs. Treatment completion was defined as opacification of the lesion (or all loculi) so that no additional injections were needed (Fig. 1). Patients who progressed poorly or who had patchy opacification (loculation) develop were reinjected at 6-week intervals. Radiographic healing and satisfactory progression were defined by opacification of the lesion with an increase in cortical thickness (to at least 50% for the bone) or a decrease in the size of the cyst (minimum of 25% from original) or remodeling (long-term followup) (Fig. 1). Recurrence was defined as development of fresh area(s) of radiolucencies in a previously opacified cyst (treatment completed) with or without increase in the size of the lesion (Fig. 2). Nontreatable lesions were lesions that failed to show opacification altogether or in part (greater than 50%) after three sequential injections or in which any new communicating lesion appeared before treatment completion. For study purposes, patients who either showed initial healing and later did not progress even after three sequential injections or who had greater than 50% but less than complete healing of the lesion (and no appearance of any new lesion) were defined as having a partial healing/response. For the purpose of calculation and comparison of primary healing rates, recurrence, nontreatable lesions, and partial healing were grouped together (treatment failure). This was based on the rationale that either alternative procedure (for failure or partial healing) or repeat procedure (for recurrence) would be required in all these cases.
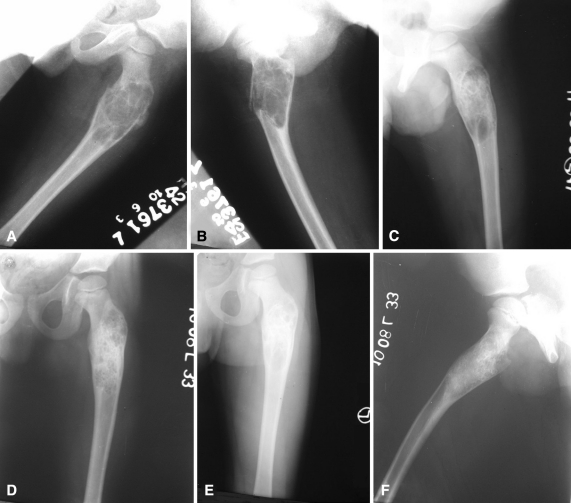
Fig. 1A–F.
(A) AP and (B) lateral radiographs show an ABC affecting the proximal femur of the left side in an 8-year-old boy with pathologic fracture. (C) An AP radiograph taken after two injections of polidocanol shows an unopacified loculation in the lower part of the lesion. (D) A radiograph was obtained at completion of treatment after three injections. (E) AP and (F) lateral radiographs show the lesion at 2 years with remodeling of the lesion.
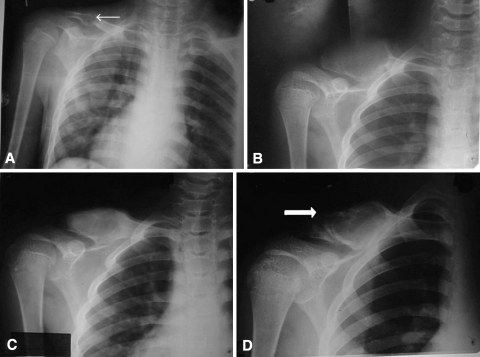
Fig. 2A–D.
(A) An AP radiograph of the right shoulder of an 11-year-old boy shows a small ABC of the clavicle (small arrow) with pathologic fracture. (B) The cyst rapidly enlarged within 4 months of presentation to us. (C) The cyst was treated with one intralesional injection of polidocanol and showed good healing and partial remodeling at 5 months of followup. (D) Recurrence (large arrow) with rapid reexpansion of the cyst was seen in the lesion at 11 months of followup.
Patients in Group 2 were treated as inpatients by a distinct surgical team (SR, SAK). Curettage of the lesion was supplemented with high-speed burring and the cavity was packed with autologous bone graft taken from the iliac crest (and fibula in some cases), or for larger cavities, autologous graft mixed with bone graft substitute (synthetic hydroxyapatite granules; G-Bone®, G. Surgiwear Ltd, Shahjahanpur, India). Wound healing and suture removal marked treatment completion for this group. Patients were followed up every 6 weeks for assessment of radiographic healing or development of any complications. Graft incorporation with evidence of sclerosis and/or increase in cortical thickness (by at least 50% for the bone) defined satisfactory progression of radiographic healing. Recurrence was defined as the appearance of a radiolucency in any part (or the entire lesion) (Fig. 3) or any pattern (geographic/patchy/circumferential) with or without graft resorption. Recurrences, if any, were treated by repeat procedure for the group.
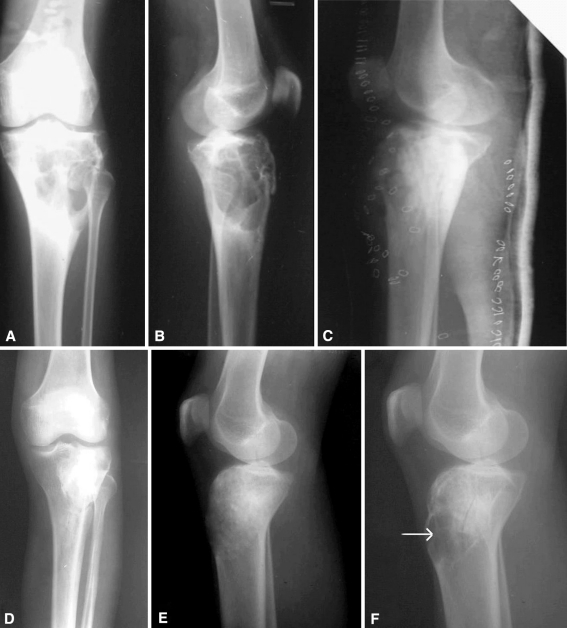
Fig. 3A–F.
(A) AP and (B) lateral radiographs show an ABC of the proximal tibia of the left leg (C) treated with extended curettage and bone grafting. (D) AP and (E) lateral radiographs taken at 9 months of followup show good healing of the lesion. (F) Recurrence can be seen on this lateral radiograph (arrow), with the appearance of clinical swelling in the region of the tibial tuberosity.
After radiographic healing (in both groups), the patients were followed up every 3 months for 1 year and then every 6 months for a minimum of 3 years (total followup). Patients from each group were assessed clinically for pain (10-point visual analog scale [VAS]) at every visit and were asked to note the score at home, specifically when the score decreased to 3 of 10 or less and improved further. Functional status was evaluated using the scoring system of Enneking et al. [15] at treatment completion and at 3 years of followup. Other outcome criteria studied were hospital stay and time to healing.
We compared the groups using Levene’s robust test for equality of variances and t test for equality of means. We used ANOVA to compare the mean size of tumors and age of patients. Healing rates were calculated as percentages and differences (null hypothesis) were explored using Fisher’s exact test. To compare functional score and improvement within a group, we used Student’s t test, whereas for intergroup comparison for improvement in functional scores at treatment completion and 3-year followup, we used the paired-samples t test. Multinomial logistic regression analysis was used to identify whether there was any association between age, gender, or juxtaphyseal location of cyst and recurrence and whether there were any differences between the two groups.
Results
We observed no difference in healing rates between Groups 1 and 2. One patient in Group 1 did not respond to treatment. There were two recurrences (4.44%) in Group 1 and seven (15.2%) in Group 2 presenting within 1 year of followup (Table 1). Retreatment with the procedure for the specific group cured the lesion at last followup (followup of 26 to 59 months after treatment of recurrence). Therefore, radiographic healing (primary healing rate) was similar (p = 0.315) in the two groups (Group 1: 42 of 45, 93.3%; Group 2: 39 of 46, 84.8%) (Table 1).
The mean hospital stay was shorter (p < 0.05) in Group 1 (< 1 day) than in Group 2 (7.5 days) (Table 1).
The mean time to treatment completion in Group 1 was 3.38 months (range, 1.5–7.5 months). The mean time to radiographic healing was similar (p = 0.118) for both groups: 5.7 months (SD, 1.8 months) for Group 1 compared with 5.1 months (SD, 1.6 months) for Group 2 (Table 1). The number of sittings (injections/procedures) required was greater (p = 0.014) in Group 1 (mean, 2.3; median, 2; range, 1–5) than in Group 2 (mean, 1.2; range, 1–3); this included retreatment for recurrence and/or complications for both groups (Table 1).
The mean functional scores (rating percentage) at treatment completion were better (p < 0.0001) in the repetitive sclerotherapy group (intergroup comparison) than in the curettage group (72 versus 57), and this trend also was reflected at 3 years of followup (91 versus 69) (p < 0.0001) (Table 1). Also in both groups (intragroup comparison), the mean functional score at 3 years of followup was considerably better (p < 0.0001) than that at treatment completion. The VAS scores reduced to 3 of 10 faster (p = 0.008) for Group 1 (mean, 7 days) than for Group 2 (mean, 41 days) (Table 1).
Three patients in Group 2 had deep infections requiring surgical exploration whereas five others had superficial infections. Two patients in Group 2 had growth disturbances. Some complications were unique to Group 1 and included local induration (37, 82.2%), hypopigmentation at the injection site (11, 24.4%), and dizziness episode (one patient), but were reversible and did not require any intervention.
There was no difference (p = 0.27) in the recurrence rates in patients younger or older than 15 years. Of the nine recurrences (two in Group 1 and seven in Group 2), three (of 39, 7.7%) were in patients younger than 15 years whereas six (of 52, 11.5%) were in patients 15 years or older. For Group 2, there were two recurrences in 16 patients younger than 15 years, which was similar (p = 0.79) to the five recurrences in 30 patients 15 years or older. The recurrence rates were similar (p = 0.88) in males and females: seven of 61 (11.5%) in males compared with three of 30 (10%) in females. Logistic regression analysis also suggested no relationship (p = 0.443) between juxtaphyseal cysts and recurrence (Group 2). Two recurrences (of seven) were noted in 14 juxtaphyseally located cysts in Group 2, whereas three (one in Group 1 and two in Group 2) recurrences were noted in all the juxtaphyseal cysts (total of 31) treated with either method.
Discussion
Surgical treatment for ABCs has been primarily intralesional curettage supplemented with various adjuvants to achieve higher overall healing rates and reduce recurrences. Limitations with the use of these methods have been hospitalization, immobilization of the part, intraoperative blood loss, and potential of physeal damage associated with vigorous curettage [3, 8–11, 29, 30, 42, 46]. Instillation of sclerosant into the lesion based on Lichtenstein’s hemodynamic theory [26] was used to circumvent these limitations and possibly achieve higher healing rates. The preliminary reports regarding the use of sclerotherapy have been encouraging [1, 14, 16, 19, 22, 38]. We compared the use of polidocanol as a sclerosant with intralesional excision performed with high-speed burring (extended curettage) and bone grafting to determine whether repetitive sclerotherapy is efficacious in terms of achieving primary healing rates and reducing recurrence. Also improvement in pain, hospital stay, time to healing, complications, and functional outcome with each method were compared to judge the acceptability of the methods. Recurrence rates were studied in detail to find any relation to age, gender, or juxtaphyseal location of lesion.
Our study is limited first by our method of randomization. Stratified randomization for age and/or gender or use of minimization technique could produce more specific data (related to patient demographics and treatment) and recommendations for the particular groups but are complicated and require large sample sizes, possible only in a multicenter study. Second, we had low power. This limited our ability to definitely answer questions regarding differences between the two groups; our finding of no difference in the healing rates achieved with repetitive sclerotherapy and extended curettage with bone grafting needs to be substantiated with large-scale multicenter trials. Nonetheless, given the size of our groups and high healing rates, we presume there would not be major differences. Third, the epidemiologic profile of our patients did not reflect that in the literature. We had a high percentage of male patients (67%; male:female = 61:30), and the age distribution in the two groups was different, with the repetitive sclerotherapy group having a younger patient population. However, the groups were comparable with respect to gender distribution and treatment outcome difference has not been clearly identified with respect to age difference in the patient population [13].
The primary healing rate (principle outcome criteria) of 93.3% with repetitive sclerotherapy was not different from the 84.8% of the curettage group. On analyzing the available literature regarding treatment of ABCs, there is a recurrence rate of approximately 29.2% (209 of 717 patients) when curettage only and bone grafting (Table 3) are attempted, which decreases to 15.4% (12 of 78) with the use of high-speed burring [13, 20]. The recurrence rate of 15.2% in our series with the use of extended curettage and bone grafting compares with published rates. Compiled analysis for the use of sclerotherapy using Ethibloc® (Table 4) suggests treatment failure (but no recurrences) in approximately 12% of patients that requires a separate procedure, which is comparable to that for extended curettage (approximately 15%). A treatment failure rate of 6.67% (two recurrences and one patient who did not respond to sclerotherapy) in our study patients is still better than the reported recurrence rates (12%) for sclerotherapy (Table 4). In a previously published series [38], there was a recurrence rate of 2.8% using polidocanol in 72 cases. All the cases that respond to sclerotherapy continue to do so whether there is partial response (reflected by improved healing with repeat procedure) and no recurrences [1, 9, 10, 12, 14, 16, 19, 22, 45], or as in our previous [38] and current study, by complete healing even in recurrences with repeat sclerotherapy.
Table 3.
Results for treatment of aneurysmal bone cysts with intralesional excision
| Study | Curettage and bone grafting | Curettage with a high-speed burr | Recurrences |
|---|---|---|---|
| Biesecker et al. [3] | 44 | 26 | |
| Bollini et al. [4] | 12 | 5 | |
| Campanacci et al. [5] | 91 | 19 | |
| Clough and Price [7] | 15 | 6 | |
| Cole [8] | 22 | 7 | |
| Dormans et al. [13] | 44 | 8 | |
| Farsetti et al. [17] | 11 | 2 | |
| Gibbs et al. [20] | 34 | 4 | |
| Koskinen et al. [23] | 14 | 2 | |
| Mankin et al. [29] | 110 | 24 | |
| Marcove et al. [30] | 44 | 26 | |
| Motamedi et al. [31] | 59 | 8 | |
| Nobler et al. [32] | 18 | 6 | |
| Ozaki et al. [34] | 30 | 11 | |
| Ramirez and Stanton [37] | 24 | 8 | |
| Ruiter et al. [40] | 82 | 28 | |
| Server Perez et al. [43] | 17 | 4 | |
| Vergel de Dios et al. [46] | 124 | 27 | |
| Current study | 46 | 7 |
Table 4.
Results of treatment of aneurysmal bone cysts with sclerotherapy
| Study | Number of patients | Sclerosant used | Number of patients requiring multiple injections | Complete initial healing | Partial healing | Secondary surgery/procedure | Recurrence |
|---|---|---|---|---|---|---|---|
| Adamsbaum et al. [1] | 17 | Ethibloc® | 3 (up to 3 injections) | 14 | 0 | 3 | 0 |
| de Gauzy et al. [12] | 12 | Ethibloc® | 1 (up to 2 injections) | 6 | 3 | 3 | 0 |
| Dubois et al. [14] | 17 | Ethibloc® | 9 (up to 5 injections) | 16 | 1 | 0 | 0 |
| Falappa et al. [16] | 13 | Ethibloc® | 9 (up to 4 injections) | 13 | 0 | 0 | 0 |
| Garg et al. [19] | 10 | Ethibloc® | 3 (up to 2 injections) | 7 | 3 | 0 | 0 |
| Guibaud et al. [22] | 16 | Ethibloc® | 6 (up to 3 injections) | 13 | 2 | 1 | 0 |
| Rastogi et al. [38] | 72 | Polidocanol | 62 (up to 5 injections) | 70 | 2 | 0 | 2 |
| Topouchian et al. [45] | 15 | Ethibloc® | 4 (up to 3 injections) | 9 | 2 | 4 | |
| Current study | 45 | Polidocanol | 31 (up to 3 injections) | 44 | 0 | 1 | 2 |
Fourteen of 45 patients (31.1%) in our repetitive sclerotherapy group achieved complete healing with one injection. Approximately 79% (31 of 45) of our patients required multiple injections. The mean number of injections (sittings) required for opacifying all the cavities in this group was 2.3, compared with 1.2 procedures required in the intralesional excision group (inclusive of treatment of complications), but the fact that no hospitalization is required for repetitive sclerotherapy makes the larger number of sittings clinically unimportant. Considering the available literature for sclerotherapy using Ethibloc® (Table 4), multiple injections (range, 2–5 injections) were required in 35 of 100 patients (35%) varying from 33% [19] to 69% [16] in different studies. In a previous study [38], 86% of patients (62 of 72) required multiple sittings (range, 2–5 injections). In a study by Dormans et al. [13], a second procedure was required in eight of 45 patients and an additional two patients required a third procedure for persistent or recurrent lesions (mean, 1.22 procedures). Gibbs et al. [20] performed three repeat procedures for recurrences and one elbow release in 34 patients (mean, 1.12 procedures). Mankin et al. [29] reported 34 second and 13 third procedures in 150 patients, related to recurrences or failed systems (mean, 1.31 procedures). This correlates to the requirement of 1.2 procedures in Group 2 in our study, although the data for additional surgery for shortening or infection if required are not readily available from the literature. Hospitalization has been recommended for 24 hours postprocedure to watch for inflammatory and allergic reactions [1, 14, 22] that may be supported by serious complications reported with Ethibloc® use [22, 36, 44, 45]. In our previous experience [38] and in the current study with polidocanol, we believe hospitalization is not required and the procedure can be completed in the outpatient setting as it induces a mild inflammatory reaction if at all to be clinically important, and even if some extravasation inadvertently occurs, it settles clinically within 6 hours.
Radiographic healing appeared somewhat later in the repetitive sclerotherapy group compared with the curettage group (5.7 months versus 5.1 months). Recurrent lesions healed with repeat procedure for the group. We had one patient in whom treatment failed with repetitive sclerotherapy; no opacification occurred even after three sequential injections although the patient had complete pain relief after one injection. Radiographs continued to show a loculated lytic cavity and the lesion was cured with extended curettage and bone grafting. During surgery, there were variable-thickness bridging columns of bone across an absolutely empty cyst. Histopathologic evaluation of these columns revealed living bone. Nonresponsiveness to sclerotherapy has been identified in approximately 12% of patients (Table 4), requires a different procedure [1, 9, 10, 12, 14, 16, 19, 22, 45] from that used in the current study, and is commonly evident after the first injection.
Pain improvement is prompt with the use of sclerotherapy using polidocanol, appearing after the first injection. An initial increase in pain requiring analgesics and observation and/or immobilization is common with the use of Ethibloc® for the first 48 hours, which is followed with gradual improvement during the next 1 to 2 weeks [1, 22, 45]. We found no increase in pain after the procedure or requirement of analgesics. Although no comparative data are available for extended curettage with bone grafting, in our study, the pain relief in this group took substantially longer, which may be an important factor affecting acceptability of the procedure.
The functional scores were consistently better for sclerotherapy and importantly the scores at final followup were substantially better in both groups compared with treatment completion. This possibly may reflect continued healing; however, psychologic improvement and associated bias in responding to the questionnaire could have a role. Repetitive sclerotherapy was cosmetically more acceptable, being a scarless outpatient procedure. Marcove et al. [30] reported a 90% functional rating (range, 63%–100%) in patients treated with cryosurgery, which was better than the scores of our patients in Group 2 (mean, 69).
The deep infection rate of 6.5% in the extended curettage group is comparable to published rates for infection. Schreuder et al. [42] reported deep infections in two of 26 patients (7.4%) (27 ABCs treated with cryosurgery with or without bone grafting) managed with débridement and antibiotic beads, whereas Papagelopoulos et al. [35] reported two deep infections in 40 patients (5%) with pelvic ABCs (treated with intralesional excision or excision curettage) of which one persisted as chronic osteomyelitis and the other was managed by débridement and antibiotic beads. Shortening was seen in two of our patients (4.3%) (one with lower limb shortening of 4 cm corrected with lengthening, the other with humerus shortening of 2.5 cm managed nonoperatively [Fig. 4]). The literature for growth disturbances associated with surgical procedures is unsettled. Although Rizzo et al. [39] reported no evidence of growth arrest in 15 patients with juxtaphyseal cysts treated with intralesional surgery and bone grafting, Capanna et al. [6] reported premature fusion in five of 39 juxtaphyseal cysts in 198 patients (2.53%). Growth arrest also was reported by Green et al. [21] (one of eight cases, 12.5%) and Lampasi et al. [24] (one of seven juxtaphyseal cysts, 14.3%). Deep infection and shortening are important, as they require additional procedures and affect acceptability of the procedure. Sclerotherapy using Ethibloc® resulted in transient local inflammatory reactions in 37.8% patients (65 of 172 patients) (Table 5). Fistulation and abscess formation observed by Topouchian et al. [45] (four of 15 patients) led them to abandon their study, and others have reported pulmonary and fatal vertebrobasilar system embolisms [22, 36, 44, 45]. We did not encounter such serious complications using polidocanol in the current or previously [38] studied patients. One study suggests higher recurrence rates in children younger than 10 years [20]. Surgeons’ reluctance to aggressively curette the region around open growth plates to avoid physeal damage is believed to leave disease behind, possibly leading to recurrence; therefore, we considered all patients younger than 15 years (open growth plate) for this analysis. Biesecker et al. [3] suggested there could be higher rates of recurrence in patients younger than 15 years, which was indirectly supported by the high recurrence rates (54%) reported by Cole [8] who studied patients 5 to 14 years of age. Gibbs et al. [20] systematically studied the factors related to local recurrence after curettage with high-speed burring and found a higher recurrence rate in children younger than 10 years. There were three recurrences in six patients younger than 10 years compared with one recurrence in 28 patients older than 10 years. However, a similar evaluation by Dormans et al. [13] showed there was no relation to younger age or juxtaphyseal location of the cyst. They found recurrence or persistence in three of 13 children younger than 10 years compared with five of 32 children older than 10 years, which is contrary to the original belief. They suggested that familiarity in working near open growth plates was an important factor to reduce recurrence rates. In a retrospective evaluation of 53 patients, Lin et al. [27] found an association of recurrence to juxtaphyseal location of the cyst (eight of 19 juxtaphyseal cysts recurred) and to younger age. Age younger than 12 years was the single predictor for recurrence (local recurrence-free survival 53% versus 100%) with 100% recurrence noted in patients younger than 5 years [27]. Biesecker et al. [3] and Gibbs et al. [20] reported a trend toward higher recurrence rates in female patients but could not statistically evaluate the finding because of small numbers. However, we found no relation between recurrence of the lesion and younger age at presentation either in the extended curettage and bone grafting group or in the overall patient population, nor did we find a relation to gender or juxtaphyseal location. These observations support those of Dormans et al. [13].
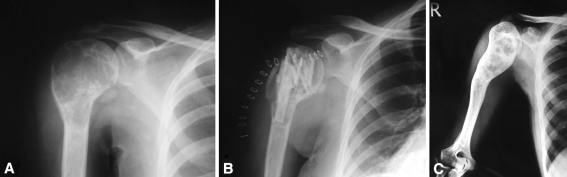
Fig. 4A–C.
(A) An AP radiograph shows an ABC of the proximal right humerus in a 13-year-old girl. (B) The lesion was treated with extended curettage and bone grafting. (C) The lesion healed completely but was marked with destruction of the proximal physis and 4 cm of shortening at 5.2 years of followup.
Table 5.
Complications of sclerotherapy
| Study | Number of patients | Local inflammatory reactions (transient) | Abscess formation, osteitis, or cutaneous fistulae |
|---|---|---|---|
| Adamsbaum et al. [1] | 17 | 16 | 3 |
| de Gauzy et al. [12] | 12 | 5 | 0 |
| Dubois et al. [14] | 17 | 2 | 0 |
| Falappa et al. [16] | 13 | 8 | 2 |
| Garg et al. [19] | 10 | 2 | 1 |
| Guibaud et al. [22] | 16 | 5 | 1 |
| Rastogi et al. [38] | 72 | 22 | 0 |
| Topouchian et al. [45] | 15 | 5 | 4 |
| Current study | 45 | 37 | 0 |
We found no difference in healing rates with the two treatment methods. However, sclerotherapy could be done as an outpatient procedure and provided faster pain relief compared with extended curettage and bone grafting. Also, the functional outcome was better with the use of repetitive sclerotherapy. Intralesional instillation of sclerosant seems safer and more cosmetically satisfactory, as it provides a scarless method of treatment and it may circumvent the problem of potential injury to the physis (growth disturbance) and infection associated with open surgical methods.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that the institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at All India Institute of Medical Sciences.
References
- 1.Adamsbaum C, Mascard E, Guinebretiere JM, Kalifa G, Dubousset J. Intralesional Ethibloc injections in primary aneurysmal bone cysts: an efficient and safe treatment. Skeletal Radiol. 2003;32:559–566. doi: 10.1007/s00256-003-0653-x. [DOI] [PubMed] [Google Scholar]
- 2.Althof PA, Ohmori K, Zhou M, Bailey JM, Bridge RS, Nelson M, Neff JR, Bridge JA. Cytogenetic and molecular cytogenetic findings in 43 aneurysmal bone cysts: aberrations of 17p mapped to 17p13.2 by fluorescence in situ hybridization. Mod Pathol. 2004;17:518–525. doi: 10.1038/modpathol.3800090. [DOI] [PubMed] [Google Scholar]
- 3.Biesecker JL, Marcove RC, Huvos AG, Mike V. Aneurysmal bone cysts: a clinicopathologic study of 66 cases. Cancer. 1970;26:615–625. doi: 10.1002/1097-0142(197009)26:3<615::AID-CNCR2820260319>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Bollini G, Jouve JL, Cottalorda J, Petit P, Panuel M, Jacquemier M. Aneurysmal bone cysts in children: analysis of twenty-seven patients. J Pediatr Orthop B. 1998;7:274–285. doi: 10.1097/01202412-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25–36. [PubMed] [Google Scholar]
- 6.Capanna R, Springfield DS, Biagini R, Ruggieri P, Giunti A. Juxtaepiphyseal aneurysmal bone cyst. Skeletal Radiol. 1985;13:21–25. doi: 10.1007/BF00349089. [DOI] [PubMed] [Google Scholar]
- 7.Clough JR, Price HG. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin Orthop Relat Res. 1973;97:52–63. doi: 10.1097/00003086-197311000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Cole WG. Treatment of aneurysmal bone cysts in childhood. J Pediatr Orthop. 1986;6:326–329. doi: 10.1097/01241398-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B. 2006;15:155–167. doi: 10.1097/01.bpb.0000210588.50899.29. [DOI] [PubMed] [Google Scholar]
- 10.Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg. 2007;127:105–114. doi: 10.1007/s00402-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 11.Cottalorda J, Kohler R, Chotel F, Gauzy JS, Lefort G, Louahem D, Bourelle S, Dimeglio A. Recurrence of aneurysmal bone cysts in young children: a multicentre study. J Pediatr Orthop B. 2005;14:212–218. doi: 10.1097/01202412-200505000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Gauzy JS, Abid A, Accadbled F, Knorr G, Darodes P, Cahuzac JP. Percutaneous Ethibloc injection in the treatment of primary aneurysmal bone cysts. J Pediatr Orthop B. 2005;14:367–370. doi: 10.1097/01202412-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Dormans JP, Hanna BG, Johnston DR, Khurana JS. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;421:205–211. doi: 10.1097/01.blo.0000126336.46604.e1. [DOI] [PubMed] [Google Scholar]
- 14.Dubois J, Chigot V, Grimard G, Isler M, Garel L. Sclerotherapy in aneurysmal bone cysts in children: a review of 17 cases. Pediatr Radiol. 2003;33:365–372. doi: 10.1007/s00247-003-0899-4. [DOI] [PubMed] [Google Scholar]
- 15.Enneking W, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 16.Falappa P, Fassari FM, Fanelli A, Genovese E, Ascani E, Crostelli M, Salsano V, Montanaro A, Di Lazzaro A, Serra F. Aneurysmal bone cysts: treatment with direct percutaneous Ethibloc injection: long-term results. Cardiovasc Intervent Radiol. 2002;25:282–290. doi: 10.1007/s00270-001-0062-2. [DOI] [PubMed] [Google Scholar]
- 17.Farsetti P, Tudisco C, Rosa M, Pentimalli G, Ippolito E. Aneurysmal bone cyst: long-term follow-up of 20 cases. Arch Orthop Trauma Surg. 1990;109:221–223. doi: 10.1007/BF00453145. [DOI] [PubMed] [Google Scholar]
- 18.Frullini A, Cavezzi A. Sclerosing foam in the treatment of varicose veins and telengiectases: history and analysis of safety and complications. Dermatol Surg. 2002;28:11–15. doi: 10.1046/j.1524-4725.2002.01182.x. [DOI] [PubMed] [Google Scholar]
- 19.Garg NK, Carty H, Walsh HP, Dorgan JC, Bruce CE. Percutaneous Ethibloc injection in aneurysmal bone cysts. Skeletal Radiol. 2000;29:211–216. doi: 10.1007/s002560050595. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs CP, Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities: factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 1999;81:1671–1678. doi: 10.2106/00004623-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Green JA, Bellemore MC, Marsden FW. Embolization in the treatment of aneurysmal bone cysts. J Pediatr Orthop. 1997;17:440–443. doi: 10.1097/00004694-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Guibaud L, Herbreteau D, Dubois J, Stempfle N, Berard J, Pracros JP, Merland JJ. Aneurysmal bone cysts: percutaneous embolization with an alcoholic solution of zein—series of 18 cases. Radiology. 1998;208:369–373. doi: 10.1148/radiology.208.2.9680561. [DOI] [PubMed] [Google Scholar]
- 23.Koskinen EV, Visuri TI, Holmström T, Roukkula MA. Aneurysmal bone cyst: evaluation of resection and curettage in 20 cases. Clin Orthop Relat Res. 1976;118:136–146. [PubMed] [Google Scholar]
- 24.Lampasi M, Magnani M, Donzelli O. Aneurysmal bone cysts of the distal fibula in children: long-term results of curettage and resection in nine patients. J Bone Joint Surg Br. 2007;89:1356–1362. doi: 10.1302/0301-620X.89B10.19375. [DOI] [PubMed] [Google Scholar]
- 25.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst: a population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;363:176–179. doi: 10.1097/00003086-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein L. Aneurysmal bone cyst: observations on fifty cases. J Bone Joint Surg Am. 1957;39:873–882. [PubMed] [Google Scholar]
- 27.Lin PP, Brown C, Raymond AK, Deavers MT, Yasko AW. Aneurysmal bone cysts recur at juxtaphyseal locations in skeletally immature patients. Clin Orthop Relat Res. 2008;466:722–728. doi: 10.1007/s11999-007-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malghem J, Maldague B, Esselinckx W, Noel H, Nayer P, Vincent A. Spontaneous healing of aneurysmal bone cysts: a report of three cases. J Bone Joint Surg Br. 1989;71:645–650. doi: 10.1302/0301-620X.71B4.2768314. [DOI] [PubMed] [Google Scholar]
- 29.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756–6762. doi: 10.1200/JCO.2005.15.255. [DOI] [PubMed] [Google Scholar]
- 30.Marcove RC, Sheth DS, Takemoto S, Healey JH. The treatment of aneurysmal bone cyst. Clin Orthop Relat Res. 1995;311:157–163. [PubMed] [Google Scholar]
- 31.Motamedi MH, Navi F, Eshkevari PS, Jafari SM, Shams MG, Taheri M, Abbas FM, Motahhari P. Variable presentations of aneurysmal bone cysts of the jaws: 51 cases treated during a 30-year period. J Oral Maxillofac Surg. 2008;66:2098–2103. doi: 10.1016/j.joms.2008.05.364. [DOI] [PubMed] [Google Scholar]
- 32.Nobler MP, Higinbotham NL, Phillips RF. The cure of aneurysmal bone cyst: irradiation superior to surgery in an analysis of 33 cases. Radiology. 1968;90:1185–1192. doi: 10.1148/90.6.1185. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira AM, Perez-Atayde AR, Inwards CY, Medeiros F, Derr V, Hsi BL, Gebhardt MC, Rosenberg AE, Fletcher JA. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so called secondary aneurysmal bone cysts. Am J Pathol. 2004;165:1773–1780. doi: 10.1016/S0002-9440(10)63432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop Relat Res. 1997;337:240–248. doi: 10.1097/00003086-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 35.Papagelopoulos PJ, Choudhury SN, Frassica FJ, Bond JR, Unni KK, Sim FH. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg Am. 2001;83:1674–1681. doi: 10.2106/00004623-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Peraud A, Drake JM, Armstrong D, Hedden D, Babyn P, Wilson G. Fatal ethibloc embolization of vertebrobasilar system following percutaneous injection into aneurysmal bone cyst of the second cervical vertebra. AJNR Am J Neuroradiol. 2004;25:1116–1120. [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez AR, Stanton RP. Aneurysmal bone cyst in 29 children. J Pediatr Orthop. 2002;22:533–539. doi: 10.1097/00004694-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Rastogi S, Varshney MK, Trikha V, Khan SA, Choudhury B, Safaya R. Treatment of aneurysmal bone cyst with percutaneous sclerotherapy using polidocanol: a review of 72 cases with long-term follow-up. J Bone Joint Surg Br. 2006;88:1212–1216. doi: 10.1302/0301-620X.88B9.17829. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo M, Dellaero DT, Harrelson JM, Scully SP. Juxtaphyseal aneurysmal bone cysts. Clin Orthop Relat Res. 1999;364:205–212. doi: 10.1097/00003086-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 40.Ruiter DJ, Rijssel TG, Velde EA. Aneurysmal bone cysts: a clinicopathological study of 105 cases. Cancer. 1977;39:2231–2239. doi: 10.1002/1097-0142(197705)39:5<2231::AID-CNCR2820390541>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Saglik Y, Kapicioglu MI, Guzel B. Spontaneous regression of aneurysmal bone cyst: a case report. Arch Orthop Trauma Surg. 1993;112:203–204. doi: 10.1007/BF00662291. [DOI] [PubMed] [Google Scholar]
- 42.Schreuder HW, Veth RP, Pruszczynski M, Lemmens JA, Koops HS, Molenaar WM. Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J Bone Joint Surg Br. 1997;79:20–25. doi: 10.1302/0301-620X.79B1.7097. [DOI] [PubMed] [Google Scholar]
- 43.Server Perez F, Gomez Bonsfills X, Mateo Montanes X, Garcia Garcia C, Casamitjana J, Vidal Homs E. [Aneurysmal bone cysts: review of 20 cases] [in French] Acta Orthop Belg. 1980;46:272–288. [PubMed] [Google Scholar]
- 44.Shisha T, Marton-Szucs G, Dunay M, Pap K, Kiss S, Nemeth T, Szendroi M, Szoke M. The dangers of intraosseous fibrosing agent injection in the treatment of bone cysts: the origin of major complications shown in a rabbit model. Int Orthop. 2007;31:359–362. doi: 10.1007/s00264-006-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topouchian V, Mazda K, Hamze B, Laredo JD, Penneçot GF. Aneurysmal bone cysts in children: complications of fibrosing agent injection. Radiology. 2004;232:522–526. doi: 10.1148/radiol.2322031157. [DOI] [PubMed] [Google Scholar]
- 46.Vergel de Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst: a clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::AID-CNCR2820691210>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]