Abstract
Background
Degenerative rotator cuff tears are increasing with the aging population, and healing is not uniform after surgery. Rotator cuffs may show improved healing when biologic factors are added during surgery.
Questions/purposes
We asked: (1) What cellular processes are involved in normal bone-to-tendon healing? (2) What approaches are being developed in tendon augmentation? (3) What approaches are being developed with the addition of growth factors?
Methods
We reviewed research in relating to biologic augmentation and cellular processes involved in rotator cuff repair, focusing on animal models of rotator cuff repair and nonrandomized human trials.
Results
Regular bone-to-tendon healing forms a fibrous junction between tendon and bone that is distinct from the original bone-to-tendon junction. Tendon augmentation with cellular components serves as scaffolding for fibroblastic cells and a possible source of growth factors and fibroblastic cells. Extracellular matrices provide a scaffold for incoming fibroblastic cells, although current research does not conclusively confirm which if any of these scaffolds enhance repair owing in part to intermanufacturer variations and the limited human research. Growth factors and platelet-rich-plasma are established in other fields of research and may enhance repair but have not been rigorously tested.
Conclusions
There is potential application of biologic augmentation to improve healing after rotator cuff repair. However, research in this field is still inconclusive and has not been sufficiently demonstrated to merit regular clinical use. Future human trials can elucidate the use of biologic augmentation in rotator cuff repairs.
Introduction
Incidence rates of degenerative rotator cuff tears increase with age [8]. A cadaveric study demonstrated the incidence of full-thickness rotator cuff tears in subjects older than 60 years was 30%, as compared to 6% in those younger than 60 years [31]. As the population increases in age, degenerative rotator cuff tears will become an increasingly prevalent clinical problem.
Despite multiple surgical techniques to improve bone-to-tendon healing, recurrent tearing of the rotator cuff is not infrequent. Postoperative rotator cuff retears occur in as little as 11% to as much as 94% of rotator cuff repair surgeries, perhaps depending on the size of the tear and the level of tendon degeneration [5, 6, 19, 21, 33, 34, 60]. Retearing correlates with decreased functional outcome after rotator cuff repair [53]. Considering the relatively high percentage of repair failure occurring with current surgical techniques, it is important to explore techniques of biologic augmentation of rotator cuff repair to reduce the retear rates and improve long-term shoulder function.
When considering an appropriate biologic treatment for rotator cuff tears, it is important to consider their etiology. Although rotator cuff tears can occur via acute injury, most rotator cuff tears occur as a result of a gradual degeneration of the tendon and the bone-to-tendon interface [54].
The normal bone-to-tendon interface involves the interdigitation of layers of intact, oriented Type I collagenous fibers to a continuous insertion on the humerus [12]. The vasculature is organized and dispersed throughout the tendon; vessels decrease in size and number as distance to the bone decreases [12]. Longitudinally, there are four distinct zones of tissue: tendon, nonmineralized fibrocartilage, mineralized fibrocartilage, and bone [24].
When the tendons within the rotator cuff begin to degenerate, the collagenous fibers undergo hyaline and myxoid degeneration, and the bone undergoes chondroid metaplasia. Inflammation, calcification, vascular proliferation, and fatty infiltration are also present [25]. Importantly, these tendinous changes are visible throughout the damaged tendon, not just at the site of rupture [36]. These degenerative changes are often macroscopically visible during surgical repair [39].
We reviewed the literature to determine what approaches were being developed in: (1) tendinous augmentation with cellular components, (2) acellular extracellular matrix (ECM) augmentation, and (3) the addition of growth factors.
Search Strategy and Criteria
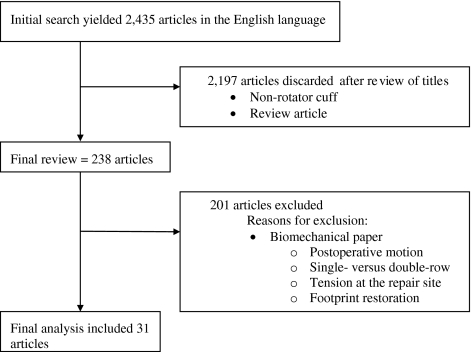
We searched all published literature in the English language from January 1966 to November 2009 using MEDLINE for the following key words: rotator cuff repair OR bone to tendon healing, which yielded 2270 articles. In addition, to ensure all relevant papers would be captured, we searched the additional terms: rotator cuff augmentation, OR rotator cuff graft, OR rotator cuff biologic. This yielded an additional 165, or a total of 2435 articles (Fig. 1). The titles of each paper were reviewed by two of us (EC, LS). We excluded papers if they were review articles, or if they involved anatomical areas other than the rotator cuff (such as Achilles tendon, ACL, flexor or extensor tendon surgery in the hand, or hamstring). This yielded 238 articles. The titles and abstracts were further reviewed, and further excluded if they investigated biomechanical factors toward rotator cuff healing (such as postoperative motion, tension at the repair site, double- versus single-row fixation, suture anchors, or issues related to footprint restoration). This yielded 31 articles (Fig. 1).
Fig. 1.
A flow diagram of the article selection process is shown.
Biology of Bone-to-Tendon Healing
In normal bone-to-tendon healing, the final bone-to-tendon insertions ultimately have little resemblance to the native insertion site; instead of four distinct zones, the bone and tendon are joined by a layer of fibrovascular scar tissue predominated by Type III collagen [20, 47]. This tissue is weaker than the original insertion site and may contribute to the substantial number of repair failures. Bone-to-tendon healing can be divided into three stages: the inflammatory stage, the repair stage, and the remodeling stage [9].
The inflammation cascade is a complex tissue response and involves a number of different cytokines and recruited cells that fluctuate in number as the healing continues [9]. The inflammatory stage begins within the first week of rotator cuff repair and is characterized by the infiltration of neutrophils and recruited macrophages. These recruited macrophages secrete transforming growth factor β1 (TGF-β1), which increases proteinase activity and collagen formation [26]. These early-recruited macrophages contribute to the formation of fibrovascular scar tissue; it is likely TGF-β1 secretion is the mechanism of scar tissue formation in the presence of recruited macrophages. Local macrophages are also present in smaller numbers in the repairing bone-to-tendon insertion point; local macrophages are thought to have a positive anabolic function opposed to the catabolic function observed in the recruited macrophages [26]. Additionally, inflammation occurs after tendon overuse injuries and may serve as a protective mechanism in this setting [43].
Several growth factors have been implicated in the repair stage of natural bone-to-tendon healing. Bone morphogenic protein (BMP) 12 to 14, expressed in active fibroblasts and heavily involved in de novo joint formation, were present throughout the healing process of the bone-to-tendon insertion [49, 57]. Seeherman et al. [49] demonstrated in a full-thickness rotator cuff tear sheep model that delivery of rhBMP-12 in sponge carriers had the potential to accelerate healing of rotator cuff repairs when compared to untreated controls.
Basic fibroblast growth factor (bFGF), which increases fibroblastic proliferation while suppressing collagen formation, is also present [13]. Insulin-like growth factor 1 (IGF-1), which stimulates protein synthesis and cell proliferation while decreasing swelling, has been observed in the healing bone-to-tendon junction [13]. Platelet-derived growth factor β (PDGF- β), correlated to increased levels of Type I collagen, is also present in low levels throughout the repair [32, 52]. Although the repair stage of healing classically begins at the end of the first week, Randelli et al. [44] report some of these cytokines appear immediately after surgical repair procedures. Fifteen minutes after performing arthroscopic acromioplasty and other shoulder procedures on patients, 3 mL of liquid was removed from the subacromial space and from the venous blood supply. Levels of TGF-β, PDGF-β, and bFGF were measured in each liquid. For each growth factor, levels in the subacromial space were markedly higher than levels in the serum. These observations indicate growth factors may be equally important during the beginning of the healing process as toward the end [44].
Vascularity of the tendinous region also contributes to rotator cuff tears and therefore also may contribute to the repair of those tears. Within a tendon, vascularity decreases at the point of the tear as compared to its interface on bone [4]. Although no studies have directly proven a causal relationship between increased vascularity and improved rotator cuff healing, a recent randomized prospective clinical trial has demonstrated a topically applied angiogenic factor, glyceryl trinitrate, reduced shoulder pain and stiffness in patients with chronic supraspinatus tendinopathy [29, 42].
Repair Strategies
Tendon Augmentation with Cellular Components
Transplanted tendon can be used to decrease the repair tension between tendon and bone. In addition, using tendons may help with the biologic process of recovery, either by providing a growth scaffold for cells or by populating the bone-to-tendon junction with donor cells and growth factors. In a rat model of acute rotator cuff tears treated with cellular grafts, Iwata et al. [29] found the host cell commenced proliferation in the interposed graft tendon. However, it is unknown if the host cells retain their active role in rotator cuff healing in the setting of a degenerative or avascular tear.
Tendon transplants can come from either allogenic or autogenic sources. Moore et al. [41] repaired massive rotator cuff tears in 28 patients with allografts of cadaveric Achilles, patellar, or quadriceps tendon. Although the patients demonstrated improved function, the improvements were not superior to those of similar patients who were treated with acromioplasty and débridement alone. Additionally, within the allograft group, there was one case of infection and one case of presumed acute rejection in which intraoperative cultures were negative.
Autograft augmentation, because of the decreased likelihood of rejection, may be preferred as a cellular graft material. Several studies have been performed investigating the efficacy of cellular autograft treatments for rotator cuff tears using the tenotomized biceps tendon [11, 45]. In addition to reducing repair tension and providing cellular material, an autograft biceps tendon has two further benefits. First, because it is in the same site of repair, it does not necessitate a primary harvest surgery. Second, because many patients present with a rotator cuff tear and a complicating lesion in the long head of the biceps, they are likely to need tenotomy anyway. Cho et al. [11] retrospectively reviewed massive rotator cuff tears with and without augmented tenotomized biceps. Both procedures were performed arthroscopically and involved standard technique with the exception of the biceps interposition. During the biceps augmentation procedure, the biceps tendon was tenotomized at its origin and passed into the subacromial space. Suture anchors were placed at the greater tuberosity, and limbs of the suture penetrated both the biceps graft and the rotator cuff. The biceps was interposed between the lateral edge of the cuff and the tuberosity. There was no difference in pain, ROM, or clinical outcome at 12 months postoperatively. However, patients who underwent biceps augmentation demonstrated greater strength and lower structural failure rate (41% failure with augmentation, 73.7% without augmentation).
ECM Augmentation
Other augmentations to rotator cuff tears minimize the potential problem of graft rejection by using acellular material. In the repair site, an extracellular matrix with no cellular components acts as a tissue bridge between shortened tendon and bone, and its matrix can act as an effective scaffold for aligned cellular growth and collagen assembly [1]. These augmentations are currently available in three forms: xenograft, allograft, and synthetic extracellular matrices.
Extracellular matrices (ECMs) formed from xenogenic or allogenic material must first be rigorously processed in order to remove any cellular material. Although this process varies among manufacturers and is often proprietary, general decellularization techniques can be organized into three approaches: physical, chemical, and enzymatic [23, 54]. The physical decellularization approach uses snap freezing or mechanical agitation to lyse the native cells in the harvested tissue [30, 35]. The chemical decellularization approach lyses native tissue cells with hypotonic solutions or detergents; the cellular remnants are then solubilized and removed from the extracellular matrix in sequential washing steps [56]. Enzymatic decellularization uses trypsin to degrade cellular material and can be used in conjunction with a solubilizing detergent [40]. Complete decellularization of a xenograft or an allograft will include one or more of the above approaches.
The use of xenograft ECM in rotator cuff repairs has yielded mixed results. One xenograft ECM, Restore® (DePuy Orthopaedics, Richmond, VA) is a collagen-based material made from porcine small intestine mucosa. In a randomized control trial of the Restore® patch, Iannotti et al. [27] demonstrated no improvement in patients with Restore® augmentation when compared with patients who underwent the same procedure without xenograft addition. More recently, Restore® was found to contain a relatively high level of DNA within its matrix (1.13 ± 0.01 ng DNA/mg dry weight) and has also resulted in an inflammatory result in 20% of patients whose rotator cuff tears were repaired with Restore® augmentation [22, 38]. In a retrospective analysis, Walton et al. [55] reported that patients whose rotator cuffs had been repaired with the Restore® patch experienced decreased post-repair strength, increased shoulder impingement, slower pain resolution after activity, and no decrease in retear rate when compared to patients whose rotator cuff tears had been repaired using standard surgical techniques. Due to a high proportion of patients with a severe inflammatory reaction to the xenograft, the authors from the study did not recommend use of this implant.
Zimmer® Collagen Repair Patch, marketed as Permacol®, (Tissue Science Laboratories PLC, Aldershot, Hampshire, UK), is a porcine dermal graft with no measurable DNA in its matrix and no reported inflammatory responses in patients [22]. One retrospective analysis of rotator cuff repair using Permacol® showed improved functional scores by 50% at 4.5 years postrepair [3]. It is unclear whether the improvements compared favorably to individuals with similar rotator cuff tears repaired without xenograft augmentation as no control group was used in this study [3]. Another study investigated the use of Permacol® in bridging gaps in massive rotator cuff tears [51]. Retear rates were not lower than massive rotator cuff repairs performed without augmentation.
TissueMend® (TEI Biosciences, Boston, MA) and CuffPatch® (Arthrotek, Warsaw, IN) are two other commercially available xenografts. A study comparing rotator cuff repairs augmented with Restore®, Cuffpatch®, TissueMend®, Permacol®, and GraftJacket® (Wright Medical Technology, Arlington, TN) demonstrated rotator cuff repairs augmented with CuffPatch® experienced substantial inflammation when compared to the other grafts [54]. Another similar study demonstrated TissueMend® had higher levels of DNA embedded in the ECM when compared to other xenograft materials [15]. No additional studies have been performed using these xenografts in rotator cuff repair.
Allogenic extracellular matrices have been developed via decellularization of cadaveric material. Despite differences between allogenic ECM and autogenic cellular tendon, equivalence has been demonstrated between autografts and allogenic ECM. Adams et al. [1] compared cellular autografts to an allogenic ECM (GraftJacket®) in a canine rotator cuff repair model. During the first 6 weeks, rotator cuffs repaired with cellular autograft augmentation showed better recovery and repair than those repaired with allogenic ECM. After 12 weeks, however, the rotator cuffs repaired with allogenic ECM and the rotator cuffs repaired with autogenic tendon were equivalent in strength and histologic measurement [1].
Two commercially available allogenic ECMs have been studied to date: Allopatch® (Musculoskeletal Tissue Foundation, Edison, NJ), an allogenic ECM made from harvested human fascia lata, and GraftJacket®, made from human dermal tissue. The two graft materials are comparable in terms of stiffness, strength, and tissue retention [2]. GraftJacket® has been studied extensively in terms of rotator cuff repair augmentation. When compared to other graft materials, GraftJacket® reportedly had a higher load-to-failure than Permacol®, TissueMend®, Restore®, and CuffPatch® but is notably weaker than autologous tendon [15].
Ide et al. [28] found rotator cuff tears repaired with allogenic ECM (GraftJacket®) augmentation had higher tendon maturing scores than an untreated control defect group, demonstrated greater mean ultimate force to failure than the defect group, and performed better histologically and mechanically at every point in the study. Cadaveric studies comparing allogenic-augmented rotator cuff repairs (GraftJacket®) to rotator cuff repairs without augmentation demonstrated that the use of human dermal allograft increased the strength of the repaired tendon; the mean failure strengths were 325 ± 74 N with allogenic ECM and 273 ± 116 N without allogenic ECM [3]. There are, however, limited human subject data comparing repair with allogenic augmentation to repair of comparable tears without allogenic augmentation. Bond et al. [7] showed repairing massive rotator cuff tears with allogenic augmentation (GraftJacket®) yielded a failure rate of 19%. This result is lower than the 38% to 95% failure rate demonstrated in several studies evaluating unaugmented massive rotator cuff repairs [5, 20, 21, 60]. A histologic assessment of one patient’s allogenically augmented rotator cuff repair (GraftJacket®) demonstrated no calcification, infection, or inflammatory response at 3 months. Collagen was well aligned and little blood vessel ingrowth was observed, demonstrating improved bone-to-tendon healing with allograft ECM augmentation [50].
However, there are potential problems with allogenic ECMs. Allogenic ECMs can still contain some DNA from their allogenic source and may induce inflammatory responses in the host [22]. These inflammatory responses can cause pain and edema at the site of repair and may increase the degeneration of the rotator cuff repair that has been documented in the initial degenerative process of the rotator cuff [59]. They are also less elastic than autogenic tendon, which may result in comparably increased retear rates due to decreased load-carrying abilities [14].
Because concern remains that allograft materials may create an inflammatory response, there is considerable interest in developing synthetic ECM grafts for surgical use. Synthetic ECMs may still serve as an adequate scaffold for cellular and fibrotic growth, while running a smaller risk of provoking an inflammatory response than allograft ECMs. Several animal studies have investigated the benefit of augmenting rotator cuff repair with synthetic ECMs.
One study used a polyglycolic acid (PGA) sheet to augment rotator cuff repairs of infraspinatus tendons in Japanese white rabbits [58] and showed histologic improvement in fibrocartilage layering but only a slight improvement in tensile strength when compared to control tendons augmented with another slowly absorbing synthetic material [58]. A similar experiment performed by Funakoshi et al. [18] demonstrated increased fibroblast presence and collagen formation when synthetic ECM was surgically applied to rotator cuff tears. In this experiment, a 10-mm surgical defect was created at the humeral insertion of the infraspinatus tendon in 21 Japanese white rabbits. In one shoulder, the 10-mm defect was covered with chitin, a biodegradable polymer, sutured into the bone trough and attached to the free end of the infraspinatus tendon. The contralateral shoulder was left untreated as a control. Throughout the experiment, tendon-to-bone junctions covered with chitin fabric demonstrated greater cell number, better collagen fiber alignment, and greater mechanical strength than the tendon-to-bone junctions left free as control [18]. A third study using polylactic acid patches in goats showed no observable difference between the treated and control groups [37]. Derwin et al. [15] performed a similar experiment using a woven poly-L-lactide device. The superior 2/3 of each infraspinatus tendon was removed from the rotator cuff and then repaired in both shoulders of mongrel dogs. In one shoulder, a woven poly-L-lactide device was placed over the repair. In the other shoulder, the repair was left unaugmented. The augmented rotator cuff repair resulted in fewer tendon retractions, greater strength, and increased stiffness when compared to the contralateral untreated rotator cuff repairs.
Several studies recently reported using ECMs as a scaffold for autogenic cells in vitro, as well as in rotator cuff repair. First, autologous cells are harvested from undamaged sites on the host such as patellar ligaments, fascia lata, or other tendinous structures by a small biopsy punch. They are then washed in an antibiotic medium, digested with collagenase, and filtered to remove any matrix debris. After the cells have been appropriately purified, they are cultured in incubation flasks alone for several days and then cultured with the ECM for up to 5 days to appropriately seed the matrix in vitro [10]. This construct of allogenic, xenogenic, or synthetic ECM and autogenic cells has several potential applications.
ECM-autogenic cell constructs can be incubated in appropriate media and result in engineered autogenic tendons. These tendons have been investigated as augmentation materials in the repair of rotator cuff tears. Chen et al. [10] modeled the effect of using such engineered tendons to augment rotator cuff tears in Japanese white rabbits. Tenocytes were harvested from patellar ligaments, purified using markers for Types I and III collagen expression, and then implanted onto xenogenic and synthetic ECMs. Fibers were cultured for 5 days to allow for appropriate tenocyte matrix formation. Rotator cuff tears were created in the infraspinatus tendon and then repaired with genetically engineered tendon, ECM, or autologous tendon. When compared to ECM augmentation alone, augmentation with engineered autologous tendon resulted in increased Types I and III collagen deposition, decreased immunologic response, and improved absorption into the host tendon. Augmentation with engineered autologous tendon was comparable to the autogenic cellular augmentation performed as a control.
ECM-autogenic cell constructs can also be manipulated via gene therapy. Dines et al. [16] investigated repair of rotator cuff tears using interposed genetically engineered autologous tendon in Sprague Dawley rats. Tenocytes were isolated from the rotator cuff and then transduced with the genes for PDGF-β or IGF-1, two factors that promote fibroblast proliferation and minimize inflammation. The transduction was performed using a retroviral vector. Successfully transduced cells were again isolated, seeded onto PGA, and used to augment rotator cuff repair. When incorporated into rotator cuff repair, the tendons transduced with PDGF-β showed no improvement over controls augmented with PGA matrix alone or simple repair. However, the fibroblastic cells transduced to express IGF exhibited an improvement in both toughness and maximum load. The growth factors Dines et al. [16] chose to transpose represent only two of a handful of known growth factors involved in de novo tendon formation and wound healing. Gene therapy in genetically engineered autogenous tendons is a promising delivery system for growth factors required for appropriate tendon healing, and further investigation here is merited.
Growth Factor Addition
The studies previously discussed have focused on adding structural support to the rotator cuff repair, with a possibly beneficial cellular contribution in some cases. However, if the cellular benefits offered by grafts can be attributed to the factors the graft’s cells secrete, the direct application of those growth factors may be a more direct therapy. The following approaches consider the addition of growth factors to healing rotator cuff repairs to provide increased biologic support.
Several individual growth factors have been tested independently for their contribution to rotator cuff healing. Osteoinductive proteins have been examined for their role in rotator cuff healing. In a sheep infraspinatus tear model, growth factors from bovine cortical bone extract (BMP-2 to BMP-7, TGF-β1 to TGF-β3, and FGF) were implanted into a healing rotator cuff with a Type I collagen sponge [48]. The investigators found increased bone volume, soft tissue volume, and failure loads in the augmented group when compared to nonaugmented control. However, when the loads were normalized for tissue volume, they observed no differences between treated and untreated shoulders. The data suggest, while the bovine cortical bone extract may have accelerated the healing process, it did not change the quality of the repair [48].
The addition of recombinant human BMP-12 to acute full-thickness tears in sheep demonstrated a similar result [49]. Repairs with recombinant human BMP augmentation showed increased strength and rigidity compared to untreated repairs by 300%. However, tissues in both augmented and control arms of the study exhibited similar mechanical properties when normalized to the size of the tendon [49].
TGF-β has also been identified as an important growth factor in bone-to-tendon healing, although one report suggests the application of TGF-β to injured tendons yielded increased proliferation without necessarily increasing tendon strength [20].
In vivo, these growth factors work in concert to create appropriate bone-to-tendon healing; it is unlikely a great change will be observable through the application of individual growth factors. Because of this, there is a great amount of interest in the creation of “platelet-rich plasma” (PRP) by centrifuging autologous blood to purify a dense, suturable plasma matrix [44]. PRP includes many of the growth factors identified previously as crucial in normal bone-to-tendon healing: TGF-β, bFGF, PDGF, vascular endothelial growth factor, connective tissue growth factor, and epidermal growth factor [17].
Although PRP has yet to prove itself as a biologic augmentation to rotator cuff tears, one human subject study has investigated the safety of PRP augmentation to rotator cuff repair. A total of 14 patients undergoing arthroscopic repair of a rotator cuff tear received an intraoperative application of autologous PRP in combination with an autologous thrombin component after tear repair. The authors concluded application of PRP during arthroscopic rotator cuff repair is safe and effective, without any adverse events [44]. Further prospective studies are needed to investigate the effect of PRP on rotator cuff healing and ultimate shoulder function.
Discussion
Degenerative rotator cuff tears are becoming increasingly frequent as the population ages. Failure to heal and retearing after surgery is not uncommon. Biologic augmentation strategies offer the potential to improve healing in large tears or in revision situations when prior repair has failed. We therefore reviewed the literature to explore and describe the approaches being developed in: (1) tendinous augmentation with cellular components, (2) acellular extracellular matrix (ECM) augmentation, and (3) the addition of growth factors.
There are several limitations to our study design. First, ours was a selective review, and not a rigorous systematic review of the literature. We did not include a search of the EMBASE database, did not utilize multiple search terms with nesting, did not include Medline field tags, or Boolean logic. Thus, our search strategy may have missed eligible scientific articles within this field of study. Second, there are limitations to what was available in the literature. All of the human studies in our literature search were small case series or retrospective studies with Level III-IV data. The inherent quality of such studies requires the conclusions of these studies to be interpreted cautiously. Third, we did not assess study quality. Fourth, most articles we identified were animal studies, and how this data can be extrapolated to humans is unclear. However, the limited information available on this subject demonstrates these applications provide potential for improved biologic healing.
Although many growth factors and cellular processes have been identified in the normal bone-to-tendon healing process, each growth factor has a multitude of functions and interactions. It may not be possible to clearly define their complex functions in the inflammation and healing cascades. Extracellular matrices are difficult to endorse without well-designed intermanufacturer comparison. Additionally, animal studies showing host cell repopulation of the bone-to-tendon junction used ECMs to repair simulated traumatic rotator cuff repairs [29]. It is unclear whether human host cells in degenerative rotator cuff tears will behave similarly to those of traumatic rotator cuff tears created in animals.
The knowledge of bone-to-tendon healing is being leveraged in several ways. One approach focuses on augmenting rotator cuff repairs with tendinous materials containing cellular components. Allogenic cellular tendon grafts have been associated with no marked improvement in functional outcome and have been associated with inflammation and graft rejection [41].
The use of ECMs as a scaffolding system for fibroblasts and collagen matrices has yielded mixed results depending on the patch manufacturer. ECMs (GraftJacket®, Permacol®, Tissuemend®, CuffPatch®) have limited research demonstrating favorable recoveries in rotator cuff repairs but have not been analyzed in any randomized control trial or indeed in any comparative trial in human patients to establish improved repair strength. An important role that ECMs may begin to play in the future is as scaffolding for implanted autologous tenocytes or fibroblasts genetically engineered to produce growth factors conducive to tendinous growth.
A contemporary technical challenge for the use of ECM and allograft patches are that rotator cuff repairs are commonly being done arthroscopically. Placing and securing the patch graft arthroscopically would be technically difficult for most surgeons. The added operative time and swelling from arthroscopic fluid extravastion might add additional morbidity for the patient without a well-defined clinical benefit. A large randomized study in this regard would be very surgeon-dependent, and results might vary depending on the tear pattern and/or size of the allograft patch. To place the graft patch with a traditional open rotator cuff repair technique would be more reproducible, but exposes the patient to the added morbidity of deltoid detachment.
The benefit of growth factor addition in the form of an injectable medium is that it is less dependent on surgical technique and is technically easier to perform. Addition of separate growth factors (BMP-2 to BMP-7, BMP-12, TGF-β1 to TGF-β3, FGF) increases bone volume, soft tissue, volume, and failure loads when compared to normal tendon in animal models [46, 49]. A case study in humans using PRP injected after rotator cuff repair demonstrated that it was safe for use but did not compare the augmented rotator cuff repairs with any control [44]. The next step in this field would be to perform a randomized control study using PRP as an augmentation to rotator cuff repair, versus a conventional repair, to assess whether PRP truly affects clinical improvement.
Another factor to consider is the added cost of the commercial equipment used for this procedure, which is quoted by various companies as approximately $400–800. This includes the disposable applicator and injection kit, as well as the prepackaged thrombin required to activate the platelets. The added operative time required to prepare the PRP is short; approximately 15 minutes or less.
In summary, many of the new and promising applications of biologic augmentation have not yet been rigorously tested in humans. Further human subject research might include multicenter prospective trials comparing the addition of ECMs, allograft, or growth factors to a standard repair technique. Future studies in this regard will define the role of biologic augmentation in rotator cuff repair and protocols for surgical technique and clinical application.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
This work was performed at Stanford University.
References
- 1.Adams JE, Zobitz ME, Reach JS, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Barber FA, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25:1233–1239. doi: 10.1016/j.arthro.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24:20–24. doi: 10.1016/j.arthro.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Biberthaler P, Wiedemann E, Nerlich A, Kettler M, Mussack T, Deckelmann S, Mutschler W. Microcirculation associated with degenerative rotator cuff lesions: in vivo assessment with orthogonal polarization spectral imaging during arthroscopy of the shoulder. J Bone Joint Surg Am. 2003;85:475–480. [PubMed] [Google Scholar]
- 5.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15:290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 7.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403–409. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Bureau. UC, 2007 American Community Survey 1-year estimates. United States-Age and Sex. 2007. Available at: http://factfinder.census.gov. Accessed January 13, 2009.
- 9.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/S1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 10.Chen JM, Willers C, Xu JK, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 11.Cho NS, Yi JW, Rhee YG. Arthroscopic biceps augmentation for avoiding undue tension in repair of massive rotator cuff tears. Arthroscopy. 2009;25:183–191. doi: 10.1016/j.arthro.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Clark JM, Harryman DT. Tendons, ligaments, and capsule of the rotator cuff—gross and microscopic anatomy. J Bone Joint Surg Am. 1992;74:713–725. [PubMed] [Google Scholar]
- 13.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23:84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair—biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 15.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. J Bone Joint Surg Am. 2009;91:1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dines JS, Grande DA, Dines DM. Tissue engineering and rotator cuff tendon healing. J Shoulder Elbow Surg. 2007;16(5 Suppl):S204–S207. doi: 10.1016/j.jse.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Everts PA, Knape JT, Weibrich G, Schönberger JP, Hoffmann J, Overdevest EP, Box HA, Zundert A. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 18.Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg. 2006;15:112–118. doi: 10.1016/j.jse.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 21.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 26.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, Rodeo SA. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 27.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears: a randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 28.Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. Reconstruction of large rotator-cuff tears with acellular dermal matrix grafts in rats. J Shoulder Elbow Surg. 2009;18:288–295. doi: 10.1016/j.jse.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Iwata Y, Morihara T, Tachiiri H, Kajikawa Y, Yoshida A, Arai Y, Tokunaga D, Sakamoto H, Matsuda K, Kurokawa M, Kawata M, Kubo T. Behavior of host and graft cells in the early remodeling process of rotator cuff defects in a transgenic animal model. J Shoulder Elbow Surg. 2008;17(1 Suppl):101S–107S. doi: 10.1016/j.jse.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Jackson DW, Grood ES, Wilcox P, Butler DL, Simon TM, Holden JP. The effects of processing techniques on the mechanical properties of bone-anterior cruciate ligament-bone allografts. An experimental study in goats. Am J Sports Med. 1988;16:101–105. doi: 10.1177/036354658801600203. [DOI] [PubMed] [Google Scholar]
- 31.Kinsella K, Velkoff VA. An Aging World: 2001. Washington, DC: US Government Printing Office; 2001. [Google Scholar]
- 32.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J Bone Joint Surg Am. 2008;90:275–286. doi: 10.2106/JBJS.H.00388. [DOI] [PubMed] [Google Scholar]
- 34.Levy O, Venkateswaran B, Even T, Ravenscroft M, Copeland S. Mid-term clinical and sonographic outcome of arthroscopic repair of the rotator cuff. J Bone Joint Surg Br. 2008;90:1341–1347. doi: 10.1302/0301-620X.90B10.19989. [DOI] [PubMed] [Google Scholar]
- 35.Lin P, Chan WC, Badylak SF, Bhatia SN. Assesing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–1053. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 36.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36:533–538. doi: 10.1177/0363546507308549. [DOI] [PubMed] [Google Scholar]
- 37.MacGillivray JD, Fealy S, Terry MA, Koh JL, Nixon AJ, Warren RF. Biomechanical evaluation of a rotator cuff defect model augmented with a bioresorbable scaffold in goats. J Shoulder Elbow Surg. 2006;15:639–644. doi: 10.1016/j.jse.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907–911. doi: 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- 39.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon—reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 40.McFetridge PS, Daniel JW, Bodamyali T, Horrocks M, Chaudhuri JB. Preparation of porcine carotid arteries for ascular tissue engineering applications. J Biomed Mater Res A. 2004;70:224–234. doi: 10.1002/jbm.a.30060. [DOI] [PubMed] [Google Scholar]
- 41.Moore DR, Cain EL, Schwartz ML, Clancy WG., Jr Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34:392–396. doi: 10.1177/0363546505281237. [DOI] [PubMed] [Google Scholar]
- 42.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806–813. doi: 10.1177/0363546504270998. [DOI] [PubMed] [Google Scholar]
- 43.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14(1 Suppl):79S–83S. doi: 10.1016/j.jse.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair: a pilot study. Disabil Rehabil. 2008;30:1584–1589. doi: 10.1080/09638280801906081. [DOI] [PubMed] [Google Scholar]
- 45.Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36:1511–1518. doi: 10.1177/0363546508316020. [DOI] [PubMed] [Google Scholar]
- 46.Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16(5 Suppl 1):S191–S197. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 49.Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, D’Augusta D, Li XJ, Smith E, Wozney JM. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 50.Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket matrix. Arthroscopy. 2009;25:329–333. doi: 10.1016/j.arthro.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 51.Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears: a report of 4 cases. Acta Orthop Belg. 2007;73:432–436. [PubMed] [Google Scholar]
- 52.Spindler KP, Murray MM, Detwiler KB, Tarter JT, Dawson JM, Nanney LB, Davidson JM. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21:245–249. doi: 10.1016/S0736-0266(02)00145-6. [DOI] [PubMed] [Google Scholar]
- 53.Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair: a prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 54.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications: a comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 55.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- 56.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 2005;26:7339–7349. doi: 10.1016/j.biomaterials.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 57.Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16(5 Suppl):S198–S203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokoya S, Mochizuki Y, Nagata Y, Deie M, Ochi M. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36:1298–1309. doi: 10.1177/0363546508314416. [DOI] [PubMed] [Google Scholar]
- 59.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mat Res B Appl Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 60.Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90:2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]