Abstract
Background
Orthopaedic oncologists often must address leg-length discrepancy after resection of tumors in growing patients with osteosarcoma. There are various alternatives to address this problem. We describe a three-stage procedure: (1) temporary arthrodesis, (2) lengthening by Ilizarov apparatus, and (3) tumor prosthesis.
Questions/Purposes
We asked (1) to what extent are affected limbs actually lengthened; (2) how many of the patients who undergo a lengthening procedure eventually achieve joint arthroplasty; and (3) can the three-stage procedure give patients a functioning joint with equalization of limb length?
Patients and Methods
We reviewed 56 patients (younger than 14 years) with osteosarcoma who had staged lengthening arthroplasty between 1991 and 2004.
Results
Thirty-five of the 56 patients (63%) underwent soft tissue lengthening, and of these 35, 28 (50% of the original group of 56) had implantation of a mobile joint. Three of the 28 prostheses were later removed owing to infection after arthroplasty. The overall average length gained was 7.8 cm (range, 4–14 cm), and 25 (71%) of the 35 patients had a mobile joint at final followup. The average Musculoskeletal Tumor Society functional score was 23.2 (range, 15–28) and limb-length discrepancy at final followup was 2.6 cm (range, 0–6.5 cm). Although most mobile joints had an acceptable ROM (average, 74.2°; range, 35°–110°), extension lag was frequent.
Conclusions
Our approach is one option for skeletally immature patients, especially in situations where an expandable prosthesis is not available. However, this technique requires multiple stages and would be inappropriate for patients who cannot accept prolonged functional deficit owing to a limited lifespan or other reasons.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Limb salvage surgery in skeletally immature patients remains a challenge [1, 25]. The distal femur and proximal tibia are the most common sites for osteosarcoma, and the epiphyses of the distal femur and proximal tibia contribute approximately 35% and 30%, respectively, to growth of the lower extremity [2]. Therefore, substantial limb-length discrepancy at maturity should be considered when contemplating reconstruction in children with osteosarcoma around the knee. Several options, including an expandable prosthesis [9, 13, 18, 21, 24] or distraction osteogenesis [19, 23], have been used to solve this problem [1, 25].
During the past three decades, internally expandable prostheses have provided the most popular means of treating patients with a clinically important limb-length inequality [2, 7, 9, 18, 21, 24]. Although the expandable prosthesis is an excellent practical option for skeletally immature patients, there are occasions when this prosthesis is not available or impractical, ie, low socioeconomic status or mismatch with prechemotherapeutic design attributable to tumor progression. In addition, previous reports suggest expandable implants have limited longevity with revision rates between 22% and 83% [2, 7, 9, 13, 18, 21, 24], primarily because of aseptic loosening, infection, or implant fracture [1].
Although quantitative models in dynamic MRI and [F-18]-fluorodeoxy-D-glucose-positron emission tomography are being developed to find new and more accurate early prognostic criteria [5, 8, 15, 22], treatment protocols currently are still based primarily on staging of the tumors and imprecise histologic grading. Therefore, it is difficult to discern with certainly which patients will have local recurrences and metastases and which will not. To circumvent the recurrence-prone period of 2 to 3 years from tumor resection, we performed a temporary resection-arthrodesis (TRA), which is a modification of an intramedullary rod embedded in bone cement after tumor resection [6, 17]. This initial procedure is relatively simple and inexpensive. If the patient survives without local recurrence, we then add two more stages: (1) soft tissue lengthening with an external fixator after 2 to 3 years after tumor resection when the possibility of metastasis was considered low, and (2) conversion to an adult-type mobile joint at maturity. This results in a three-stage procedure for appropriate patients.
Specifically, we asked the following three questions. (1) To what extent are affected limbs actually lengthened? (2) How many of the patients who undergo a lengthening procedure eventually achieve joint arthroplasty? (3) Can the three-stage procedure give patients a functioning joint with equalization of limb length?
Patients and Methods
We retrospectively reviewed 56 skeletally immature patients (boys younger than 14 years, girls younger than 12 years) with osteosarcoma treated with the three-stage approach between 1991 and 2004. During this time, we treated a total of 74 patients (boys younger than 14 years, girls younger than 12 years) for osteosarcoma around the knee. Of the 74 patients, nine underwent wide resection and prosthesis reconstruction, four had amputation, two had rotationplasties, and three had hemiarthroplasties at the knee. We performed hemiarthroplasty to retain the unaffected opposing joint cartilage (in case of distal femoral osteosarcoma, we saved the proximal tibia cartilage). For the remaining 56 patients, we planned staged lengthening arthroplasty. There were 33 boys and 23 girls with an average age of 10.5 years (range, 6.9–13.9 years) at the time of tumor resection. Staging included radiographs of the lesion, a bone scan, MRI of the lesion, and CT of the chest. All patients had Stage IIB tumors according to the Musculoskeletal Tumor Society (MSTS) classification [11]. All underwent preoperative chemotherapy, surgery, and postoperative chemotherapy and were followed as described previously [16]. Thirty-five of the 56 (63%) patients underwent the second-stage soft tissue lengthening (Table 1). The remaining 21 patients did not have the lengthening procedure for the following reasons: 10 patients (18%) died of disease (average interval from index operation to death, 25 months; range 9–54 months), three underwent amputation owing to local recurrence at 9, 12, and 16 months postoperatively, two refused additional intervention, two were lost to followup, and four patients with a discrepancy less than 3 cm at maturity had implantation of a modular prosthesis. Tumor locations in the 35 patients who underwent soft tissue lengthening were the distal femur (28 patients) and the proximal tibia (seven patients). The average followup after tumor resection for all patients was 138 months (range, 9–213 months). This study was approved by our institutional research review board.
Table 1.
Demographic data for 35 patients requiring soft tissue lengthening
| Patient | Age (years) | Gender | Anatomic site | Final reconstruction type | Final status | Followup (months) |
|---|---|---|---|---|---|---|
| 1 | 6.9 | Male | Distal femur | Tumor prosthesis | CDF | 147 |
| 2 | 7.4 | Female | Distal femur | Tumor prosthesis | CDF | 155 |
| 3 | 7.8 | Female | Distal femur | Arthrodesis | CDF | 151 |
| 4 | 7.8 | Female | Distal femur | Tumor prosthesis | CDF | 100 |
| 5 | 7.9 | Male | Distal femur | Arthrodesis | CDF | 201 |
| 6 | 8.0 | Female | Proximal tibia | Arthrodesis | NED | 120 |
| 7 | 8.1 | Male | Distal femur | Tumor prosthesis | CDF | 80 |
| 8 | 8.5 | Male | Distal femur | Arthrodesis | CDF | 191 |
| 9 | 8.6 | Female | Distal femur | Tumor prosthesis | CDF | 98 |
| 10 | 8.9 | Female | Proximal tibia | Tumor prosthesis | CDF | 60 |
| 11 | 9.0 | Female | Distal femur | Tumor prosthesis | CDF | 63 |
| 12 | 9.5 | Female | Distal femur | Arthrodesis | CDF | 194 |
| 13 | 9.8 | Male | Distal femur | Arthrodesis | CDF | 154 |
| 14 | 9.8 | Male | Distal femur | Tumor prosthesis | CDF | 95 |
| 15 | 9.8 | Female | Distal femur | Arthrodesis | CDF | 63 |
| 16 | 10.0 | Male | Distal femur | Tumor prosthesis | NED | 131 |
| 17 | 10.0 | Male | Distal femur | Tumor prosthesis | CDF | 175 |
| 18 | 10.1 | Female | Distal femur | Tumor prosthesis | CDF | 213 |
| 19 | 10.5 | Female | Distal femur | Tumor prosthesis | CDF | 181 |
| 20 | 10.6 | Male | Proximal tibia | Tumor prosthesis | CDF | 165 |
| 21 | 10.7 | Male | Distal femur | Rotationplasty | CDF | 175 |
| 22 | 10.9 | Male | Proximal tibia | Tumor prosthesis | CDF | 201 |
| 23 | 10.9 | Female | Distal femur | Tumor prosthesis | CDF | 195 |
| 24 | 10.9 | Female | Distal femur | Tumor prosthesis | CDF | 187 |
| 25 | 11.0 | Female | Distal femur | Tumor prosthesis | CDF | 171 |
| 26 | 11.0 | Male | Proximal tibia | Arthrodesis | CDF | 134 |
| 27 | 11.5 | Female | Distal femur | Tumor prosthesis | CDF | 157 |
| 28 | 11.6 | Male | Distal femur | Tumor prosthesis | CDF | 123 |
| 29 | 11.7 | Female | Distal femur | Tumor prosthesis | CDF | 165 |
| 30 | 11.9 | Male | Proximal tibia | Arthrodesis | CDF | 112 |
| 31 | 12.0 | Male | Distal femur | Tumor prosthesis | CDF | 148 |
| 32 | 12.0 | Female | Distal femur | Tumor prosthesis | CDF | 174 |
| 33 | 12.1 | Male | Distal femur | Tumor prosthesis | CDF | 62 |
| 34 | 12.1 | Male | Proximal tibia | Tumor prosthesis | CDF | 64 |
| 35 | 13.2 | Male | Distal femur | Tumor prosthesis | CDF | 133 |
CDF = continuously disease free; NED = no evidence of disease.
At the time of the initial tumor resection (first stage), we performed a TRA using multiple Ender nails and a bone cement spacer, which had enough stability to allow the patient to bear weight a few days after surgery (Fig. 1A–B) [6]. We performed the second-stage lengthening procedure for patients who met the following criteria: (1) relapse free for at least 2 years from tumor resection and (2) shortening greater than 4 cm. The average time from tumor resection to first lengthening was 34 months (range, 24–58 months). To correct limb shortening, soft tissue lengthening without bone transport was performed using an Ilizarov apparatus (Fig. 1C). This procedure can be summarized as follows: (1) removal of the Ender nails-bone cement composite to facilitate lengthening, insert the Ilizarov wires and pins, and perform extensive scarectomy; (2) filling of the defect with bone cement spacer in fully distracted position to maintain space for a subsequent megaprosthesis; and (3) application of the Ilizarov apparatus under the guidance of an image intensifier. Seven days after the procedure, distraction was started at a rate of 0.25 mm four times a day [3]. We used this distraction rate because no serious complications were reported for this method [3]. After lengthening, patients with expected growth greater than another 4 cm were switched to TRA for the following reasons: (1) to minimize neurovascular complications, we planned to lengthen the limb 5 to 6 cm in procedure [3], and (2) it is difficult for patients to carry limb-length inequality greater than 4 cm with nonoperative methods until skeletal maturity. Seventeen of 35 patients with initial TRA underwent two or more of the same procedures after lengthening. Eleven of 17 patients with multiple lengthenings finally achieved the final stage of a mobile joint.
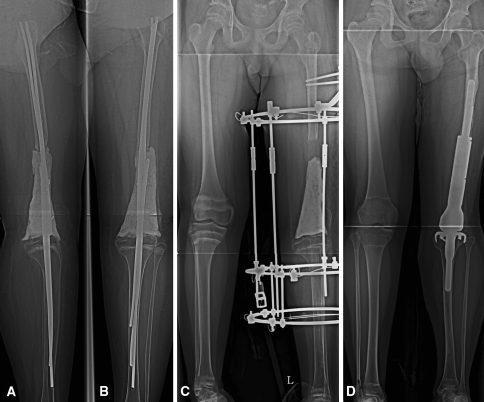
Fig. 1A–D.
Patient 33 was a 12-year-old boy with a diagnosis of metaphyseal osteosarcoma. (A) The lateral and (B) AP radiographs were taken after TRA using multiple Ender nails and bone cement. (C) This AP radiograph was taken after the soft tissue lengthening procedure was finished. The gap between bone cement and the distal end of the femur reveals the amount of length gained. The cement spacer was inserted to maintain room for subsequent implantation. (D) This AP radiograph shows successful conversion to an adult-type implant.
Patients who had reached skeletal maturity had a static adult prosthesis implanted (Fig. 1D). After the conversion to a mobile joint, the average angle of the flexion contracture and additional flexion were 1.4° (range, 0°–15°) and 75.6° (range, 40°–110°). The mean extension lag was 32.4° (range, 5°–80°). Our seemingly complicated procedure enabled us to use a stepwise approach in pediatric patients with osteosarcoma. Within 2 years after tumor resection, we focus on complete treatment of the cancer. After that, we concentrate on functional restoration with equalization of limb length.
For patients who underwent TRA (first stage) or soft tissue lengthening (second stage), we permitted walking with support within 2 weeks of surgery. Partial or complete weightbearing was encouraged according to the patient’s condition. Patients with tumor prostheses (third stage) started continuous passive motion (CPM) after the drains were removed. Active movement began after patients had gained 60° flexion, and they were allowed partial weightbearing. Thereafter, patients were discharged when they were able to transfer independently (approximately 3 weeks after surgery). Before discharge, they were instructed on ROM and strengthening exercises (30 minutes each, three times per day) and were recommended to visit the local clinic for supervision. Eight weeks postoperatively the patients returned for functional assessment and radiographic evaluation. Patients were allowed to wean from use of walking assists as tolerated thereafter.
All patients were checked every month during postoperative chemotherapy with conventional radiographs of the chest and the primary lesion. After completion of chemotherapy, patients were followed for 2 years with monthly radiographs of the chest and the operated limb. Thereafter, the same protocol was repeated twice yearly up to the fifth year and annually after that time.
After the 8-week visit we followed patients twice yearly for followup of the surgery. At each visit we determined MSTS scores [10]. We made the following assessments of limb lengths: measurement of spine-malleolar distance, wood block test, and teleroentgenograms showing the entire legs in one film. Final limb-length discrepancy, knee ROM, and extension lag were analyzed separately. We collected age, gender, site involved, heights at index operation and maturity, surgical procedures (the number of lengthening procedures and the total length gained), complications, and outcome information from the patients’ records.
All radiographs were reviewed by one radiologist (JYY) and two of the authors (SYL, DGJ). Using AP and lateral views of the entire extremity and scanograms, we evaluated (1) limb-length discrepancy and the amount of length gained, (2) limb-length inequality at skeletal maturity, and (3) presence of aseptic loosening. The radiographic interpretation of loosening was categorized into three grades: definite loosening, probable loosening, and possible loosening.
A major complication was defined as one that necessitated removal of hardware as a revision procedure. A minor complication was defined as problems other than the ones described above that necessitated additional surgical procedures or nonoperative management.
Results
The mean length gained was 7.8 cm (range, 4–14 cm) (Table 2). The average height at the time of the index operation was 140 cm (range, 119–155 cm), and the average limb-length discrepancy at the beginning of lengthening was 6.2 cm (range, 4.0–9.5 cm). Seventeen (49%) of the 35 patients who underwent soft tissue lengthening required more than one course of lengthening (range, 2–4 times). The average limb-length discrepancy at final followup was 2.6 cm (range, 0–6.5 cm). The major complication related to soft tissue lengthening was wound infection. Four patients (Patients 3, 6, 13, 26) experienced wound infections, which prevented conversion to a tumor prosthesis. Minor complications included transient peroneal nerve palsy in one patient and pin tract infection in three.
Table 2.
Surgical procedures
| Patient | Height at index operation (cm) | Height at maturity (cm) | Number of lengthenings | Amount of length gained (cm) | Surgical procedures | Time from index surgery to TP (months) | Time from TP to final followup (months) |
|---|---|---|---|---|---|---|---|
| 1 | 130 | 175 | 4 | 14 | RA → STL → TP | 134 | 13 |
| 2 | 121 | 151 | 1 | 7 | RA → STL → TP | 44 | 111 |
| 3 | 129 | 159 | 2 | 10 | RA → STL → RA | ND | ND |
| 4 | 122 | 146 | 1 | 5 | RA → STL → TP | 89 | 11 |
| 5 | 124 | 163 | 3 | 13 | RA → STL → RA | ND | ND |
| 6 | 129 | 159 | 2 | 7 | RA → STL → RA | ND | ND |
| 7 | 136 | 173 | 3 | 13 | RA → STL → TP | 73 | 7 |
| 8 | 135 | 167 | 3 | 12 | RA → STL → RA | ND | ND |
| 9 | 129 | 161 | 2 | 9 | RA → STL → TP | 85 | 13 |
| 10 | 127 | 151 | 1 | 4 | RA → STL → TP | 54 | 6 |
| 11 | 119 | 146 | 2 | 10 | RA → STL → TP | 54 | 9 |
| 12 | 136 | 158 | 1 | 7 | RA → STL → TP → RA | 50 | NA |
| 13 | 140 | 169 | 2 | 9 | RA → STL → RA | ND | ND |
| 14 | 135 | 168 | 2 | 10 | RA → STL → TP | 59 | 36 |
| 15 | 134 | 157 | 2 | 11 | RA → STL → RA | ND | ND |
| 16 | 140 | 169 | 2 | 11 | RA → STL → TP | 69 | 62 |
| 17 | 140 | 169 | 1 | 5 | RA → STL → TP | 91 | 84 |
| 18 | 152 | 157 | 1 | 4 | RA → STL → TP | 58 | 155 |
| 19 | 143 | 156 | 3 | 9 | RA → STL → TP | 78 | 103 |
| 20 | 150 | 169 | 2 | 8 | RA → STL → TP | 117 | 48 |
| 21 | 144 | 178 | 3 | 12 | RA → STL → TP → Rotationplasty | 78 | NA |
| 22 | 143 | 163 | 1 | 5 | RA → STL → TP | 54 | 147 |
| 23 | 147 | 160 | 1 | 6 | RA → STL → TP | 33 | 162 |
| 24 | 139 | 154 | 1 | 7 | RA → STL → TP | 57 | 130 |
| 25 | 149 | 159 | 1 | 7 | RA → STL → TP | 39 | 132 |
| 26 | 133 | 166 | 1 | 7 | RA → STL → RA | ND | ND |
| 27 | 149 | 162 | 1 | 6 | RA → STL → TP | 85 | 72 |
| 28 | 152 | 170 | 1 | 4 | RA → STL → TP | 31 | 92 |
| 29 | 155 | 163 | 1 | 5 | RA → STL → TP | 35 | 130 |
| 30 | 152 | 170 | 1 | 5 | RA → STL → TP → RA | 58 | NA |
| 31 | 152 | 173 | 2 | 7 | RA → STL → TP | 130 | 18 |
| 32 | 155 | 165 | 1 | 4 | RA → STL → TP | 35 | 139 |
| 33 | 152 | 167 | 2 | 10 | RA → STL → TP | 43 | 19 |
| 34 | 148 | 168 | 1 | 5 | RA → STL → TP | 56 | 8 |
| 35 | 159 | 172 | 1 | 6 | RA → STL → TP | 30 | 103 |
TP = tumor prosthesis; RA = resection arthrodesis; STL = soft tissue lengthening; ND = not done; NA = not assessed.
Of the 35 patients having a second stage procedure, 28 (80%) underwent the third stage and had implantation of a mobile joint (Table 2). Seven patients did not have a prosthesis implanted; four did not have a prosthesis implanted because of recurrent wound infection (Patients 3, 6, 13, 26), and conversion surgery was contraindicated in three owing to poor soft tissue condition (Patients 5, 15, 33). The mean age of the 28 patients at third-stage implantation was 17 years (range, 14–24 years). The average time from tumor resection to implantation of a mobile joint was 65 months (range, 30–134 months) and from prosthesis insertion to final followup was 72 months (range, 6–162 months). Three of the 28 prostheses later were removed owing to infections 8, 9, and 45 months after arthroplasty.
At final followup, the average MSTS functional score of 25 patients with mobile joints was 25.0 (range, 19–28). Among them, 10 patients had a limb-length inequality of 1 cm or less and seven patients had limb-length inequality between 1 and 2 cm (Table 3). Seventeen patients with limb-length inequality of 2 cm or less had an average MSTS score of 26.2 (range, 20–28), whereas eight patients (32%) with a discrepancy greater than 2 cm had a score of 22.5 (range, 19–26). The average functional score of the 10 patients who remained in the TRA stage was 18.9 (range, 15–21). Although most mobile joints had acceptable ROM (average, 74.2°; range, 35°–110°), extension lag frequently was observed (Table 3). Sixteen patients with greater than 30° extension lag had a mean 7.75-cm lengthening procedure whereas nine patients with less than 30° extension lag had a 6.3-cm lengthening (Table 3). No patient had aseptic loosening develop during the followup. One patient (Patient 18) had polyethylene liner wear at 62 months postoperatively, which was resolved by a liner change. Two patients (Patients 28, 35) had a periprosthetic fracture after a traffic accident and a fall, respectively. Internal fixation was performed in both and solid bony union was achieved (Fig. 2).
Table 3.
Functional outcome of tumor prostheses
| Patient | Surgical procedures | Final LLD (cm) | MSTS score | Knee ROM (°) | ||
|---|---|---|---|---|---|---|
| FC | FF | Extension lag | ||||
| 1 | RA → STL → TP | 5.5 | 21 | 0 | 40 | 30 |
| 2 | RA → STL → TP | 4 | 19 | 0 | 80 | 10 |
| 3 | RA → STL → RA | 4 | 17 | 0 | 0 | 0 |
| 4 | RA → STL → TP | 4 | 24 | 10 | 50 | 30 |
| 5 | RA → STL → RA | 3 | 21 | 0 | 0 | 0 |
| 6 | RA → STL → RA | 0 | 19 | 0 | 0 | 0 |
| 7 | RA → STL → TP | 5 | 20 | 0 | 50 | 40 |
| 8 | RA → STL → RA | 6.5 | 20 | 0 | 0 | 0 |
| 9 | RA → STL → TP | 1 | 27 | 0 | 70 | 20 |
| 10 | RA → STL → TP | 4.5 | 20 | 15 | 50 | 40 |
| 11 | RA → STL → TP | 0 | 20 | 0 | 50 | 40 |
| 12 | RA → STL → TP → RA | 5 | 17 | 0 | 0 | 0 |
| 13 | RA → STL → RA | 4 | 15 | 0 | 0 | 0 |
| 14 | RA → STL → TP | 2 | 28 | 0 | 70 | 5 |
| 15 | RA → STL → RA | 3 | 20 | 0 | 0 | 0 |
| 16 | RA → STL → TP | 2 | 26 | 0 | 90 | 60 |
| 17 | RA → STL → TP | 4 | 26 | 0 | 80 | 10 |
| 18 | RA → STL → TP | 1 | 28 | 0 | 100 | 20 |
| 19 | RA → STL → TP | 1 | 24 | 0 | 100 | 80 |
| 20 | RA → STL → TP | 2 | 28 | 0 | 90 | 40 |
| 21 | RA → STL → TP → Rotationplasty | NA | 18 | 0 | 0 | 0 |
| 22 | RA → STL → TP | 0 | 28 | 0 | 90 | 30 |
| 23 | RA → STL → TP | 1 | 25 | 0 | 60 | 10 |
| 24 | RA → STL → TP | 2 | 27 | 0 | 90 | 60 |
| 25 | RA → STL → TP | 2 | 27 | 0 | 110 | 60 |
| 26 | RA → STL → RA | 6 | 21 | 0 | 0 | 0 |
| 27 | RA → STL → TP | 1 | 28 | 0 | 90 | 10 |
| 28 | RA → STL → TP | 1.5 | 28 | 0 | 80 | 10 |
| 29 | RA → STL → TP | 1 | 26 | 0 | 80 | 40 |
| 30 | RA → STL → TP → RA | 2 | 21 | 0 | 0 | 0 |
| 31 | RA → STL → TP | 3 | 26 | 0 | 50 | 30 |
| 32 | RA → STL → TP | 1 | 25 | 0 | 70 | 50 |
| 33 | RA → STL → TP | 3 | 24 | 0 | 70 | 40 |
| 34 | RA → STL → TP | 2 | 22 | 10 | 80 | 40 |
| 35 | RA → STL → TP | 1 | 28 | 0 | 100 | 5 |
LLD = limb-length discrepancy; MSTS = Musculoskeletal Tumor Society; FC = flexion contracture; FF = further flexion; RA = resection arthrodesis; STL = soft tissue lengthening; TP = tumor prosthesis.
Fig. 2.
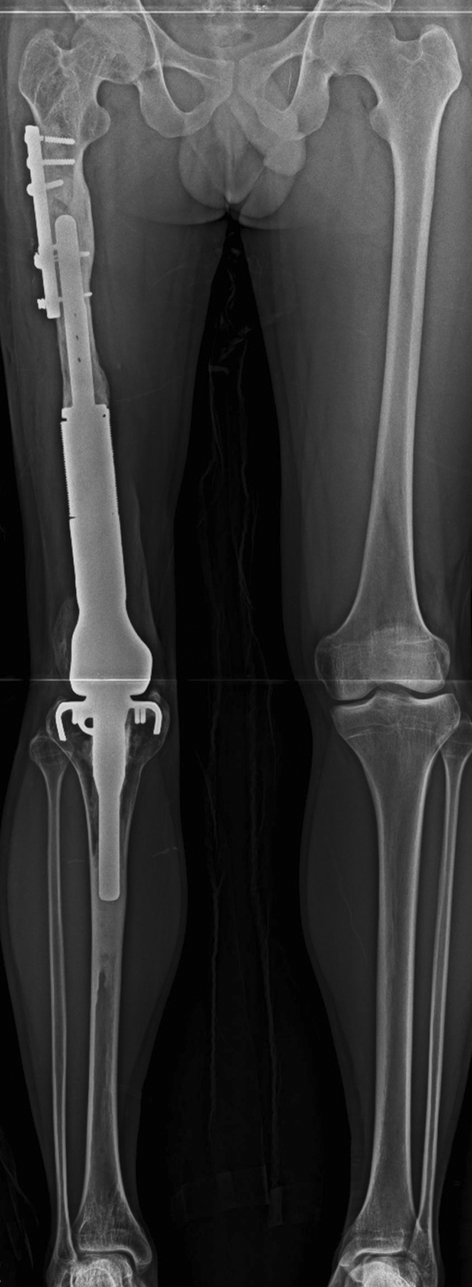
Patient 35 was a 13-year-old boy with an osteosarcoma in the distal femur. The patient had a periprosthetic fracture at the proximal femur, which was treated with open reduction and plate fixation. Solid bony union of the fracture is seen on this AP radiograph taken 6 months after internal fixation.
Discussion
At the time of epiphysis-ablating resection for skeletally immature patients with osteosarcoma around the knee, orthopaedic oncologists must determine the best reconstructive method. Currently, the most popular and accepted modality is an extendible prosthesis [1, 2, 13]. However, there are some concerns regarding metastatic probability and limb-length discrepancy at skeletal maturity, as suggested in previous reports (Table 4). Also, in some situations or locations, an expandable prosthesis is not available and therefore not an option. Therefore, we designed a three-stage lengthening procedure. We postponed the leg-length equalization procedure until the relapse potential of the patient reached a plateau and limb shortening was greater than 4 cm. Our stepwise approach could be cost-effective because the lengthening procedure is performed only when necessary.
Table 4.
Comparison with previous studies using expandable prostheses
| Study | Number of patients | Age (years)* | Number of patients who underwent lengthening (%) | Deaths (%) | Amputations (%) | Amount extended (cm)* | Final limb shortening (cm)* | Revisions (%) | Followup (years)* |
|---|---|---|---|---|---|---|---|---|---|
| Current study | 56 | 10.5 (6.9–13.9) | 35 (63%) | 10 (18%) | 3 (5%) | 7.8 (4–14) | 2.6 (0–6.5) | 0 (0%) | 11.8 (0.8–17.8) |
| Unwin and Walker [24] (1996) | 168 | 10.5 (3–18) | 96 (57%) | 28 (16.7%) | 13 (7.8%) | 3.1 | Not described | 38/123 (31%) | 2.4 (0.2–9.2) |
| Cool et al. [7] (1997) | 24 | 10.1 (5.8–14) | 22 (92%) | 1 (5%) | Excluded | Not described | Not described | 5/23 (22%) | 4.7 (2.5–7.9) |
| Schindler et al. [21] (1997) | 18 | 11 (8–14) | 12 (67%) | 4 (22%) | 2 (11%) | 5.2 (3.0–7.0) | < 3.5 | 10/12 (83%) | 8.7 (6.0–13.2) |
| Grimer et al. [13] (2000) | 20 | 9.9 (5–14) | 11 (55%) | 5 (25%) | 4 (20%) | Not described | 1.0 | 9/11 (82%) | > 5 |
| Eckardt et al. [9] (2000) | 32 | 9.7 (3–15) | 16 (50%) | 10 (31%) | 3 (9%) | 2 (1–9) | < 3 | 13/16 (81%) | 8.8 (4.5–13) |
| Neel et al. [18] (2003) | 18 | 11 (7–15) | 13 (87%) | 1 (7%) | 1 (7%) | 3.2 (0.2–5.6) | Not described | 8/16 (50%) | 1.8 (1–2.8) |
| Arkader et al. [2] (2007) | 12 | 11.6 (5.9–15.5) | 8 (67%) | 0 (0%) | 1 (8%) | 4.9 | Not described | 8/12 (67%) | 6.3 (1–12.7) |
* Values are expressed as means, with ranges in parentheses.
Our retrospective study has two limitations. First, we could not always perform the staged procedure as we planned. In some patients, lengthening or arthroplasty was postponed owing to a minor infection or problems with compliance. This might have affected the functional result. Second, the resection of soft tissue and its amount at tumor removal could not be standardized. The amount of soft tissue to be excised is determined by the extent of the tumor and this also might affect the subsequent infection rate or functional outcome [4].
Restoration of leg length is one of the primary goals in immature patients with osteosarcoma [1]. In our series, the average actual length gained was 7.8 cm (range, 4–14 cm), which is slightly larger than reported by others using an expandable prosthesis (Table 4). Using extensive scarectomy followed by gradual soft tissue lengthening, we can safely distract the soft tissues and avoid neurovascular complications [12, 20]. Because the maximal tolerable rate of soft tissue lengthening has not been clarified, we followed the well-established distraction rate (1 mm/day), which was applied to distraction osteogenesis. Patient 1 achieved 14 cm of lengthening through four separate lengthening procedures with no neurovascular complications. This might be difficult to achieve using an expandable tumor prosthesis.
Although an expandable prosthesis seems a reasonable way to equalize limb lengths in a growing child, among 289 reported patients receiving an expandable prosthesis, only 158 (54%) patients actually underwent lengthening (Table 4). The main reasons for patients not undergoing lengthening are early relapse of the tumor or overestimation of limb-length discrepancy. We found a similar percentage of patients who were actual candidates for lengthening: 35 of 56 patients (63%). Also, 28 of the 35 patients (80%) having soft tissue lengthening (and 50% of the total group of 56) ultimately had a mobile joint implanted. Major obstacles in switching to tumor prostheses were infection and incompetence of soft tissue coverage. These problems are two sides of the same coin. Infection may be influenced by the soft tissue dissection at tumor resection, number of lengthenings, and amount of lengthening [14]. The soft tissue condition can be compromised by repeated lengthening procedures and in turn may increase the risk of infection. The average lengthening amount of the patients who did not receive prostheses (four patients with infections, three with poor soft tissue coverage) was 9.6 cm (range, 7–13 cm), which is larger than that of patients receiving prostheses (average, 7.4 cm; range, 4–14 cm). Moreover, all but one patient in the group that did not receive prostheses had more than two lengthening procedures. Infection and local recurrence are the main reasons for limb loss [26]. Previous reports suggest 24 of 156 patients (15.4%) had amputations (Table 4). Given the reported local recurrence rates are 8% to 14% [13, 21, 26], the remaining portion (as much as 5% to 10%) of amputations would be an end result of infection. Another obstacle in converting to a mobile joint was soft tissue incompetence. After tumor extirpation, the axial space replaced by the temporary cement spacer remains unchanged owing to the loss of appositional bone growth. Moreover, soft tissue gradually becomes thinner through the limb lengthening. In patients with an excessive soft tissue defect, an additional flap surgery might be necessary.
Considering our staged procedure required a substantial amount of time, we believed the average MSTS functional score of 25.0 (range, 19–28) would be acceptable compared with previous reports (range, 23–25) [13, 21]. One might presume the amount of lengthening and duration of TRA could be inversely correlated with the functional outcome but we found no such trend. In terms of joint function, our approach has several disadvantages. First, TRA might have a negative influence on functional results over an expandable prosthesis owing to the TRA period with relatively poor function. Although we found passive knee ROM after arthroplasty was almost equivalent to that provided with a tumor prosthesis, the majority of patients complained of difficulty in climbing stairs owing to extension lag [13, 21]. Second, patients must accept a temporary functional deficit until they reach the final step of arthroplasty. Especially for the patients who can undergo direct conversion to an adult prosthesis without soft tissue lengthening, TRA might be an unnecessary procedure [17]. In fact, four of our patients had a prosthesis implanted without intervening fusion. Third, the conceptual advantage of a delayed tumor prosthesis could be a disadvantage: as we cannot predict who will die within a few years after presentation, patients may have been better off with one limb-salvage procedure to enhance the quality of life. Finally, the patient may end up with an arthrodesis for the remainder of his or her life if he or she is not a candidate for the third-stage conversion of implanting a mobile prosthesis.
Compared with the relatively high revision rate of expandable prostheses, a recently introduced novel noninvasive expandable prosthesis reportedly showed a mean MSTS score of 27 (range, 21–30) with low revision rates [25]. However, Wilkins et al. [25] expressed concern regarding the mechanical weakness of a “growing prosthesis” compared with the adult-type static prosthesis. Nevertheless, we suspect this kind of new device ultimately will reduce the number of operations and the complication rate.
The three-stage lengthening arthroplasty we described seems to be an option for skeletally immature patients, especially when the expandable prosthesis is not available. However, this technique has limitations for patients who cannot accept a prolonged functional deficit.
Acknowledgments
We thank Dr. J.Y. Yoo for assistance with radiographic measurement.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might constitute a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abudu A, Grimer R, Tillman R, Carter S. The use of prostheses in skeletally immature patients. Orthop Clin North Am. 2006;37:75–84. doi: 10.1016/j.ocl.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Arkader A, Viola DC, Morris CD, Boland PJ, Healey JH. Coaxial extendible knee equalizes limb length in children with osteogenic sarcoma. Clin Orthop Relat Res. 2007;459:60–65. doi: 10.1097/BLO.0b013e3180514c37. [DOI] [PubMed] [Google Scholar]
- 3.Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 1997;79:1243–1258. doi: 10.2106/00004623-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti MG, Catani F, Donati D, Simoncini L, Giannini S. Muscle performance about the knee joint in patients who had distal femoral replacement after resection of a bone tumor: an objective study with use of gait analysis. J Bone Joint Surg Am. 2000;82:1619–1625. doi: 10.2106/00004623-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Brisse H, Ollivier L, Edeline V, Pacquement H, Michon J, Glorion C, Neuenschwander S. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34:595–605. doi: 10.1007/s00247-004-1192-x. [DOI] [PubMed] [Google Scholar]
- 6.Capanna R, Biagini R, Ruggieri P, Bettelli G, Casadei R, Campanacci M. Temporary resection-arthrodesis of the knee using an intramedullary rod and bone cement. Int Orthop. 1989;13:253–258. doi: 10.1007/BF00268507. [DOI] [PubMed] [Google Scholar]
- 7.Cool WP, Carter SR, Grimer RJ, Tillman RM, Walker PS. Growth after extendible endoprosthetic replacement of the distal femur. J Bone Joint Surg Br. 1997;79:938–942. doi: 10.1302/0301-620X.79B6.7868. [DOI] [PubMed] [Google Scholar]
- 8.Costelloe CM, Macapinlac HA, Madewell JE, Fitzgerald NE, Mawlawi OR, Rohren EM, Raymond AK, Lewis VO, Anderson PM, Bassett RL, Jr, Harrell RK, Marom EM. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50:340–347. doi: 10.2967/jnumed.108.058461. [DOI] [PubMed] [Google Scholar]
- 9.Eckardt JJ, Kabo JM, Kelley CM, Ward WG, Sr, Asavamongkolkul A, Wirganowicz PZ, Yang RS, Eilber FR. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;373:51–61. doi: 10.1097/00003086-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 11.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed] [Google Scholar]
- 12.Goldfarb CA, Murtha YM, Gordon JE, Manske PR. Soft-tissue distraction with a ring external fixator before centralization for radial longitudinal deficiency. J Hand Surg Am. 2006;31:952–959. doi: 10.1016/j.jhsa.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Grimer RJ, Belthur M, Carter SR, Tillman RM, Cool P. Extendible replacements of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2000;82:255–260. doi: 10.1302/0301-620X.82B2 .9863. [DOI] [PubMed] [Google Scholar]
- 14.Hardes J, Gebert C, Schwappach A, Ahrens H, Streitburger A, Winkelmann W, Gosheger G. Characteristics and outcome of infections associated with tumor endoprostheses. Arch Orthop Trauma Surg. 2006;126:289–296. doi: 10.1007/s00402-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins DS, Conrad EU, 3rd, Butrynski JE, Schuetze SM, Eary JF. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009;115:3519–3525. doi: 10.1002/cncr.24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MS, Cho WH, Song WS, Lee SY, Jeon DG. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin Orthop Relat Res. 2007;463:157–165. doi: 10.1097/BLO.0b013e318142b27d. [DOI] [PubMed] [Google Scholar]
- 17.Muschler GF, Ihara K, Lane JM, Healey JH, Levine MJ, Otis JC, Burstein AH. A custom distal femoral prosthesis for reconstruction of large defects following wide excision for sarcoma: results and prognostic factors. Orthopedics. 1995;18:527–538. doi: 10.3928/0147-7447-19950601-04. [DOI] [PubMed] [Google Scholar]
- 18.Neel MD, Wilkins RM, Rao BN, Kelly CM. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003;415:72–81. doi: 10.1097/01.blo.0000093899.12372.25. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki T, Nakatsuka Y, Kunisada T, Kawai A, Dan’ura T, Naito N, Inoue H. High complication rate of reconstruction using Ilizarov bone transport method in patients with bone sarcomas. Arch Orthop Trauma Surg. 1998;118:136–139. doi: 10.1007/s004020050333. [DOI] [PubMed] [Google Scholar]
- 20.Sabharwal S, Finuoli AL, Ghobadi F. Pre-centralization soft tissue distraction for Bayne type IV congenital radial deficiency in children. J Pediatr Orthop. 2005;25:377–381. doi: 10.1097/01.bpo.0000152907.31293.00. [DOI] [PubMed] [Google Scholar]
- 21.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Stanmore custom-made extendible distal femoral replacements: clinical experience in children with primary malignant bone tumours. J Bone Joint Surg Br. 1997;79:927–937. doi: 10.1302/0301-620X.79B6.7164. [DOI] [PubMed] [Google Scholar]
- 22.Torricelli P, Montanari N, Spina V, Manfrini M, Bertoni F, Saguatti G, Romagnoli R. Dynamic contrast enhanced magnetic resonance imaging subtraction in evaluating osteosarcoma response to chemotherapy. Radiol Med. 2001;101:145–151. [PubMed] [Google Scholar]
- 23.Tsuchiya H, Abdel-Wanis ME, Sakurakichi K, Yamashiro T, Tomita K. Osteosarcoma around the knee: intraepiphyseal excision and biological reconstruction with distraction osteogenesis. J Bone Joint Surg Br. 2002;84:1162–1166. doi: 10.1302/0301-620X.84B8.13330. [DOI] [PubMed] [Google Scholar]
- 24.Unwin PS, Walker PS. Extendible endoprostheses for the skeletally immature. Clin Orthop Relat Res. 1996;322:179–193. doi: 10.1097/00003086-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins RM, Camozzi AB, Gitelis SB. Reconstruction options for pediatric bone tumors about the knee. J Knee Surg. 2005;18:305–309. doi: 10.1055/s-0030-1248197. [DOI] [PubMed] [Google Scholar]
- 26.Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74. doi: 10.1097/00003086-199901000-00009. [DOI] [PubMed] [Google Scholar]