Abstract
A controversy has existed in the literature for the past several years regarding the number of vertebrate genes encoding the mitochondrial protein that initiates the first step in heme biosynthesis, delta-aminolevulinate synthase [ALAS; succinyl-CoA: glycine C-succinyltransferase (decarboxylating), EC 2.3.1.37]. By analysis of chicken ALAS cDNA clones isolated from both liver and erythroid cells, we show that at least two separate genes encode ALAS mRNAs. These experiments show that (i) two different genes encode the ALAS isozymes found in erythroid and in liver tissues, and (ii) while the product of the erythroid gene (ALASE) is expressed exclusively in erythroid cells, the hepatic form of the enzyme is expressed ubiquitously, suggesting that this is the nonspecific form (ALASN) found in all chicken tissues.
Full text
PDF


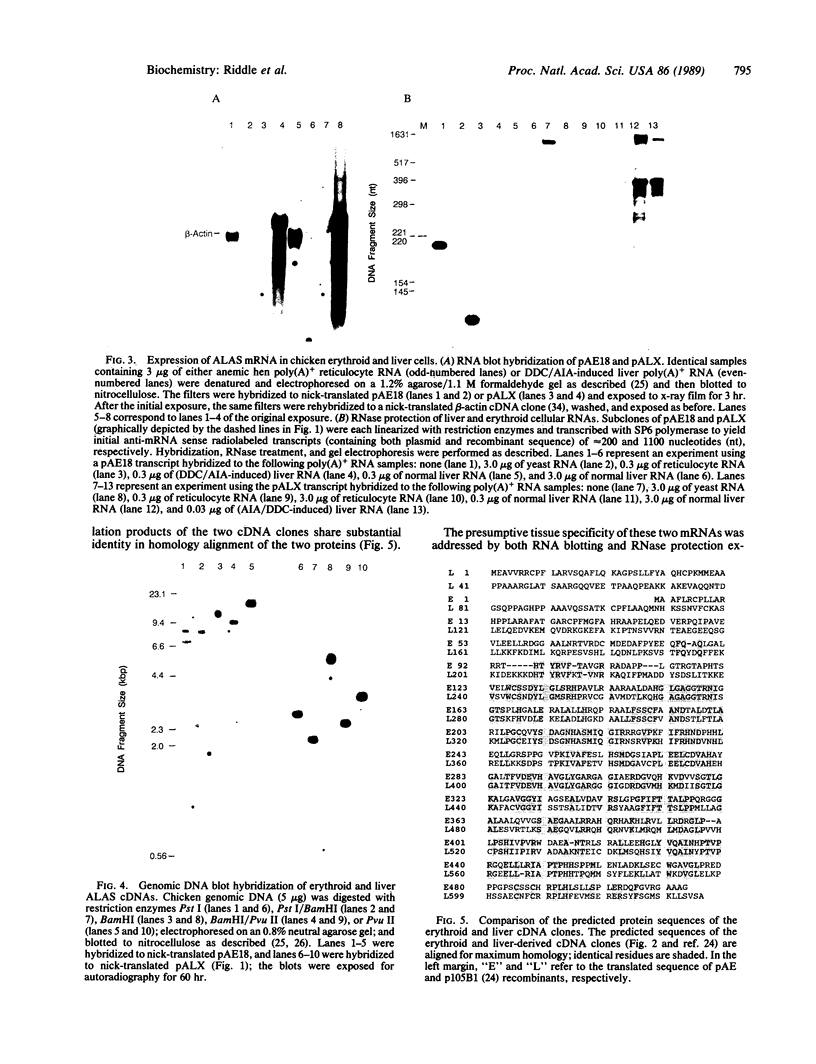

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. F., Kitchen H., Wood W. A. Evidence for erythroid and nonerythroid forms of delta-aminolevulinate synthetase. Arch Biochem Biophys. 1981 Feb;206(2):380–391. doi: 10.1016/0003-9861(81)90105-3. [DOI] [PubMed] [Google Scholar]
- Borthwick I. A., Srivastava G., Brooker J. D., May B. K., Elliott W. H. Purification of 5-aminolaevulinate synthase from liver mitochondria of chick embryo. Eur J Biochem. 1983 Jan 1;129(3):615–620. doi: 10.1111/j.1432-1033.1983.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Borthwick I. A., Srivastava G., Day A. R., Pirola B. A., Snoswell M. A., May B. K., Elliott W. H. Complete nucleotide sequence of hepatic 5-aminolaevulinate synthase precursor. Eur J Biochem. 1985 Aug 1;150(3):481–484. doi: 10.1111/j.1432-1033.1985.tb09047.x. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Smithee G. A. Characterization and measurement of delta-aminolaevulinate synthetase in bone marrow cell mitochondria. Biochim Biophys Acta. 1968 Apr 24;159(1):27–37. doi: 10.1016/0005-2744(68)90241-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Elferink C. J., Srivastava G., Maguire D. J., Borthwick I. A., May B. K., Elliott W. H. A unique gene for 5-aminolevulinate synthase in chickens. Evidence for expression of an identical messenger RNA in hepatic and erythroid tissues. J Biol Chem. 1987 Mar 25;262(9):3988–3992. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Birch H., Dickson P. W., Schreiber G. Determination of the molecular weight of detergent-solubilized enzymes by sedimentation equilibrium in an air-driven ultracentrifuge. Biochem Biophys Res Commun. 1982 Apr 14;105(3):895–901. doi: 10.1016/0006-291x(82)91054-3. [DOI] [PubMed] [Google Scholar]
- KIKUCHI G., KUMAR A., TALMAGE P., SHEMIN D. The enzymatic synthesis of delta-aminolevulinic acid. J Biol Chem. 1958 Nov;233(5):1214–1219. [PubMed] [Google Scholar]
- Kikuchi G., Hayashi N. Regulation by heme of synthesis and intracellular translocation of delta-aminolevulinate synthase in the liver. Mol Cell Biochem. 1981 Jun 9;37(1):27–41. doi: 10.1007/BF02355885. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Williams P. H., Ditta G. S. Analysis of the 5' regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res. 1985 Aug 26;13(16):5965–5976. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire D. J., Day A. R., Borthwick I. A., Srivastava G., Wigley P. L., May B. K., Elliott W. H. Nucleotide sequence of the chicken 5-aminolevulinate synthase gene. Nucleic Acids Res. 1986 Feb 11;14(3):1379–1391. doi: 10.1093/nar/14.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C. R., Somerville J. E., Guerinot M. L., Chelm B. K. Structure of the Bradyrhizobium japonicum gene hemA encoding 5-aminolevulinic acid synthase. Gene. 1987;54(1):133–139. doi: 10.1016/0378-1119(87)90355-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S. Induction of -aminolevulinic acid synthetase in chick embryo liver cells in cluture. Proc Natl Acad Sci U S A. 1970 Oct;67(2):517–522. doi: 10.1073/pnas.67.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P. W., Dodgson J. B., Engel J. D. Structure and expression of the chicken ferritin H-subunit gene. Mol Cell Biol. 1987 May;7(5):1751–1758. doi: 10.1128/mcb.7.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. L., Marks G. S. Drug-induced porphyrin biosynthesis. V. Effect of protohemin on the transcriptional and post-transcriptional phases of -aminolevulinic acid synthetase induction. Biochem Pharmacol. 1972 Aug 1;21(15):2077–2093. doi: 10.1016/0006-2952(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Wada O., Sassa S., Takaku F., Yano Y., Uratta G., Nakao K. Different responses of the hepatic and erythropoietic delta-aminolevulinic acid synthetase of mice. Biochim Biophys Acta. 1967 Nov 28;148(2):585–587. doi: 10.1016/0304-4165(67)90165-1. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Hayashi N., Kikuchi G. delta-Aminolevulinate synthase isozymes in the liver and erythroid cells of chicken. Biochem Biophys Res Commun. 1983 Jun 15;113(2):377–383. doi: 10.1016/0006-291x(83)91737-0. [DOI] [PubMed] [Google Scholar]
- Whiting M. J. Synthesis of delta-aminolaevulinate synthase by isolated liver polyribosomes. Biochem J. 1976 Aug 15;158(2):391–400. doi: 10.1042/bj1580391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Dixon R. L. Studies of the perinatal differences in the activity of hepatic -aminolevulinic acid synthetase. Biochem Pharmacol. 1972 Jun 15;21(12):1735–1744. doi: 10.1016/0006-2952(72)90080-9. [DOI] [PubMed] [Google Scholar]
- Woods J. S. Studies on the role of heme in the regulation of delta-aminolevulinic acid synthetase during fetal hepatic development. Mol Pharmacol. 1974 May;10(3):389–397. [PubMed] [Google Scholar]
- Yamamoto M., Fujita H., Watanabe N., Hayashi N., Kikuchi G. An immunochemical study of delta-aminolevulinate synthase and delta-aminolevulinate dehydratase in liver and erythroid cells of rat. Arch Biochem Biophys. 1986 Feb 15;245(1):76–83. doi: 10.1016/0003-9861(86)90191-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Regulation of synthesis and intracellular translocation of delta-aminolevulinate synthase by heme and its relation to the heme saturation of tryptophan pyrrolase in rat liver. Arch Biochem Biophys. 1981 Jul;209(2):451–459. doi: 10.1016/0003-9861(81)90302-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Hayashi N., Kikuchi G. Translational inhibition by heme of the synthesis of hepatic delta-aminolevulinate synthase in a cell-free system. Biochem Biophys Res Commun. 1983 Aug 30;115(1):225–231. doi: 10.1016/0006-291x(83)90993-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kure S., Engel J. D., Hiraga K. Structure, turnover, and heme-mediated suppression of the level of mRNA encoding rat liver delta-aminolevulinate synthase. J Biol Chem. 1988 Nov 5;263(31):15973–15979. [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Hayashi N., Kikuchi G. Translocation of delta-aminolevulinate synthase from the cytosol to the mitochondria and its regulation by hemin in the rat liver. J Biol Chem. 1980 Feb 25;255(4):1746–1751. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]