Abstract
Purpose
The anaplastic large cell kinase gene (ALK) is rearranged in approximately 5% of lung adenocarcinomas within the Asian population. We evaluated the incidence and the characteristics of ALK-rearranged lung adenocarcinomas within the Western population and the optimal diagnostic modality to detect ALK rearrangements in routine clinical practice.
Experimental Design
We tested 358 lung adenocarcinomas from three institutions for ALK rearrangements by fluorescent in-situ hybridization (FISH) and immunohistochemistry (IHC) with and without tyramide amplification (TA). The clinicopathologic characteristics of tumors with and without ALK rearrangements were compared.
Results
We identified 20 lung adenocarcinomas (5.6%) with ALK rearrangements within our cohort of Western patients. ALK rearrangement was associated with younger age (P = 0.0002), never smoking (P < 0.0001), advanced clinical stage (P = 0.0001), and a solid histology with signet-ring cells (P < 0.0001). ALK rearrangement was identified by FISH in 95% of cases, IHC with and without TA in 80% and 40% of cases, respectively, but neither FISH nor IHC alone detected all cases with ALK rearrangement on initial screening. None of the ALK-rearranged tumors harbored coexisting EGFR mutations.
Conclusions
Lung adenocarcinomas with ALK rearrangements are uncommon in the Western population and represent a distinct entity of carcinomas with unique characteristics. For suspected cases dual diagnostic testing, with FISH and IHC, should be considered to accurately identify lung adenocarcinomas with ALK rearrangement.
Introduction
Lung cancer is the leading cause of death from cancer in both men and women(1). Despite advances in treatment, the five-year overall survival rate is approximately 15.7%(2). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases (3, 4). Currently, pathologic stage is the most important system to predict survival in patients with NSCLC and to define groups with similar treatment strategies (5, 6). However, the discovery of novel molecular alterations may help identify therapeutic targets that are more effective and with fewer side effects than current treatments.
The current classification of lung adenocarcinoma by the World Health Organization recognizes several distinct morphologic subtypes of adenocarcinoma: papillary, acinar, solid and bronchioloalveolar (7). However, the majority of lung adenocarcinomas exhibit combinations of morphologic patterns and are categorized as mixed subtype (7-9). While the biologic basis for the histologic subtypes remains an area of active investigation (9), there is evidence that some subtypes may be associated with specific molecular alterations (9-14) or a better outcome (15-17).
Anaplastic large cell lymphoma kinase gene (ALK) was originally identified through cloning of the t(2;5)(p23;35) translocation found in a subset of anaplastic large cell lymphomas (ALCLs), a tumor of T-cell lineage (18, 19). ALK encodes a tyrosine kinase receptor that is normally expressed only in select neuronal cell types. In ALK-rearranged anaplastic large cell lymphomas (ALCLs), the intracytoplasmic portion of ALK is fused to the N-terminal portion of nucleophosmin (NPM) resulting in a chimeric protein with constitutive kinase activity. Several other balanced translocations involving ALK have been discovered in ALCLs; however the various resulting chimeric proteins all retain the ALK kinase domain (20). The importance of the kinase activity is exemplified by ALK-rearranged ALCL cell lines which are dependent upon ALK enzymatic activity for growth and survival.
Recently, ALK rearrangements were identified in rare non-small cell lung cancer (NSCLC) cell lines and in isolated primary adenocarcinomas from Japanese and Chinese populations (21, 22). The majority of the ALK rearrangements within NSCLCs result from an interstitial deletion and inversion in chromosome 2p and result in the EML4-ALK fusion gene product (21, 22). EML4 encodes Echinoderm microtubule associated protein-like 4- a protein that may function in microtubule assembly. Transgenic mice expressing EML4-ALK within the lung epithelium develop numerous tumors, thereby confirming the oncogenic nature of the mutant protein (23). Murine tumors and human cell lines expressing EML-ALK are sensitive to inhibitors of ALK kinase activity (23, 24). Together these data indicate that, like EGFR, ALK is an important molecular target in lung carcinoma. Thus, it will be critical to efficiently and accurately identify those lung adenocarcinomas that harbor ALK rearrangements in routine practice in order to guide the appropriate clinical therapy.
In this study, we evaluated 358 lung adenocarcinomas from three institutions and identified 20 harboring an ALK rearrangement. None of ALK-rearranged adenocarcinomas in our study showed coexistent mutations in EGFR. We found that ALK-rearranged adenocarcinomas are more likely to present in younger patients with a history of never-smoking, and at higher stage relative to those without ALK rearrangements (ALK germline). The majority of ALK-rearranged adenocarcinomas had a distinct histology represented by solid tumor growth and frequent signet-ring cells with abundant intracellular mucin. Finally, we illustrate that the routine screening for ALK rearrangements in lung adenocarcinomas is challenging and suggest that screening by both FISH and IHC will accurately identify patients with this uncommon molecular abnormality.
Materials and Methods
Case selection
Three hundred and fifty-eight cases of lung adenocarcinoma from three participating institutions were examined (Supplementary Table 1). The first group consists of 116 consecutive lung adenocarcinoma patients treated with surgery with or without postoperative adjuvant chemotherapy at Brigham & Women’s Hospital (BWH) between March 1997 and December 1999 (Supplementary Table 1). Each resection specimen was evaluated with standard pathologic methods as described in the Surgical Pathology Dissection Manual of the Department of Pathology (25). The cases were reviewed and staged according to the Sixth Edition of the American Joint Committee on Cancer manual (5). Patients were selected for study with the following inclusion criteria: lung adenocarcinoma; first treatment by surgery alone, with or without postoperative adjuvant treatment; no other malignant tumors in five years prior to the diagnosis of lung adenocarcinoma except squamous cell or basal cell carcinoma of the skin or carcinoma in situ of the uterine cervix; and no deaths in the perioperative period less than 30 days after surgery.
The second group consists of 111 consecutive surgically resected adenocarcinomas from the University of Pittsburgh (UP) that were subjected to EGFR and KRAS mutational analysis between February of 2005 and July of 2007 using the same inclusion criteria as for the BWH group.
The third group consists of 131 patients with lung adenocarcinoma who were referred for treatment at Massachusetts General Hospital (MGH). These patients were screened for an ALK translocation by FISH as part of routine clinical care along with EGFR mutation analysis. The cohort was enriched for clinical characteristics associated with EGFR mutation including young age, and never or light smoking history (Supplemental Table 1). The pathologic characteristics were tumor size, pathologic stage, tumor and lymph node status (Supplemental Table 1). Protocol reviews and approvals were obtained from the Dana Farber Harvard Cancer Center, University of Pittsburgh, and Massachusetts General Hospital Institutional Review Boards.
Histologic Analysis
For each case, multiple slides corresponding to whole tissue sections were reviewed simultaneously by at least two pathologists and classified according to WHO criteria (7). In mixed subtype adenocarcinomas we assessed the percentage of each histologic pattern (acinar, papillary, solid and bronchioloalveolar) in 10-percent increments and recorded the predominant histologic pattern (9). Cases with differences between the two reviewers were reevaluated and a consensus interpretation was rendered. Poorly differentiated adenocarcinomas with a pure solid growth pattern were confirmed by a positive mucicarmine stain and negative p63 immunohistochemical stain.
Tissue microarrays (TMA) used for FISH and IHC analyses were constructed from a representative block from formalin-fixed, paraffin-embedded archival tissue specimens from the BWH and UP cohorts, as previously described (8). Three tumor samples (0.6 mm cores) from each case were included into paraffin recipient blocks using a manual arrayer (Beecher Instruments, Inc.). The three cored areas on each donor block were randomly selected from different parts of the tumor tissue based on a histologic characterization of the H&E-stained slide.
Immunohistochemistry
Immunohistochemistry was performed on 4μm-thick formalin-fixed, paraffin-embedded tissue sections as described (26). Briefly, slides were deparaffinized and pretreated with 1 mM EDTA, pH 8.0 (Zymed, South San Francisco, CA) and heat-mediated antigen retrieval in a steam pressure cooker (Decloaking Chamber, BioCare Medical, Walnut Creek, CA). All further steps were performed at room temperature in a hydrated chamber. Endogenous peroxidase activity was quenched with Peroxidase Block (DAKO USA, Carpinteria, CA) for 5 minutes and slides were pre incubated in 20% normal goat serum in 50-mM Tris-HCl (pH 7.4). Mouse monoclonal anti-human CD246, ALK culture supernatant (clone: ALK1, DAKO USA, Carpinteria, CA) was applied at 1:2 in DAKO diluent overnight (for unamplified detection), washed in Tris-HCl, and detected with horse radish peroxidase (HRP) conjugated anti-mouse Envision+ kit (DAKO).
Alternatively, for tyramide amplification, anti-CD246 culture supernatant (DAKO) was applied at 1:10 in DAKO diluent for one hour, and then further amplified using the catalytic activity of HRP to bind biotin-labeled tyramide (TSA, Perkin Elmer, Waltham, MA) diluted 1:250 for 10 minutes (26, 27). Chromogenic visualization of amplified slides was accomplished through the use of HRP conjugated streptavidin (DAKO), followed by DAB. All slides were then counterstained with hematoxylin.
All cases were evaluated and scored as either positive or negative for ALK expression by two pathologists (SJR and LRC).
Fluorescent in-situ hybridization
FISH was performed on formalin-fixed, paraffin-embedded tumor tissues using a break-apart probe to the ALK gene (Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe, Abbott Molecular) per manufacturer’s instructions. FISH positive cases were defined as >15% split signals in tumor cells.
Statistical analysis
Fisher’s exact test was used to compare categorical data for clinicopathologic characteristics between ALK-rearranged and ALK germline subgroups, and Wilcoxon rank-sum test was used to compare differences in the distribution of continuous data. All p-values are based on a two-sided hypothesis test.
Results
ALK rearrangements are rare among surgically resected lung adenocarcinomas
The vast majority of ALK-rearranged NSCLCs identified in the Asian population to date have been adenocarcinomas, with rare cases exhibiting squamous components (21, 28). To establish the prevalence of ALK-rearranged adenocarcinomas in the Western population, we screened 227 consecutive, unselected patients with lung adenocarcinoma from the archives of two institutions in the United States (Brigham & Women’s Hospital, Boston, MA, and University of Pittsburgh Hospital, Pittsburgh, PA). The two cohorts demonstrated similar clinicopathologic characteristics (Supplementary Table 1). The average patient age and proportion with a positive smoking history in these groups is comparable to that observed nationally (compared with SEER statistics, data not shown) (1, 2). However, because both cohorts consisted of surgically resected tumor specimens, these cases were also enriched for low stage (74% stages I and II) without involvement of regional lymph nodes (66% node-negative).
Each case was tested for ALK rearrangements by IHC and FISH. Both techniques independently identified the same single positive case from the BWH group and no positive cases from the UP group (1 case of 227; 0.45%). The patient with an ALK rearranged adenocarcinoma was a 62 year old woman with a history of 1.5 pack-year smoking.
ALK rearrangements are enriched among adenocarcinomas from a selected patient population
We next examined a group of cases collected and analyzed simultaneously for EGFR mutations as part of a clinical trial at a third institution based on case referral to a thoracic oncology practice (Massachusetts General Hospital, Boston, MA)(29). This group consists of patients (predominantly stage IV 64% vs. 4% from BWH and UP, Supplemental Table 1), as expected from an oncology clinic population. In addition, the MGH patients were younger at diagnosis (mean age 63 years), and more likely to have had a history of never-smoking (40% for MGH vs. 15% for BWH and UP).
Among 131 MGH cases examined by FISH analysis, 19 cases (14%) were positive for an ALK rearrangement. A subset of these cases was confirmed to express ALK protein by IHC (see below, Table 3). The difference in the incidence of ALK-rearranged tumors between the combined unselected BWH and UP cohorts and the MGH cohort is significant (P < 0.001) and suggests differences between the selection criteria.
Table 3.
Correlation of FISH and IHC results*
| Immunohistochemistry | FISH Test Result |
|
|---|---|---|
| ALK rearrangement | ALK germline | |
| Without Tyramide Amplification | ||
| Positive | 4 | 0 |
| Negative | 6 | 233 |
| With Tyramide Amplification | ||
| Positive | 8 | 0 |
| Negative | 2 | 233 |
Ten of twenty cases with ALK rearrangement by FISH had available tissue for IHC analysis
Characteristics of lung adenocarcinomas with ALK rearrangements
The clinicopathologic characteristics of patients with tumors with ALK rearrangement are illustrated in Table 1. Compared to national statistics (SEER database, data not shown) and to the patients with ALK germline (Table 1), patients with lung adenocarcinomas with an ALK rearrangement are younger at diagnosis and have a history of never-smoking. Strikingly, ALK-rearranged tumors presented at higher stage, most commonly at stage IV, relative to ALK germline tumors (Table 1).
Table 1.
Clinicopathologic characteristics of ALK-rearranged and ALK germline NSCLC*
| Characteristic | ALK rearrangement | ALK germline | P value |
|---|---|---|---|
| Number of cases | 20 (6) | 338 (94) | |
| Sex - n (%) | 0.16 | ||
| Male | 11 (55) | 127 (38) | |
| Female | 9 (45) | 211 (62) | |
| Age (years) | 0.0002 | ||
| Median | 51 | 66 | |
| Range | 29-76 | 29-90 | |
| Smoking Status - n (%) | <0.0001 | ||
| Never smoker | 14 (70) | 71 (21) | |
| Smoker | 6 (30) | 237 (70) | |
| Unknown | 30 (9) | ||
| Tumor Status** - n (%) | 0.15 | ||
| pT1 | 4 (20) | 103 (30) | |
| pT2 | 2 (10) | 119 (35) | |
| pT3 | 0 (0) | 10 (3) | |
| pT4 | 0 (0) | 37 (11) | |
| Not evaluated | 14 (70) | 69 (20) | |
| Lymph Node Status** - n (%) | 0.13 | ||
| Negative | 4 (20) | 176 (52) | |
| Positive | 6 (30) | 81 (24) | |
| Not evaluated | 10 (50) | 81 (24) | |
| Stage** - n (%) | 0.0001 | ||
| I | 4 (15) | 165 (49) | |
| II | 0 (0) | 29 (9) | |
| III | 0 (0) | 67 (20) | |
| IV | 16 (85) | 77 (23) |
Due to rounding not all percentages total 100.
Tumor and node status were based on pathology evaluation (pStage). Stage was based on both radiology and pathology evaluation (cStage).
The characteristics of NSCLC with ALK rearrangements in our study are very similar to those for NSCLC harboring mutations in EGFR (30, 31). Specifically, patients with NSCLC with ALK rearrangements are young patients without a significant smoking history. We found that the MGH cohort included 28 cases of NSCLC with EGFR mutations (21%). Of the 19 MGH ALK-rearranged NSCLCs, we found that none showed co-existing EGFR mutations. The mutual exclusivity of ALK rearrangements and EGFR mutations is significant (P = 0.013, 0/19 in ALK-rearranged vs, 28/112 in ALK germline). The details of this association and its significance are reported elsewhere (29). Of note, the MGH group includes a percentage of EGFR mutant cases comparable to that found within the UP cohort (22 cases, 20%; data not shown).
Morphologic profile of ALK-rearranged adenocarcinomas
The morphologic characteristics of ALK-rearranged tumors are illustrated in Table 2. Among cases with sufficient tissue for adequate analysis, the majority (11 of 16 cases, 69%) showed tumor cells with a solid or sheet-like pattern easily distinguishable from the acinar, papillary or bronchioloalveolar patterns. Of the remaining 5 cases, four showed a predominantly acinar pattern and one showed a predominantly bronchioloalveolar pattern.
Table 2.
Histologic characteristics of lung adenocarcinomas with ALK rearrangement*
| Characteristics | Total Samples |
ALK Rearrangement |
ALK Germline |
P value |
|---|---|---|---|---|
| Signet-ring cells in tumor - n (%) | 342 | 17 (100) | 325 (100) | <0.0001 |
| None | 295 | 3 (18) | 292 (90) | |
| ≤10% | 21 | 2 (12) | 19 (6) | |
| >10% | 26 | 12 (71) | 14 (4) | |
| Dominant histologic pattern - n (%) ** | 326 | 16 (100) | 310 (100) | 0.11 |
| Bronchioloalveolar | 22 | 1 (6) | 21 (7) | |
| Acinar | 124 | 4 (25) | 120 (39) | |
| Papillary | 46 | 0 (0) | 46 (15) | |
| Solid | 134 | 11 (69) | 123 (40) | |
|
Solid pattern and >10% signet-ring
cells - n (%) ** |
9 (56) | 9 (3) | <0.0001 | |
Due to rounding not all percentages total 100.
Tumor architecture was not evaluated in 16 cases where the diagnosis was made only by cytology.
Among tumors without ALK rearrangements only a minority (123 of 310 cases, 40%) showed a solid growth as the predominant pattern, which is significantly different from ALK-rearranged tumors (P = 0.034). The remaining cases showed a variety of histologic patterns predominated by acinar, papillary, or bronchioloalveolar morphology (data not shown).
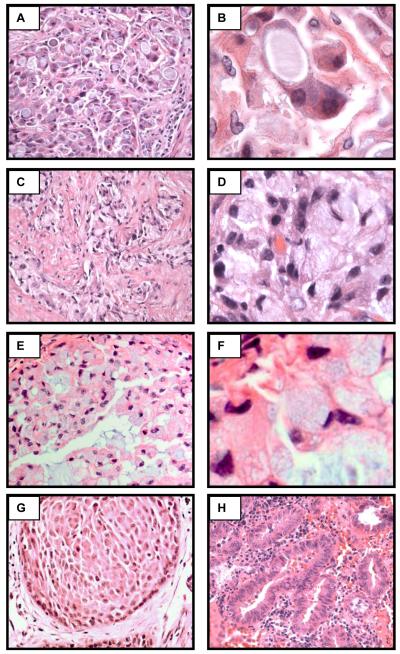
Even more striking were the cytologic features of tumors harboring ALK rearrangements. ALK-rearranged tumors (82%) showed at least focally tumor cells with abundant intracellular mucin and small, marginalized nuclei (Figure 1, Table 2). In a majority of cases (71%), cells with abundant intracellular mucin comprised >10% of the overall tumor cellularity. In contrast, the majority of ALK germline tumors (90%) showed no tumor cells with this morphology. This distinct cytologic characteristic, unusual for lung carcinoma, is reminiscent of the “signet-ring” cells more commonly seen in gastric, colonic, and breast adenocarcinomas.
Figure 1.
ALK-rearranged NSCLC have distinct histologic characteristics. Representative examples of ALK-rearranged NSCLC showing the distinct solid growth pattern (A, C, E, 400x original magnification), and ≥10% signet-ring cells (B, D, F, 1000x original magnification). An ALK-rearranged adenocarcinoma with focal squamous differentiation (G, 400x), and an ALK-rearranged adenocarcinoma with the typical acinar histologic features (H, 400x).
The majority of ALK-rearranged tumors (56%) demonstrate a solid growth pattern with >10% signet-ring cells (Figure1, Table 2). In contrast only a small minority of ALK-germline tumors (3%) demonstrate a solid growth pattern with >10% signet ring cells (p<.0001). Importantly, we identified two ALK-rearranged tumors with a predominantly acinar growth pattern and no signet-ring cells. The morphology of these two cases is, to us, indistinguishable from tumors without ALK rearrangement (data not shown). Intriguingly, we also observed a single ALK-rearranged tumor with morphologic evidence for focal squamous differentiation (Figure 1G). This sample arose as a recurrence of a primary tumor originally classified as an adenocarcinoma. Unfortunately, material from the original tumor was unavailable for complete pathologic re-review.
Detection of ALK rearrangements in lung adenocarcinoma
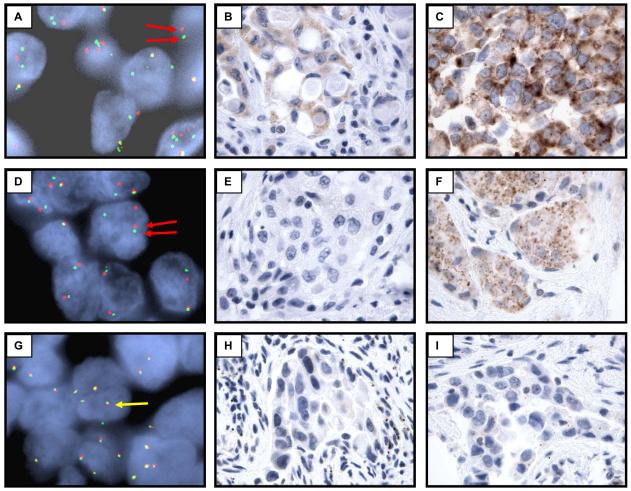
Among cases with an ALK rearrangement verified by FISH, only a subset (4 of 10 cases, 40%) showed detectable ALK protein expression by standard IHC techniques with monoclonal antibody clone ALK1 (DAKO, Figure 2, Table 3). In contrast, ALK protein was readily detectable in an ALK-rearranged anaplastic large cell lymphoma (data not shown). For the additional ALK-rearranged lung adenocarcinomas (10 cases), there was not sufficient tissue available to us for IHC analysis (missing cases are a combination of cytology and core biopsy specimens, and cases referred for FISH analysis only). In an attempt to increase the sensitivity of our IHC assay, we explored a wide variety of antibody concentrations and antigen retrieval methods (data not shown). Of the methods tested, we found that diluting the primary antibody and secondarily amplifying the signal with a tyramide-biotin (TSA) based protocol resulted in the highest sensitivity for detecting ALK protein without excessive loss of specificity (Figure 2)(26, 27). With this method we were able to detect ALK protein in 8 of 10 FISH positive cases (sensitivity = 80%; Table 3). Because of the modest sensitivity of tyramide amplified IHC for ALK-rearranged adenocarcinomas we considered FISH analysis as a more reliable diagnostic test. However, during the course of the study, we discovered one case originally classified as ALK negative by FISH, but consistently positive for ALK protein by both unamplified and TSA-based IHC (data not shown). This tumor was further examined because it demonstrated abundant signet-ring cells and a solid growth pattern characteristic of ALK-rearranged tumors. Subsequent reassessment of the FISH analysis from this patient confirmed that the case was indeed ALK-rearranged. This case demonstrates that the FISH finding for rearrangement of the ALK locus can manifest as a modest, split signal that is subtle and can be misinterpreted as normal. Thus IHC staining for ALK serves as a useful ancillary test for detecting ALK rearrangements despite a lower overall sensitivity than FISH analysis.
Figure 2.
Standard diagnostic techniques are not optimal for the routine detection of ALK-rearranged NSCLC. Representative ALK-rearranged (A-F) and ALK germline (G-I) tumors analyzed by fluorescent in-situ hybridization using probes flanking the ALK gene (A, D, G), standard immunohistochemical staining for ALK protein (B, E, H), and tyramide amplified immunohistochemical staining for ALK protein (C, F, I). Red arrows: split FISH probes characteristic of an ALK rearrangement. Yellow arrows: non-split FISH probes characteristic of ALK germline.
Discussion
The EML4-ALK fusion was recently identified as a novel molecular abnormality in 5% of lung adenocarcinomas within Asian populations (21, 22). The clinical and histopathological characteristics of lung cancers with the EML4-ALK fusion gene and the relationship with ALK protein expression have not been established in the Western population in detail (28, 32).
In this study, we assessed the prevalence of ALK rearrangement in a group of patients from three institutions and found a total of 20 ALK-rearranged adenocarcinomas. We found that the incidence of ALK-rearranged tumors in our surgically resected specimens from unselected groups of patients is 0.45%, which is lower that that reported for the Asian cohorts also consisting of surgically resected cases (28, 33). Importantly, however, the incidence of ALK-rearranged tumors in biopsy specimens from the selected cohort of patients (generally younger, with a history of never-smoking, and with high stage disease) is 14%, much higher than that of the surgical cohorts (generally older, with a smoking history, and early stage disease). Indeed, we found that young age, history of never-smoking, and high stage disease are all statistically different between patients with ALK-rearranged and ALK germline lung adenocarcinomas (Table 1). Despite this general difference, we did identify cases of older patients (oldest age 76 years) with a smoking history with ALK-rearranged tumors. Therefore the clinical characteristics alone are not sufficient to predict the genetic aberration with absolute certainty.
Given that a younger age at presentation and a lack of smoking history are characteristic of patients with tumors harboring EGFR mutations, we screened our group of ALK-rearranged tumors for coexisting EGFR. Within the group of 20 ALK-rearranged tumors we found no cases with coexisting mutations. Furthermore, no patient with an ALK-rearranged tumor treated with an EGFR inhibitor showed clinical response (data reported in Shaw et al., in press). These findings suggest that ALK rearrangements may be mutually exclusive with EGFR mutations and will require a distinct group of TKIs that specifically target ALK enzymatic activity.
One of the striking findings of this study is the unique histopathologic characteristics of the ALK-rearranged NSCLC in our cohort. The tumors in a majority of cases (56%) showed a solid pattern of growth and a significant (≥10%) component of signet-ring cells. Although this cytologic pattern is a well recognized variant of adenocarcinomas of the stomach, colon, and breast, it is reported to be only rarely observed in lung adenocarcinoma (34-37). In agreement with published findings, we found that within the same group of patients (MGH) a solid growth pattern with signet-ring cells was found in only a minority of cases (6/113 cases, 5%) of ALK germline NSCLC.
Among the cases without the unique histologic pattern, i.e. a solid growth with signet-ring cells, we did not find overt features distinguishing ALK-rearranged from non-rearranged NSCLC. These cases included lung adenocarcinomas, mixed subtype, with acinar, papillary and bronchioloalveolar patterns. Interestingly, one case demonstrated the coexistence of adenocarcinoma and, focal squamous cell carcinoma, with ALK rearrangement also present in the squamous cell component. The combined squamous and glandular morphology has been reported in rare ALK-rearranged cases (38). Despite the distinct histologic pattern found in the majority of ALK-rearranged NSCLC, our finding that ALK rearrangements can occur in adenocarcinomas with a variety of histologic patterns suggests that, in isolation, histologic characteristics alone are not sufficient for selecting individual cases for further testing for an ALK rearrangement. However, if prominent signet ring cells are observed in a lung adenocarcinoma, especially a non-smoker, ALK testing will yield a high rate of positivity.
Intriguingly, our histopathologic findings differ from those reported for a group of 11 ALK-rearranged NSCLCs from Japan, in which either acinar or papillary histologic patterns were the predominant histology (33). Whether regional or ethnic differences account for this discrepancy, whether it is attributed to the difference in clinical presentation (early stages vs. stage IV), or whether it is a reflection of the very small sample size (11 cases) in this prior report compared with ours remains to be determined.
With the emergence of molecularly-targeted therapies, it is reasonable to assume that many, and perhaps all, NSCLCs will be screened for ALK rearrangements. Given the tremendous number of cases of NSCLC and the relative rarity of this genetic alteration, any screening procedure will need to be highly sensitive, specific, reproducible, and cost effective. Our experience suggests the need for more effective diagnostic procedures than those that currently exist. We screened all of our cases for an ALK rearrangement by FISH, using a commercially available set of probes. However given that the ALK rearrangements in lung cancer involve target loci relatively close to one another and on the same chromosome, interpretation of a positive rearrangement is more difficult than in other ALK-rearranged tumors, such as anaplastic large cell lymphoma (ALCL) or inflammatory myofibroblastic tumor (IMT), where the target loci are on different chromosomes. One possible solution is to add an additional fluorescent probe targeting the deleted portion of chromosome 2 in ALK-rearranged NSCLC. This type of 3 probe combination has been successfully developed for detecting the TMPRSS2-ERG rearrangement in prostate cancer (39, 40).
For the majority of cases, we also screened for ALK rearrangement by IHC. Using a commonly used commercially available antibody for ALK and standard techniques, we found IHC to be specific, but not sensitive for detecting ALK rearrangements among FISH-confirmed, ALK-rearranged cases (considered as the “gold-standard” for this analysis). Furthermore, the level of ALK protein expression was significantly lower in the ALK-rearranged NSCLC cases than in the ALK-rearranged ALCLs that we used as positive controls. In attempts to improve detection of ALK, we found that tyramide signal amplification (TSA), a technique developed several years ago, was sufficient to increase the sensitivity without decreasing specificity for detecting ALK (27). This modified method could improve the detection of ALK protein from 40% to 80% among FISH-confirmed, ALK-rearranged cases tested, but the sensitivity of 80% may not be sufficient to justify the ALK IHC to be a sole modality for detecting ALK rearrangements. Additional antibodies recognizing ALK are commercially available, and although we have not tested all of them, it has been reported that none are sufficient to detect ALK in all cases of ALK-rearranged adenocarcinomas (38, 41). It is notable that the ALK antibody used in this study is the standard reagent used by pathology labs worldwide and one used in a prior study of a cohort of Japanese patients with lung adenocarcinoma (33). Although our results may suggest that FISH testing is the preferred method of screeningand considered to be the “gold standard” in our evaluation of IHC, we encountered one case that was originally interpreted as non-ALK-rearranged by FISH, but the subsequent FISH analysis prompted by concurrent, positive ALK protein expression by IHC and the characteristic tumor morphology revealed ALK rearrangement. Therefore, given the current limitations of both FISH and IHC we believe that both methods should be employed to facilitate the detection of ALK-rearranged lung adenocarcinomas. Given that IHC is a routine methodology in most pathology laboratories around the world, better antibodies recognizing ALK are likely needed to facilitate the large-scale screening of NSCLC in the future. An alternative and complementary approach in screening NSCLC is through a multiplexed RT-PCR-based analysis to detect the various ALK-fusion transcripts. This approach is currently under investigation in our lab as well as others (38). It is be important to determine whether RT-PCR based screening can detect ALK-rearranged tumors missed by both IHC and FISH.
Since their discovery in ALCL, ALK rearrangements have been identified in inflammatory myofibroblastic tumors, a subset of diffuse large B cell lymphomas (42-45), and a subset of neuroblastomas (46, 47). These findings have prompted the development of inhibitors of ALK enzymatic activity for therapeutic use (48). With the discovery of ALK rearrangements in NSLCL (21), the number of potential patients who might benefit from such drugs has increased dramatically. Importantly our findings indicate that ALK-rearranged NSCLC comprise a unique subgroup of adenocarcinoma with distinct clinicopathologic characteristics. Compared with non-ALK-rearranged NSCLC, this group is significantly enriched for young, non-smoking patients with tumors that show distinct solid growth pattern and signet-ring cell histology. These patients typically present in late stages not amenable to surgical resection and are therefore candidates for aggressive and novel chemotherapeutic regimens that target the mutated ALK protein. Thus, it is important to raise a suspicion about the possibility of ALK rearrangements based on the unique clinicopathologic characteristics and identify ALK-rearranged tumors by dual testing with IHC and FISH.
Statement of Translational Relevance.
With the recent discovery that a subset of lung adenocarcinomas derived from the Asian populations harbor ALK gene rearrangements, we sought the characteristics of tumors with this genetic abnormality within a large cohort of Western patients from three institutions. We show that lung adenocarcinomas with ALK rearrangements are very rare among patients amenable to surgical resection (prevalence 0.45%), but significantly higher among young non-smoking patients with advanced clinical stage (prevalence 15%). The majority of the ALK-rearranged lung adenocarcinomas we identified have unique histopathologic features characterized by solid growth and signet-ring cell morphology. Detection of ALK rearrangements in lung adenocarcinomas by either fluorescent in-situ hybridization or immunohistochemical assays poses technical and interpretive challenges and suggests dual testing to ensure accurate diagnosis. These findings show that ALK-rearranged lung adenocarcinomas are rare and have distinct clinicopathologic characteristics that can provide guidance to health care professionals for considering the optimal treatment options.
Supplementary Material
Acknowledgments
This work was supported by Dana-Farber/Harvard Cancer Center Specialized Programs of Research Excellence (SPORE) in Lung Cancer 2P50 CA090578-06. We are indebted to Ms. Brittany Macfarland for secretarial support.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Bepler G, Bueno R, et al. Non-small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:548–82. doi: 10.6004/jnccn.2006.0046. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL, Sobin LH. The TNM system: our language for cancer care. J Surg Oncol. 2002;80:119–20. doi: 10.1002/jso.10114. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC. Lumping, splitting, and sorting. J Thorac Oncol. 2007;2:581–2. doi: 10.1097/JTO.0b013e31807a2fae. [DOI] [PubMed] [Google Scholar]
- 7.Travis W. Pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC Press; Lyon: 2004. [Google Scholar]
- 8.Barletta JA, Perner S, Iafrate AJ, et al. Clinical Significance of TTF-1 Protein Expression and TTF-1 Gene Amplification in Lung Adenocarcinoma. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810–27. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 10.Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9:320–6. doi: 10.2353/jmoldx.2007.060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haneda H, Sasaki H, Shimizu S, et al. Epidermal growth factor receptor gene mutation defines distinct subsets among small adenocarcinomas of the lung. Lung Cancer. 2006;52:47–52. doi: 10.1016/j.lungcan.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh RK, Lim KH, Kuo HT, Tzen CY, Huang MJ. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest. 2005;128:317–21. doi: 10.1378/chest.128.1.317. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–9. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Iwakawa R, Kohno T, et al. Frequent EGFR mutations in noninvasive bronchioloalveolar carcinoma. Int J Cancer. 2006;118:2498–504. doi: 10.1002/ijc.21670. [DOI] [PubMed] [Google Scholar]
- 15.Jackman DM, Chirieac LR, Janne PA. Bronchioloalveolar carcinoma: a review of the epidemiology, pathology, and treatment. Semin Respir Crit Care Med. 2005;26:342–52. doi: 10.1055/s-2005-871993. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–52. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Sakao Y, Miyamoto H, Sakuraba M, et al. Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: the impact of nonbronchioloalveolar carcinoma components. Ann Thorac Surg. 2007;83:209–14. doi: 10.1016/j.athoracsur.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Lamant L, Meggetto F, al Saati T, et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin’s disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996;87:284–91. [PubMed] [Google Scholar]
- 19.Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–4. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow SH, International Agency for Research on Cancer. World Health Organization . WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. International Agency for Research on Cancer; Lyon, France: 2008. [Google Scholar]
- 21.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 22.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105:19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester S. Manual of surgical pathology. 2 ed. Elsevier Churchill Livingstone; Edinburgh; New York: 2006. [Google Scholar]
- 26.Sabattini E, Bisgaard K, Ascani S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–11. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–62. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 28.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009 doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 29.Shaw A, Yeap B, Mino-Kenudson M, et al. Clinical Features and Outcome of Patients with Non-Small Cell Lung Cancer Harboring EML4-ALK. 2009 doi: 10.1200/JCO.2009.22.6993. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 31.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 32.Perner S, Wagner PL, Demichelis F, et al. EML4-ALK fusion lung cancer: a rare acquired event. Neoplasia. 2008;10:298–302. doi: 10.1593/neo.07878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009 doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 34.Sarma DP, Hoffmann EO. Primary signet-ring cell carcinoma of the lung. Hum Pathol. 1990;21:459–60. doi: 10.1016/0046-8177(90)90214-p. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi H, Kitamura H, Nakatani Y, Inayama Y, Ito T. Primary signet-ring cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol. 1999;30:378–83. doi: 10.1016/s0046-8177(99)90111-9. [DOI] [PubMed] [Google Scholar]
- 36.Merchant SH, Amin MB, Tamboli P, et al. Primary signet-ring cell carcinoma of lung: immunohistochemical study and comparison with non-pulmonary signet-ring cell carcinomas. Am J Surg Pathol. 2001;25:1515–9. doi: 10.1097/00000478-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Castro CY, Moran CA, Flieder DG, Suster S. Primary signet ring cell adenocarcinomas of the lung: a clinicopathological study of 15 cases. Histopathology. 2001;39:397–401. doi: 10.1046/j.1365-2559.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 38.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3:13–7. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 39.Perner S, Demichelis F, Beroukhim R, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–41. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- 40.Demichelis F, Setlur SR, Beroukhim R, et al. Distinct genomic aberrations associated with ERG rearranged prostate cancer. Genes Chromosomes Cancer. 2009;48:366–80. doi: 10.1002/gcc.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinmura K, Kageyama S, Tao H, et al. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer. 2008;61:163–9. doi: 10.1016/j.lungcan.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–80. [PubMed] [Google Scholar]
- 43.Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157:377–84. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gascoyne RD, Lamant L, Martin-Subero JI, et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102:2568–73. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 45.Lamant L, Pulford K, Bischof D, et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–21. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–8. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–4. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 48.Li R, Morris SW. Development of anaplastic lymphoma kinase (ALK) small-molecule inhibitors for cancer therapy. Med Res Rev. 2008;28:372–412. doi: 10.1002/med.20109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.