Abstract
Heparanase is an endo–β–D-glucuronidase that cleaves heparan sulfate (HS) saccharide chains. The enzyme promotes cell adhesion, migration and invasion and plays a significant role in cancer metastasis, angiogenesis and inflammation. The present study focuses on the involvement of heparanase in autoimmunity, applying the murine experimental autoimmune encephalitis (EAE) model, a T cell dependent disease often used to investigate the pathophysiology of multiple sclerosis (MS). Intraperitoneal administration of recombinant heparanase ameliorated, in a dose dependent manner, the clinical signs of the disease. In vitro and in vivo studies revealed that heparanase inhibited mitogen induced splenocyte proliferation and mixed lymophocyte reaction (MLR) through modulation of their repertoire of cytokines indicated by a marked increase in the levels of IL-4, IL-6 and IL-10, and a parallel decrease in IL-12 and TNF-α. Similar results were obtained with active, latent, or point mutated inactive heparanase, indicating that the observed inhibitory effect is attributed to a non-enzymatic activity of the heparanase protein. We suggest that heparanase induces upregulation of Th2 cytokines, resulting in inhibition of the inflammatory lesion of EAE.
Keywords: Heparanase, experimental autoimmune encephalitis, inflammation, cytokines
1. Introduction
Heparan sulfate proteoglycans (HSPGs) are ubiquitous macromolecules associated with the cell surface and extracellular matrix (ECM) of a wide range of cells (Bernfield et al., 1999; Esko and Lindahl, 2001; Iozzo, 1998). The basic HSPG structure consists of a protein core to which several linear heparan sulfate (HS) chains are covalently O-linked (Bernfield et al., 1999; Esko and Lindahl, 2001; Iozzo, 1998). HS chains, unique in their ability to bind a multitude of proteins, ensure that a wide variety of bioactive molecules bind to the cell surface and ECM and thereby function in the control of diverse normal and pathological processes (Bernfield et al., 1999; Esko and Lindahl, 2001; Iozzo, 1998). The majority of studies on cell interaction with the microenvironment focused, among other approaches, on proteolytic enzymes (Liotta and Kohn, 2001). The involvement of glycosaminoglycan (e.g., heparan sulfate) degrading enzymes (e.g., heparanase) was underestimated, primarily due to a lack of appropriate molecular probes to explore their causative role in cell-ECM interactions and related effects. A long-term research on the biology of the heparanase enzyme led to the cloning of a single gene encoding a HS-degrading endoglycosidase (heparanase) (Hulett et al., 1999; Toyoshima and Nakajima, 1999; Vlodavsky et al., 1999) which plays important roles in cancer metastasis, angiogenesis and inflammation (Edovitsky et al., 2004; Ilan et al., 2006; McKenzie, 2007; Parish et al., 2001; Sanderson et al., 2004; Vreys and David, 2007). Heparanase is synthesized as a 65 kDa non-active precursor that subsequently undergoes proteolytic cleavage, primarily by cathepsin L (Abboud-Jarrous et al., 2008), yielding 8 kDa and 50 kDa protein subunits that heterodimerize to form an active enzyme (Levy-Adam et al., 2003; McKenzie et al., 2003). The enzyme has been identified in invasive normal and malignant cells, including activated cells of the immune system (T-cells, dendritic cells, monocytes, mast cells), cytotrophoblasts, keratinocytes, lymphoma, melanoma and carcinoma cells (Benhamron et al., 2006; Bernfield et al., 1999; Bitan et al., 2002; Ilan et al., 2006; Vlodavsky et al., 1992).
Extravasation of circulating hematopoietic and immune cells is accompanied by degradation of various components of the subendothelial ECM. Activated immune cells produce and secrete a variety of ECM degrading enzymes, including heparanase (Benhamron et al., 2006; Naparstek et al., 1984; Vlodavsky et al., 1992). Degradation of HS disintegrates the supramolecular structure of the subendothelial basal lamina, consequently facilitating trans-endothelial migration of neutrophils and activated lymphocytes, thereby mediating their extravasation during immune responses (Bartlett et al., 1995; Vlodavsky et al., 1992). Apart of the well studied catalytic feature of the enzyme, heparanase was noted to exert biological functions (i.e., Akt, Src and EGF-receptor phosphorylation), apparently independent of its enzymatic activity (Cohen-Kaplan et al., 2008; Fux, 2009a; Fux et al., 2009b; Ilan et al., 2006).
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system (CNS) characterized by the presence of patchy inflammatory infiltrates, containing autoreactive T cells that, in turn, lead to demyelination and axonal loss (Hemmer et al., 2002). It is thought that T cells specific for myelin components are polarized towards a Th1-like phenotype, target the pathologic process to the CNS and cause recruitment into the organ of antigen-nonspecific T cells, B cells and monocytes, which act as effector cells by releasing myelinotoxic mediators (Kieseier et al., 1999; Martino and Hartung, 1999).
Murine experimental autoimmune encephalitis (EAE) is a T cell dependent disease commonly used as a model to investigate the pathophysiology of MS. In this model, antigen-specific CD4+ Th1 cells mediate inflammatory damage in the CNS, resulting in demyelination manifested clinically by progressive paralysis (Begolka et al., 1998). On the other hand, increased production of Th2-type cytokines and a parallel suppression of Th1-type cytokines, leads to amelioration of EAE (Moss et al., 2004). The present study was undertaken to elucidate the potential involvement of exogenously administered heparanase in the pathogenesis of EAE. We observed a profound inhibitory effect of heparanase on EAE progression in vivo. This effect was attributed to suppression of T cell activation, increased production of Th2-type cytokines and a parallel decrease in IL-12 and TNF-α.
2. Methods
2.1 Animals
Six week old SJL/J female mice weighing 20 g were obtained from Harlan Ltd (Rehovot, Israel). The animals were fed Purina chow and acidified water ad libitum. The mice were housed under SPF conditions with a 12 h cycling of light. All procedures were conducted using facilities and protocols approved by the Animal Care and Use Committee of the Hadassah-Hebrew University School of Medicine (NIH approval # OPRR-AO1-5011).
2.2 Immunization schedule
Animals were immunized subcutaneously on day 0 with a 1:1 emulsion comprised of 200 μg proteolipid protein (PLP) (aa 139–151 peptide) in phosphate buffered saline (PBS), pH 7.4, and complete Freund’s adjuvant (CFA) containing 200 μg of Mycobacterium H37R (BD Biosciences Clontech, Palo Alto, CA) in a final volume of 100 μl. On the same day and on day +2, pertusis toxin (Sigma Chemicals, St. Louis, MO) was administered (250 ng/mouse) intraperitonealy (i.p.) in a volume of 200 μl saline (Chitnis et al., 2001).
2.3 Clinical evaluation of EAE
The animals were monitored daily, starting on day +7. In untreated mice, the first signs of EAE appeared on day +9 to +10 post immunization. These were scored as previously described, applying a 6 point scale: 0-normal behavior, 1-tale low tonus, 2-hind legs weakness, 3-hind legs paralysis, 4-full paralysis, 5-death (Chitnis et al., 2001).
2.4 Heparanase
Active recombinant human heparanase was produced in insect cells and purified as described (McKenzie et al., 2003). The construct encoding the 8 and 50 kDa heparanase subunits was kindly provided by Dr. E. McKenzie (Faculty of Life Sciences, University of Manchester, UK). Recombinant mouse heparanase was kindly provided by Dr. H-Q Miao (ImClone Systems Inc. New York, N.Y) (Miao et al., 2002). Recombinant 65 kDa latent human heparanase and inactive heparanase mutated in glutamic acid residues 225 and 343 that comprise the enzyme active site (Hulett et al., 2000) were purified from the culture medium of transfected HEK-293 cells, essentially as described (Zetser et al., 2004).
2.5 Modified heparin
Compound ST1514 was kindly provided by Dr. Claudio Pisano (Sigma-Tau, Research Department, Pomezia, Rome, Italy). Briefly, heparin was subjected to controlled alkali-catalyzed removal of sulfate groups of iduronic acid 2-O-sulfate residues, giving rise to the corresponding epoxide derivative. The epoxide rings were opened, followed by oxidative glycol-splitting of the newly formed (and the preexisting) nonsulfated uronic acid residues (Casu et al., 2002; Naggi et al., 2005; Vlodavsky et al., 2007). The ST1514 compound is 50% glycol-split modified heparin (H50gs; MW 11,200) (Casu et al., 2002; Naggi et al., 2005; Vlodavsky et al., 2007).
2.6 Treatment
Each of the heparanase preparations was diluted in saline to the desired final concentration (active heparanase: 25 μg/kg/day; latent heparanase: 75 μg/kg/day) in the injectable volume of 200 μl. Injections were initiated on the day of immunization with PLP, followed by a daily (except when stated otherwise) injection (i.p) of heparanase until day +7 or +17. Signs of neurological deficits were observed in the control group starting on day +9. The peak of the disease was noted on days +10 to +12, and the symptoms declined 3–4 days afterwards. Thus, injections of heparanase covered the entire period of clinical signs.
2.7 Histology
The entire spinal cords from control and heparanase treated mice were fixed in 4% formaldehyde. Serial sections of the specimens were transferred to plastic cassettes and paraffinised using an automatic device (Tissue-Tek VIP, SAKURA). Five micrometer sections were cut using semi-automated microtome (Jung Autocut 2055, Leica, Germany) and attached to SuperFrost-Plus Slides. The slides were stained with hematoxilin and eosin (H&E) using automatic devices (Leica autostainer XL and Leica slide coverer CV5000, Leica, Germany) (Friedmann et al., 2000). Stained slides were screened with a light microscope (Axioplan2, Zeiss, Germany). Tissue samples were fixed with 4% formaldehyde in PBS, embedded in paraffin and sectioned (5 μm). Following deparaffinization and rehydration, sections were washed (3x) with PBS and stained with hematoxyline/eosine, as described (Friedmann et al., 2000). Tissue sections were then washed, mounted with 90% glycerol in PBS and visualized with a Zeiss axioscope microscope
2.8 T-cell activation
a) Concavalin A (ConA)
Mouse spleen cells were cultured in RPMI-1640 medium, supplemented with 10% heat-inactivated human AB serum in flat-bottomed 96-well microtiter plates (Nunc, Wiesbaden, Germany) containing 0.5 × 106 cells/well/0.2 ml. Responses to 2 μg/ml concanvalin-A (Sigma, St. Louis, MO) were assessed by 3H-thymidine incorporation, as described (Slavin et al., 1977).
b) Mixed lymphocyte culture (MLC)
Splenocytes from BALB/c and C57BL/6 mice were activated against each other in one way mixed lymphocyte culture (MLC), in the absence or presence of the 65 kDa proheparanase. Briefly, mouse spleen cells were cultured in RPMI-1640 medium, supplemented with 10% heat-inactivated human AB serum in 96-well flat-bottomed microtiter plates (Nunc, Wiesbaden, Germany) containing 1 × 106 responding cells and 1 × 106 irradiated (3000 cGy) stimulating cells per 0.2 ml per well. Cells were cultured for 5 days in 5% CO2 in air, in a humidified incubator. Twenty h before harvesting, 1 μCi 3H-thymidine was added to each well and thymidine incorporation was measured, as described (Slavin et al., 1977).
2.9 Cytokine analysis
a) In vivo assay
SJL/J mice were treated with saline or recombinant heparanase as described above. Spleen lymphocytes were then harvested and subjected to ConA activation for 24, 48 or 72 h, as described above. The culture medium was taken for ELISA analysis of IL-4, IL-6, IL-10, IL-12 and TNF-α, as described (Weiss et al., 2002).
b) In vitro assay
Spleen lymphocytes (2.5–5.0 × 106 cells/ml) cultured in 75 ml flasks were stimulated for 24 h with rIL-2 (6000 IU/ml) in the absence and presence of heparanase in RPMI 1640 medium supplemented with 10 % FCS. The culture supernatant was then subjected to ELISA analysis of IL-4, IL-6, IL-10, IL-12 and TNF-α, as described (Weiss et al., 2002).
2.10 Statistics
Student’s t test was used for statistical analysis of the results.
3. Results
3.1 ffect of heparanase on EAE progression
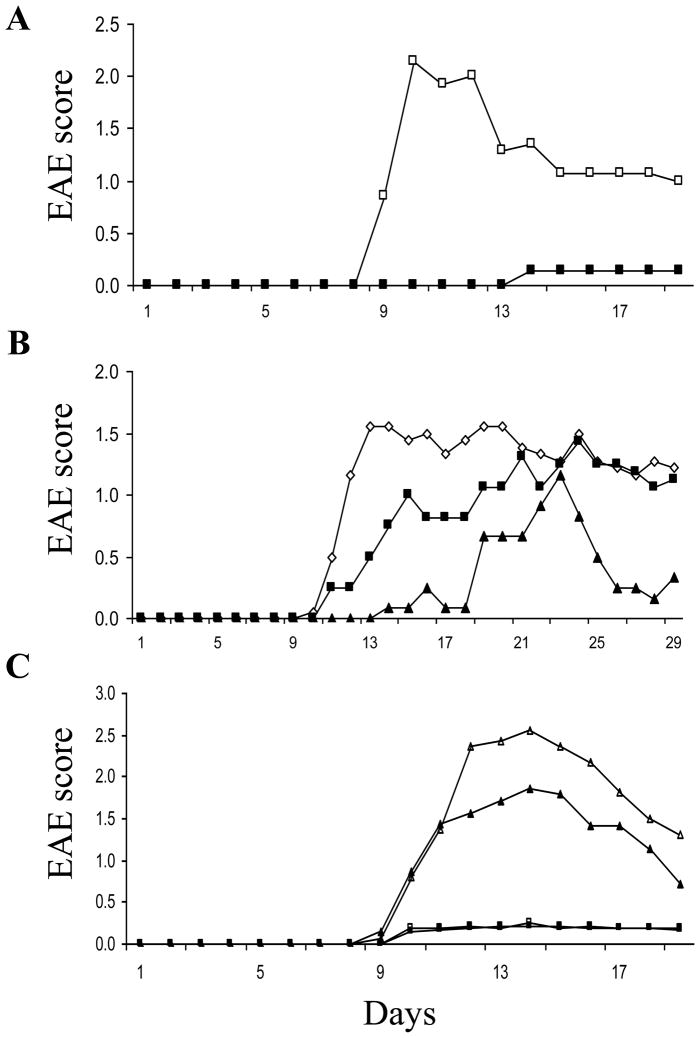
SJL/J mice were treated with PLP, CFA containing mycobacterium, and pertusis toxin to induce EAE, as described (Chitnis et al., 2001). Starting on the day of immunization, the mice were subjected to a daily injection (i.p) of active (8 + 50 kDa) recombinant heparanase (25 μg/kg/day) or saline alone. In control mice, signs of disease were first noted 9 days post immunization, reaching a maximal level on days 10–12 and declining thereafter. In contrast, an almost complete ablation of the disease symptoms was evident in the heparanase treated mice. A representative experiment showing the mean daily score of EAE severity is presented in figure 1A. Treating the mice with the latent 65 kDa recombinant human heparanase (75 μg/kg/day) yielded a significant, albeit less pronounced inhibitory effect (not shown). In a subsequent experiment, the experimental group was treated with active heparanase (25 μg/kg/day) for only 7 consecutive days, starting on the day of immunization and maintained untreated thereafter. As demonstrated in figure 1B, the inhibitory effect persisted for additional 9 days when symptoms of the disease were starting to emerge. Moreover, the heparanase inhibitory effect was dose dependent as can be seen by the intermediate inhibition of EAE in mice that were treated with 12.5 vs. 25 μg/kg active heparanase per day (Fig. 1B). Similar results were obtained regardless of whether human (Fig. 1) or mouse (not shown) recombinant active (8 +50 kDa) heparanase were applied.
Figure 1. Effect of heparanase on clinical signs of EAE. A. Continous treatment.
Female, SJL/J mice (n = 8 mice per group) were sensitized subcutaneously on day 0 and injected with pertussis on day 0 and +2. Active (50+8) heparanase was administered (i.p., 25 μg/kg/day) starting on day 0 (■). On day +9, clinical signs of encephalitis were first observed in the control group receiving saline alone (□), reaching a maximal score on day 10–12. In contrast, there were no signs of encephalitis in the heparanase treated mice. B. Treatment for 7 days only. Mice were immunized as described in A. Heparanase was administered daily from day 0 to day +7. Mice were divided to 3 groups (n = 8 mice per group): control group receiving saline alone (◇); mice receiving low dose (12.5 μg/kg/day) (■); or high dose (25 μg/kg/day) (▲) of active (50+8) heparanase. A dose dependent suppression of EAE was noted. Each data point represents the mean score of 8 mice. C. Heparanase inhibitor. Mice were immunized as described in A. Active (50+8) heparanase was administered (i.p., 25 μg/kg/day) starting on day 0. Mice were divided to 4 groups (n = 8 mice per group): control group receiving saline alone (△); mice receiving a daily i.p injection of compound ST1514 (100 μg/mice/day) alone (▲); mice receiving heparanase alone (i.p., 25 μg/kg/day) (■); and mice receiving a daily i.p injection of both compound ST1514 (100 μg/mice/day) and active (50+8) heparanase (i.p., 25 μg/kg/day) (□). Complete ablation of EAE was noted in mice treated with heparanase with or without compound ST1514. A partial inhibition of EAE was noted in mice receiving the inhibitor alone. Each data point represents the mean score of 8 mice.
In order to evaluate whether heparanase inhibits the EAE inflammatory process through its enzymatic activity, mice were treated with both active heparanase (25 μg/kg/day) and a potent inhibitor of its activity. For this purpose, we applied a 50% glycol split modified, non-anticoagulant heparin (ST1514) (Casu et al., 2002) shown to efficiently inhibit the heparanase enzyme in vitro (50% inhibition at 0.5 μg/ml) (Naggi et al., 2005; Vlodavsky et al., 2007). Daily i.p injection of compound ST1514 (100 μg/mouse/day) in parallel with the heparanase enzyme did not alter the inhibitory effect of heparanase on EAE (Fig. 1C). This result suggests that the protective effect of heparanase may involve, at least in part, non-enzymatic activities of the heparanase enzyme such as induction of cell adhesion (Fux et al., 2009b; Gilat et al., 1995; Goldshmidt et al., 2003; Ilan et al., 2006; Sotnikov et al., 2004) and/or cell survival associated with stimulation of the Akt/PI3K pathway (Gingis-Velitski et al., 2004; Ilan et al., 2006).
3.2 Spinal cord histology and morphology
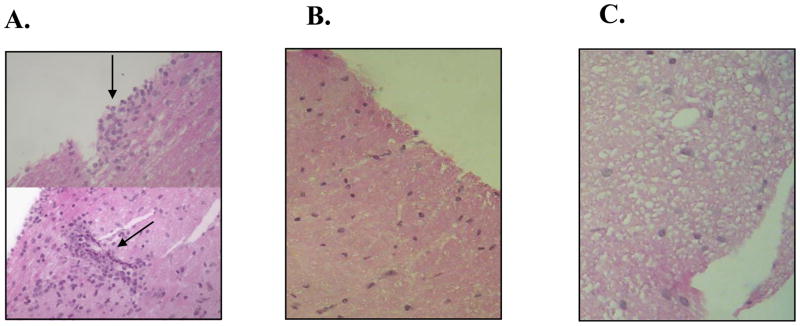
Spinal cord tissue sections taken from heparanase-treated and untreated SJL/J mice were processed for histological evaluation in order to detect changes associated with clinical signs of EAE. Hematoxylin/eosin staining of tissue sections from untreated mice revealed a marked infiltration of inflammatory cells in the white matter (Fig 2a black arrow) compared with no detectable infiltration in the spinal cord tissue of heparanase-treated mice (Fig. 2b). Moreover, a readily detectable difference was noted in spinal cord tissue derived from heparanase-treated vs. untreated mice. Whereas spaces of missing tissue (i.e., spongy appearance) were clearly seen in the white matter derived from untreated mice (Fig. 2c), sections taken from heparanase-treated mice appeared normal and were densely packed with cells and white matter (Fig. 2b). The nearly normal appearance reflects the protective effect of heparanase against inflammatory damage to the myelin sheaths of the neuronal axons residing in the white matter and shown to be affected in MS and EAE (Kieseier et al., 1999).
Figure 2. Histological examination of spinal cord tissue derived from control vs. heparanase treated mice.
Paraffin embedded sections of spinal cord specimens were taken from control and heparanase treated mice, 10 days after the initial induction of EAE. The sections were stained with hematoxylin/eosin and subjected to histological evaluation. Cellular infiltration. Spinal cord tissue taken from control mice appeared spongy and degenerated (C) with clusters of inflammatory cells in the white matter zone (A, arrows) as compared to dense, health appearance and little or no cellular infiltration in tissue samples from heparanase treated mice (B).
3.3 Effect of heparanase on T-cell activation in vitro
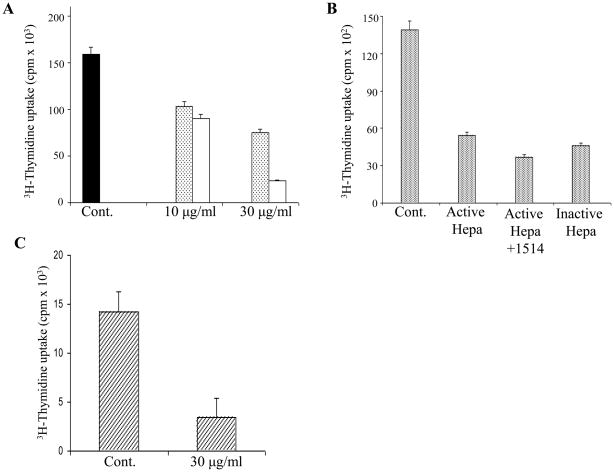
Autoimmune diseases are characterized by an attack of host tissues and organs by host T-cells. In the case of EAE, CD4+ cells are being polarized toward the Th1 phenotype and thereby mediate the inflammatory process through which demyelination occurs (Moss et al., 2004). To elucidate the involvement of heparanase in this process, we investigated its effect on activation of splenocytes. For this purpose, mouse spleen cells were cultured (12 h, 37°C) without or with increasing amounts of either the active (8+50 kDa) or latent (65 kDa) heparanase forms, and subjected to ConA induced cell proliferation. As demonstrated in figure 3A, addition of heparanase to the culture medium resulted in a significant, dose dependent decrease in ConA induced proliferation (i.e. thymidine incorporation) of the splenocytes. Active (8+50 kDa) heparanase was more potent than the latent 65 kDa pro-enzyme (Fig. 3A). A similar decrease in ConA activation was obtained in the absence or presence of the heparanase inhibitor ST1514 (Fig. 3B), suggesting that heparanase enzymatic activity is not required for the observed anti-proliferative effect. In order to substantiate this observation, we utilized heparanase in which glutamic acid residues 225 and 343 that comprise the enzyme active site (Hulett et al., 2000) were point mutated, yielding an inactive enzyme (Cohen-Kaplan et al., 2008; Goldshmidt et al., 2003). As shown in figure 3B, the inactive enzyme inhibited the ConA induced cell proliferation to an extent comparable in magnitude to that of active (8 + 50 kDa) heparanase. In a subsequent experiment, spleen cells were taken from BALB/c and C57BL/6 mice. The cells were then activated against each other in one way mixed lymphocyte culture (MLC), in the absence or presence of 30 μg/ml of the 65 kDa proheparanase. As shown in figure 3C, heparanase markedly inhibited (~ 4 fold) the activation of BALB/c derived spleen cells reacting against splenic lymphocytes obtained from C57BL/6 mice. Similar results were obtained using 5 μg/ml of the active (8+50 kDa) heparanase enzyme (not shown).
Figure 3. Effect of heparanase on activation of splenocytes. A, B. ConA activation.
A. Mouse spleen cells were isolated and subjected to activation with ConA in the absence (Cont.) and presence of 10 and 30 μg/ml recombinant latent ( ) and active (□) heparanase, followed by measurements of 3H-thymidine incorporation, as described in “Materials and Methods”. B. The splenocytes were also subjected to ConA activation in the presence of active heparanase, active heparanase plus glycol split heparin (100 μg/ml, compound ST1514), or inactive heparanase (point mutated in glutamic residues 325 and 343). Thymidine incorporation was inhibited in response to active, latent and inactive heparanase. There was no effect to compound ST1514 alone. C. Mixed lymphocyte culture (MLC). Mixed lymphocyte culture (MLC) reaction was performed in the absence (Cont.) or presence of 30 μg/ml 65 kDa heparanase, as described in “Materials & Methods”. A marked reduction in activation (3H-thymidine incorporation) of BALB/c-derived lymphocytes against C57BL/6-derived lymphocytes was noted in the heparanase treated culture. Each data point is the mean ± SD of triplicate wells.
) and active (□) heparanase, followed by measurements of 3H-thymidine incorporation, as described in “Materials and Methods”. B. The splenocytes were also subjected to ConA activation in the presence of active heparanase, active heparanase plus glycol split heparin (100 μg/ml, compound ST1514), or inactive heparanase (point mutated in glutamic residues 325 and 343). Thymidine incorporation was inhibited in response to active, latent and inactive heparanase. There was no effect to compound ST1514 alone. C. Mixed lymphocyte culture (MLC). Mixed lymphocyte culture (MLC) reaction was performed in the absence (Cont.) or presence of 30 μg/ml 65 kDa heparanase, as described in “Materials & Methods”. A marked reduction in activation (3H-thymidine incorporation) of BALB/c-derived lymphocytes against C57BL/6-derived lymphocytes was noted in the heparanase treated culture. Each data point is the mean ± SD of triplicate wells.
3.4 Effect of heparanase on cytokine production
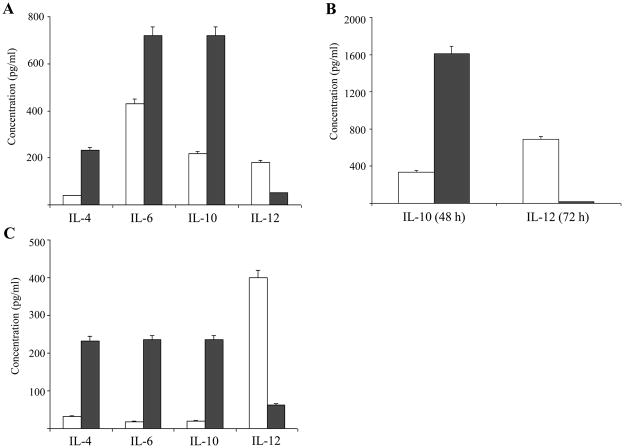
CD4+ T-cells with polarization towards the Th1 phenotype exhibit accelerated autoimmune activity against components of the neuronal system, causing the symptoms observed in the EAE model (Begolka et al., 1998; Moss et al., 2004). Increasing the level of cytokines characteristic of Th2 cells and a parallel decrease in the amount of Th1-type cytokines, ameliorate the signs and symptoms of the disease (Begolka et al., 1998; Moss et al., 2004). IL-12, known to drive differentiation of uncommitted T-cells towards a Th1 phenotype, was demonstrated to aggravate the course of adoptively transferred EAE (Gran et al., 2004). Conversely, IL-4, IL-6 and IL-10 are known to be produced by Th2 cells (Chitnis and Khoury, 2003). SJL/J mice were subjected to a daily injection of active (8 + 50 kDa) heparanase (7 days, 25 μg/kg/day). Splenocytes were then harvested, activated with ConA (24 h, 37°C, RPMI + 10% FCS) and aliquots of the culture medium were subjected to ELISA analysis of IL-4, IL-6, IL-10 and IL-12. As shown in figure 4A, the amounts of secreted Th2 cytokines such as IL-4, IL-6 and IL-10, were increased 6.0, 1.67, and 3.32 folds, respectively, following exposure to heparanase in vivo. In contrast, under the same conditions, there was a marked decrease (3.57 fold) in the level of IL-12, representing a Th1-associated and pro-EAE cytokine (Fig. 4A). The effect was more pronounced following an extended incubation time (48–72 h) of the harvested splenocytes with ConA, as demonstrated by a 4.77 fold increase in the amount of IL-10 and an almost complete abolishment of IL-12 secretion (34.3 folds decrease) (Fig. 4B).
Figure 4. Effect of heparanase on the level of Th1 and Th2-type cytokines secreted by activated T-cells.
A. In vivo. SJL/J mice were subjected to daily injections of heparanase (■) or saline (□) injections. Spleen lymphocytes were then isolated, activated with ConA for 24 h and aliquots of the culture medium were analyzed by ELISA for the amounts of IL-4, IL-6, IL-10 and IL-12. A marked increase (1.67–6 folds) in the levels of Th2 cytokines (IL-4, IL-6 and IL-10) was noted in cells derived from heparanase treated mice. In contrast, a marked decrease (3.57 folds) in the level of IL-12 (Th1 cytokine) was observed in those cells derived from heparanase treated mice, as compared to cells obtained from control, saline injected mice. B. Time course. SJL/J mice were subjected to daily injections of heparanase (■) or saline (□). Splenocytes were then isolated, activated with ConA for more than 24h and aliquots of the culture medium were analyzed for the amounts of IL-10 (48h) and IL-12 (72h). Levels of IL-10 were increased by 4.77 fold and the levels of IL-12 were decreased by 34.3 fold, as compared to the levels of those cytokines taken from control, saline injected mice.
C. In vitro. Spleen lymphocytes were isolated from SJL/J mice and incubated (24 h, 37°C) with rIL-2 (6000 IU/ml) in the absence (■) or presence (□) of 30 μg/ml 65 kDa heparanase. Aliquots of the incubation medium were analyzed by ELISA for the amounts of IL-4, IL-6, IL-10 and IL-12. A marked increase (7.35–11.75 folds) in the levels of IL-4, IL-6 and IL-10 and a 6.34 fold decrease in the amount of IL-12 were noted when heparanase was present in the incubation medium.
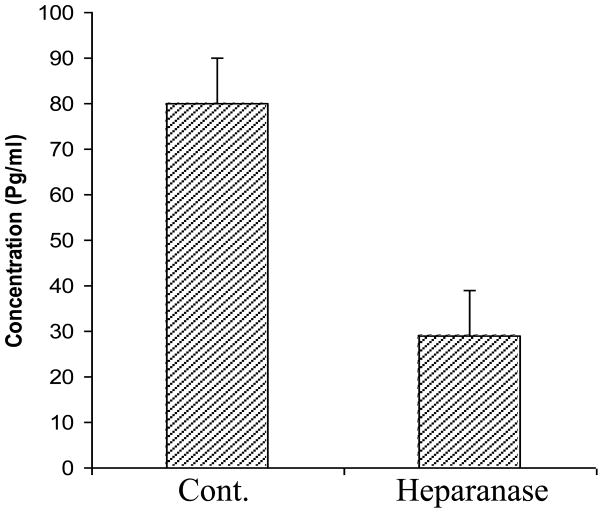
In subsequent experiments, SJL/J derived spleen lymphocytes were isolated and incubated (24 h, 37°C) in vitro with IL-2, with or without the latent 65 kDa heparanase (30 μg/ml). Aliquots of the culture medium were subjected to ELISA analysis of IL-4, IL-6, IL-10 and IL-12. As demonstrated in figure 4C, the amounts of secreted Th2 cytokines (IL-4, IL-6 and IL-10) were increased 7.35, 13.05, and 11.75 folds, respectively, while the level of secreted IL-12 was markedly decreased (6.34 folds) following treatment of the mice with heparanase, indicating that the observed shift in cytokine profile toward a Th2 phenotype is independent of the stimulatory agent. Similar results were obtained with active heparanase (5 μg/ml) (not shown). The main function of TNF-α is to stimulate inflammation by turning on gene transcription through the IKK/NFkappaB and JNK/AP-1 signaling cascades (Leong and Karsan, 2000). Neutralization of TNF-α is therefore applied to suppress a broad spectrum of inflammatory autoimmune diseases (Abuzakouk et al., 2002). The effect of heparanase on the levels of secreted TNF-α was measured in vitro. As demonstrated in figure 5, the amounts of TNF-α produced by IL-2 stimulated splenic lymphocytes and determined by ELISA, were 3–4 folds lower in cells that were treated with heparanase, either latent (30 μg/ml) (Fig. 5) or active (5 μg/ml) (not shown), as compared to untreated cells.
Figure 5. Effect of heparanase on TNF-α secretion.
SJL/J spleen lymphocytes were incubated (24 h, 37°C) without (Cont.) or with 30 μg/ml 65 kDa heparanase (heparanase). The amounts of TNF-α were determined (ELISA) in aliquots of the culture medium. A marked decrease in TNF-α was noted in the presence of heparanase. Bars, represent mean ± SD of triplicate determinations.
4. Discussion
Traditionally, the heparanase enzyme is known to facilitate cell invasion through tissue barriers. The enzyme has also been shown to release various growth factors and cytokines sequestered by heparan sulfate (HS) in the ECM, basement membrane and cell surfaces (Fux et al., 2009b; Ilan et al., 2006; Parish et al., 2001; Vreys and David, 2007). The released factors mediate processes such as angiogenesis and cell proliferation that often accompany the inflammatory response (Li and Vlodavsky, 2009; McKenzie, 2007). It was therefore expected that administration of heparanase to mice that were triggered to develop EAE, will enhance the encephalomyelitis symptoms through increased recruitment of T cells to the affected tissue. Moreover, it was previously demonstrated that heparanase-inhibiting species of heparin suppress the progression of EAE and other autoimmune disorders (Bartlett et al., 1995; Hershkoviz et al., 1995; Lider et al., 1989). Surprisingly, an opposite effect was obtained. Not only that the symptoms did not increase, they were almost completely ablated in mice that were treated with heparanase and this effect was dose dependent. The protective effect of heparanase was also noted by histological examination of spinal cord tissue sections, showing infiltration of inflammatory cells and tissue degeneration in the control, heparanase untreated cells vs. little or no infiltration and healthy appearance of the tissue following heparanase treatment. The protective effect of heparanase was further substantiated by showing that exogenously administered heparanase was able to reach and bind to the brain tissue as indicated by immunostaining applying anti-heparanase antibodies. A diffused localization of heparanase was observed in the gray matter and vasculature of the CNS in the treated mice, as compared to a very weak basal staining of the endogenous heparanase in control mice (not shown).
The pathogenesis of most autoimmune diseases is mediated mainly through effector T cells penetrating the affected tissue and causing a specific damage (O’Garra and Vieira, 2004). To better understand the mode of action of heparanase, we investigated its effect on T cells in vitro. Applying the ConA and MLC lymphocyte activation assays, we demonstrated a direct inhibitory effect of heparanase, either active (8 + 50 kDa) or latent (65 kDa) on T cell activation and proliferation. Likewise, T-cell activation by ConA was inhibited also by an inactive enzyme in which the active site proton donor (Glu-225) and nucleophil (Glu-343) were replaced by inert residues, supporting the notion that heparanase enzymatic activity is not involved in its ability to ameliorate the clinical signs of EAE. Moreover, co-administration of heparanase and a potent heparanase-inhibiting non-anticoagulant glycol split modified heparin (ST1514) (Naggi et al., 2005; Vlodavsky et al., 2007) did not alter the inhibitory effect of heparanase on EAE, indicating again that heparanase enzymatic activity is not necessary for its protective effect. It has also been demonstrated that the enzymatic activity of heparanase is optimal in an acidic environment vs. almost no activity at pH >7.4 (Gilat et al., 1995; McKenzie et al., 2003). Hence, little or no enzymatic activity is expected at the physiological pH of body fluids, lymph nodes and the CNS tissue before the onset of the disease. Moreover, we have demonstrated that the anti-EAE effect of heparanase was exerted regardless of whether the mice were treated with the active or latent forms of the enzyme, again indicating that the observed protective effect may not involve actual degradation of HS. In fact, we have demonstrated that heparanase elicits several non-enzymatic activities, including EGF receptor phosphorylation, activation of Src, Akt/PI3K, stimulation of VEGF and tissue factor gene expression, and induction of cell spreading (Cohen-Kaplan et al., 2008; Fux et al., 2009b; Gingis-Velitski et al., 2004; Ilan et al., 2006), suggesting an involvement of heparanase in cell-ECM interaction and signal transduction, possibly through binding to HS and/or specific cell surface receptors (Fux et al., 2009a; Ilan et al., 2006; Vreys and David, 2007).
Th2-shifting of lymphocytes is known to be associated with suppression of disease signs in the EAE model (Begolka et al., 1998; Chitnis and Khoury, 2003; Moss et al., 2004). Furthermore, several studies on the role of natural killer T (NKT) cells in the pathogenesis of EAE focus on their ability to alter the polarity of T cells from a Th1 toward Th2 phenotype (Miyamoto et al., 2001). In fact, we have demonstrated that stimulation of T cells by either ConA or IL2 resulted in increased production of Th2 cytokines, including IL-4, IL-6 and IL-10, and a marked decrease in IL-12 secreted by the activated cells. A similar shift was noted in splenocytes derived from SJL/J mice that were treated with heparanase. Notably, the cytokine release assays in vitro were performed with non-separated lymphocytes, resembling the situation in vivo. FACS analysis of activated T cells exposed to heparanase in vitro, demonstrated no significant change in the percentage of T, NKT and NK cell phenotypes as compared to untreated cells (not shown), indicating that the heparanase-induced shift toward the Th2 repertoire of cytokines, is not mediated by an effect on the percentage of NKT cells. Altogether, these results support a role for heparanase in determining the polarity status of lymphocytes in a way that suppresses their ability to promote the auto-inflammation process of EAE. It should also be noted that a third T cell subset, Th17, has been recognized and shown to play a role in autoimmunity (Kappel et al., 2009). Thus, the Th1/Th2 theory may be somewhat oversimplified.
The mechanism by which heparanase induces a shift from Th1 toward a Th2 phenotype is not clear, but may, as mentioned above, involve binding to and activation of a putative cell surface receptor (Fux et al., 2009a). Apart of HSPGs, several cell surface proteins have been shown to bind heparanase and mediate its uptake. These include mannose 6-phosphate receptor (MPR) and low density lipoprotein receptor-related protein (LRP) (Ben-Zaken et al., 2008; Vreys et al., 2005) which potentially can mediate heparanase signaling and non-enzymatic effects. The existence of cell surface heparanase receptor(s) is supported by binding experiment, reinforcing the notion that while HSPGs serve as low affinity, high abundant binding sites, heparanase also associates with high affinity, low abundant cell surface receptor(s) (Ben-Zaken et al., 2008). A first indication for the protein nature of this receptor and its molecular weight emerged from cross-linking experiments applying several cell types and revealing two distinct complexes representing 110 and 150 kDa proteins associated with the heparanae C-terminus domain (C-domain) (Fux et al., 2009b). The nature of these cell membrane protein(s)/receptor(s) and their occurrence in cells of the immune system, is being investigated.
Heparanase releases from ECM a trisulfated HS-derived disaccharide that inhibits the activity of TNF-α (Lider et al., 1995). Since TNF-α is a major inflammatory cytokine, such an effect may also contribute to the observed decrease in the clinical signs of EAE. In fact, we have demonstrated reduced levels of TNF-α following exposure of lymphocytes to heparanase in vitro. It has recently been shown that TNFα stimulates synthesis and secretion of heparanase in vascular endothelial cells (Chen et al., 2004) and T lymophcytes (Sotnikov et al., 2004). The decrease in TNF levels in heparanase treated T-cells, suggests a possible feed back mechanism, co-regulating expression of the two molecules.
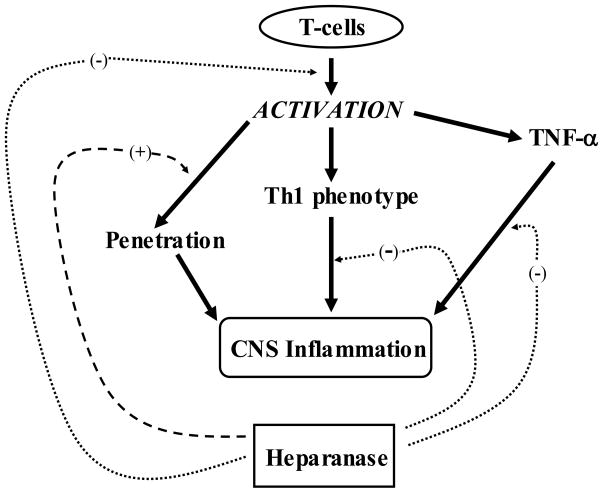
Heparanase appears to play a dual role in EAE. On the one hand, as previously reported (Lider et al., 1989; Naparstek et al., 1984; Vlodavsky et al., 1992), it may facilitate the ability of lymphocytes to extravasate, reach their target tissue and release ECM-bound pro-inflammatory mediators. On the other hand, as demonstrated in the present study, heparanase appears to suppress the inflammatory process through a decreased secretion of pro-inflammatory cytokines (i.e., TNF-α IL-12) and increased production/secretion of Th2 cytokines such as IL-4, IL-6 and IL-10. The Th1 to Th2 shift induced in the T cell population, results in inhibition of T cell activation and proliferation, as schematically presented in figure 6. Under the experimental conditions described in the present study, the net effect of heparanase was a marked suppression of EAE. The role of heparanase as an anti-inflammatory rather than pro-inflammatory molecule in vivo, as well as its ability to shift the nature of T cells towards a Th2 phenotype, as was shown by the change in cytokine profile, is a new and unexpected feature. These effects may be elicited by direct interaction of heparanase with cell surface signaling receptors and/or release and activation of HS-bound cytokines and signaling molecules. Heparanase may also modulate gene expression following its translocation into the cell nucleous (Nobuhisa et al., 2005; Schubert et al., 2004). Our studies, applying recombinant heparanase in other cognate (type 1 diabetes) (Bitan et al., 2008) and acquired autoimmune models (i.e., GVHD, our unpublished results) revealed an inhibitory effect, similar to that obtained with the EAE model, suggesting a more general role of heparanase in the regulation of autoimmunity. The protective effect of heparanase may provide a new therapeutic approach to halt the progression of EAE and other autoimmune disorders.
Figure 6. Scheme illustrating the possible involvement of heparanase in the pathogenesis of EAE.
T-cells secrete upon activation pro-inflammatory cytokines such as TNF-α and are directed toward expression of pro-inflammatory cytokines characteristic of the Th1 phenotype. The activated cells also gain ability to extravasate and reach their target brain tissue. While heparanase may stimulate the latter process, it markedly inhibits the activation of T-cells and shifts their cytokine profile toward an anti inflammatory phenotype. Altogether, the net effect is a significant amelioration of EAE.
Acknowledgments
We thank Drs. Claudio Pisano and Sergio Penco (Sigma-Tau Research Department, Pomezia, Rome, Italy) for providing the ST1514 glycol-split heparin and for their continuous support and assistance.
This work was supported by grants from the Israel Science Foundation (grant 549/06); National Cancer Institute (RO1-CA106456) and the Rappaport Family Institute Fund. I. Vlodavsky is a Research Professor of the Israel Cancer Research Fund (ICRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abboud-Jarrous G, Atzmon R, Peretz T, Palermo C, Gadea BB, Joyce JA, Vlodavsky I. Cathepsin L is responsible for processing and activation of proheparanase through multiple cleavages of a linker segment. J Biol Chem. 2008 doi: 10.1074/jbc.M801327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuzakouk M, Feighery C, Jackson J. Tumour necrosis factor blocking agents: a new therapeutic modality for inflammatory disorders. Br J Biomed Sci. 2002;59:173–9. doi: 10.1080/09674845.2002.11783656. [DOI] [PubMed] [Google Scholar]
- Bartlett MR, Cowden WB, Parish CR. Differential effects of the anti-inflammatory compounds heparin, mannose-6-phosphate, and castanospermine on degradation of the vascular basement membrane by leukocytes, endothelial cells, and platelets. J Leukoc Biol. 1995;57:207–13. doi: 10.1002/jlb.57.2.207. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Vanderlugt CL, Rahbe SM, Miller SD. Differential expression of inflammatory cytokines parallels progression of central nervous system pathology in two clinically distinct models of multiple sclerosis. J Immunol. 1998;161:4437–46. [PubMed] [Google Scholar]
- Ben-Zaken O, Shafat I, Gingis-Velitski S, Bangio H, Kelson IK, Alergand T, Amor Y, Maya RB, Vlodavsky I, Ilan N. Low and high affinity receptors mediate cellular uptake of heparanase. Int J Biochem Cell Biol. 2008;40:530–42. doi: 10.1016/j.biocel.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamron S, Nechushtan H, Verbovetski I, Krispin A, Abboud-Jarrous G, Zcharia E, Edovitsky E, Nahari E, Peretz T, Vlodavsky I, Mevorach D. Translocation of active heparanase to cell surface regulates degradation of extracellular matrix heparan sulfate upon transmigration of mature monocyte-derived dendritic cells. J Immunol. 2006;176:6417–24. doi: 10.4049/jimmunol.176.11.6417. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bitan M, Polliack A, Zecchina G, Nagler A, Friedmann Y, Nadav L, Deutsch V, Pecker I, Eldor A, Vlodavsky I, Katz BZ. Heparanase expression in human leukemias is restricted to acute myeloid leukemias. Exp Hematol. 2002;30:34–41. doi: 10.1016/s0301-472x(01)00766-4. [DOI] [PubMed] [Google Scholar]
- Bitan M, Weiss L, Zeira M, Reich S, Pappo O, Vlodavsky I, Slavin S. Heparanase prevents the development of type 1 diabetes in non-obese diabetic mice by regulating T-cell activation and cytokines production. Diabetes Metab Res Rev. 2008;24:413–21. doi: 10.1002/dmrr.868. [DOI] [PubMed] [Google Scholar]
- Casu B, Guerrini M, Naggi A, Perez M, Torri G, Ribatti D, Carminati P, Giannini G, Penco S, Pisano C, Belleri M, Rusnati M, Presta M. Short heparin sequences spaced by glycol-split uronate residues are antagonists of fibroblast growth factor 2 and angiogenesis inhibitors. Biochemistry. 2002;41:10519–28. doi: 10.1021/bi020118n. [DOI] [PubMed] [Google Scholar]
- Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ. Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression. Biochemistry. 2004;43:4971–7. doi: 10.1021/bi0356552. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Khoury SJ. Cytokine shifts and tolerance in experimental autoimmune encephalomyelitis. Immunol Res. 2003;28:223–39. doi: 10.1385/IR:28:3:223. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, Khoury SJ. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;108:739–47. doi: 10.1172/JCI12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kaplan V, Doweck I, Naroditsky I, Vlodavsky I, Ilan N. Heparanase augments epidermal growth factor receptor phosphorylation: correlation with head and neck tumor progression. Cancer Res. 2008;68:10077–85. doi: 10.1158/0008-5472.CAN-08-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I. Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis. J Natl Cancer Inst. 2004;96:1219–30. doi: 10.1093/jnci/djh230. [DOI] [PubMed] [Google Scholar]
- Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest. 2001;108:169–73. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Y, Vlodavsky I, Aingorn H, Aviv A, Peretz T, Pecker I, Pappo O. Expression of heparanase in normal, dysplastic, and neoplastic human colonic mucosa and stroma: evidence for its role in colonic tumorigenesis [In Process Citation] Am J Pathol. 2000;157:1167–75. doi: 10.1016/S0002-9440(10)64632-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, Feibish N, Cohen-Kaplan V, Gingis-Velitski S, Feld S, Geffen C, Vlodavsky I, Ilan N. Structure-function approach identifies a C-terminal domain that mediates heparanase signaling. Cancer Res. 2009a:69. doi: 10.1158/0008-5472.CAN-08-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fux L, Ilan N, Sanderson RD, Vlodavsky I. Heparanase: busy at the cell surface. Trends Biochem Sci. 2009b;34:511–9. doi: 10.1016/j.tibs.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O. Molecular behavior adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH. J Exp Med. 1995;181:1929–34. doi: 10.1084/jem.181.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279:23536–41. doi: 10.1074/jbc.M400554200. [DOI] [PubMed] [Google Scholar]
- Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, Katz BZ, Geiger B, Vlodavsky I. Heparanase mediates cell adhesion independent of its enzymatic activity. Faseb J. 2003;17:1015–25. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Rostami A. Role of the IL-12/IL-23 system in the regulation of T-cell responses in central nervous system inflammatory demyelination. Crit Rev Immunol. 2004;24:111–28. doi: 10.1615/critrevimmunol.v24.i2.20. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R, Mor F, Miao HQ, Vlodavsky I, Lider O. Differential effects of polysulfated polysaccharide on experimental encephalomyelitis, proliferation of autoimmune T cells, and inhibition of heparanase activity. J Autoimmun. 1995;8:741–50. doi: 10.1006/jaut.1995.0055. [DOI] [PubMed] [Google Scholar]
- Hulett MD, Freeman C, Hamdorf BJ, Baker RT, Harris MJ, Parish CR. Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nat Med. 1999;5:803–9. doi: 10.1038/10525. [DOI] [PubMed] [Google Scholar]
- Hulett MD, Hornby JR, Ohms SJ, Zuegg J, Freeman C, Gready JE, Parish CR. Identification of active-site residues of the pro-metastatic endoglycosidase heparanase. Biochemistry. 2000;39:15659–67. doi: 10.1021/bi002080p. [DOI] [PubMed] [Google Scholar]
- Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int J Biochem Cell Biol. 2006;38:2018–39. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–52. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–52. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier BC, Storch MK, Archelos JJ, Martino G, Hartung HP. Effector pathways in immune mediated central nervous system demyelination. Curr Opin Neurol. 1999;12:323–36. doi: 10.1097/00019052-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Leong KG, Karsan A. Signaling pathways mediated by tumor necrosis factor alpha. Histol Histopathol. 2000;15:1303–25. doi: 10.14670/HH-15.1303. [DOI] [PubMed] [Google Scholar]
- Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308:885–91. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- Li JP, Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost. 2009;102:823–8. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- Lider O, Baharav E, Mekori YA, Miller T, Naparstek Y, Vlodavsky I, Cohen IR. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989;83:752–6. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lider O, Cahalon L, Gilat D, Hershkoviz R, Siegel D, Margalit R, Shoseyov O, Cohen IR. A disaccharide that inhibits tumor necrosis factor alpha is formed from the extracellular matrix by the enzyme heparanase. Proc Natl Acad Sci U S A. 1995;92:5037–41. doi: 10.1073/pnas.92.11.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Martino G, Hartung HP. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol. 1999;12:309–21. doi: 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- McKenzie E, Young K, Hircock M, Bennett J, Bhaman M, Felix R, Turner P, Stamps A, McMillan D, Saville G, Ng S, Mason S, Snell D, Schofield D, Gong H, Townsend R, Gallagher J, Page M, Parekh R, Stubberfield C. Biochemical characterization of the active heterodimer form of human heparanase (Hpa1) protein expressed in insect cells. Biochem J. 2003;373:423–35. doi: 10.1042/BJ20030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. Br J Pharmacol. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HQ, Navarro E, Patel S, Sargent D, Koo H, Wan H, Plata A, Zhou Q, Ludwig D, Bohlen P, Kussie P. Cloning, expression, and purification of mouse heparanase. Protein Expr Purif. 2002;26:425–31. doi: 10.1016/s1046-5928(02)00558-2. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Moss RB, Moll T, El-Kalay M, Kohne C, Soo Hoo W, Encinas J, Carlo DJ. Th1/Th2 cells in inflammatory disease states: therapeutic implications. Expert Opin Biol Ther. 2004;4:1887–96. doi: 10.1517/14712598.4.12.1887. [DOI] [PubMed] [Google Scholar]
- Naggi A, Casu B, Perez M, Torri G, Cassinelli G, Penco S, Pisano C, Giannini G, Ishai-Michaeli R, Vlodavsky I. Modulation of the heparanase-inhibiting activity of heparin through selective desulfation, graded N-acetylation, and glycol splitting. J Biol Chem. 2005;280:12103–13. doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]
- Naparstek Y, Cohen IR, Fuks Z, Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984;310:241–4. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- Nobuhisa T, Naomoto Y, Takaoka M, Tabuchi Y, Ookawa K, Kitamoto D, Gunduz E, Gunduz M, Nagatsuka H, Haisa M, Matsuoka J, Nakajima M, Tanaka N. Emergence of nuclear heparanase induces differentiation of human mammary cancer cells. Biochem Biophys Res Commun. 2005;331:175–80. doi: 10.1016/j.bbrc.2005.03.129. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- Parish CR, Freeman C, Hulett MD. Heparanase: a key enzyme involved in cell invasion. Biochim Biophys Acta. 2001;1471:M99–108. doi: 10.1016/s0304-419x(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biol. 2004;23:341–52. doi: 10.1016/j.matbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Schubert SY, Ilan N, Shushy M, Ben-Izhak O, Vlodavsky I, Goldshmidt O. Human heparanase nuclear localization and enzymatic activity. Lab Invest. 2004;84:535–44. doi: 10.1038/labinvest.3700084. [DOI] [PubMed] [Google Scholar]
- Slavin S, Strober S, Fuks Z, Kaplan HS. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikov I, Hershkoviz R, Grabovsky V, Ilan N, Cahalon L, Vlodavsky I, Alon R, Lider O. Enzymatically quiescent heparanase augments T cell interactions with VCAM-1 and extracellular matrix components under versatile dynamic contexts. J Immunol. 2004;172:5185–93. doi: 10.4049/jimmunol.172.9.5185. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Nakajima M. Human heparanase. Purification, characterization, cloning, and expression. J Biol Chem. 1999;274:24153–60. doi: 10.1074/jbc.274.34.24153. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Eldor A, Haimovitz-Friedman A, Matzner Y, Ishai-Michaeli R, Lider O, Naparstek Y, Cohen IR, Fuks Z. Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis. 1992;12:112–27. [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis [see comments] Nat Med. 1999;5:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Ilan N, Naggi A, Casu B. Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Curr Pharm Des. 2007;13:2057–73. doi: 10.2174/138161207781039742. [DOI] [PubMed] [Google Scholar]
- Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–52. doi: 10.1111/j.1582-4934.2007.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreys V, Delande N, Zhang Z, Coomans C, Roebroek A, Durr J, David G. Cellular uptake of mammalian heparanase precursor involves low density lipoprotein receptor-related proteins, mannose 6-phosphate receptors, and heparan sulfate proteoglycans. J Biol Chem. 2005;280:33141–8. doi: 10.1074/jbc.M503007200. [DOI] [PubMed] [Google Scholar]
- Weiss L, Barak V, Zeira M, Abdul-Hai A, Raibstein I, Reich S, Hirschfeld E, Gross D, Slavin S. Cytokine production in Linomide-treated nod mice and the potential role of a Th (1)/Th(2) shift on autoimmune and anti-inflammatory processes. Cytokine. 2002;19:85–93. doi: 10.1006/cyto.2002.1956. [DOI] [PubMed] [Google Scholar]
- Zetser A, Levy-Adam F, Kaplan V, Gingis-Velitski S, Bashenko Y, Schubert S, Flugelman MY, Vlodavsky I, Ilan N. Processing and activation of latent heparanase occurs in lysosomes. J Cell Sci. 2004;117:2249–58. doi: 10.1242/jcs.01068. [DOI] [PubMed] [Google Scholar]