Abstract
Background
Medical therapies for hidradenitis suppurativa are often ineffective. TNF-alpha inhibitors may be a potential treatment for patients with moderate to severe hidradenitis suppurativa.
Objectives
We sought to evaluate the safety and efficacy of etanercept for patients with severe hidradenitis suppurativa.
Methods
We conducted a phase II clinical trial of etanercept 50 mg SC per week in patients with moderate to severe hidradenitis suppurativa. Efficacy was measured using a Physician’s Global Assessment (PGA) and several secondary physician and patient reported outcome measures. Responders were classified as those achieving at least a 50% reduction on the PGA at week 12 compared to baseline.
Results
Only 3 out of the 15 patients who entered the study were classified as responders (response rate of 20%; 95% CI: 4.3–48.1) based on the intention to treat analysis. DLQI scores improved slightly from a median of 19 to 15 (P=0.02). Comparison of baseline to week 12 PGA scores, as well as secondary outcome measures of lesion counts and patient pain scores, failed to show statistically significant improvement. Etanercept was generally well tolerated; however, 2 patients discontinued the study due to skin infections at the site of hidradenitis lesions requiring oral antibiotics.
Limitations
Lack of a control group and a small number of participants.
Conclusions
Our study demonstrated minimal evidence of clinically significant efficacy of etanercept 50mg SC once weekly in the treatment of hidradenitis. Future studies using higher doses of etanercept are indicated, however, patients need to be carefully monitored for infection and other adverse events. Randomized, controlled trials will be necessary to demonstrate the risk to benefit ratio of TNF-α inhibitors in the treatment of hidradenitis.
BACKGROUND
Hidradenitis suppurativa (HS) is a common inflammatory disease characterized by painful, recurrent abscesses and nodules primarily in intertriginous areas[1, 2]. HS has a point prevalence of 1–4% in the general population, is more common in women than men, and has an average age of onset in the mid twenties to early thirties[3–6]. Chronic inflammation can lead to sinus tract formation, scarring, discharge, pain, the development of squamous cell carcinoma, and serious impairments in health-related quality of life[7]. Current treatments are often unsatisfactory. Medical therapies, such as systemic antibiotics, provide only temporary relief of symptoms. Surgical interventions can be curative but are associated substantial morbidity and a high risk of recurrence of hidradenitis.
TNF-α is a proinflammatory cytokine that has numerous effects at the cellular level, and these effects may be relevant to the inflammatory aspects of HS[8–10]. Initially, patients with Crohn’s disease with concomitant HS showed improvement of their HS lesions when treated with the anti- TNF-α agent infliximab[11–13]. Subsequently, over 70 patients have been reported in the literature that have been treated with a TNF-α inhibitor (infliximab, etanercept, adalimumab)[14–23]. Most of these patients showed some clinical response while receiving treatment, with some patients demonstrating significant periods of remission. However, most of these studies were case reports or case series and few were derived from prospectively conducted clinical trials.
Etanercept is a TNF-α inhibitor that is FDA approved for the treatment of multiple inflammatory conditions including rheumatoid arthritis, psoriatic arthritis, and psoriasis. To better estimate the safety and potential efficacy of etanercept for treatment of HS, we performed an open label prospective clinical trial in patients with severe hidradenitis who had not responded adequately to existing standard treatment regimens.
METHODS
Study Patients
Institutional review board approval was obtained and all patients gave informed consent to participate. The study was conducted in accordance with the Declaration of Helsinki and was registered at ClinicalTrials.gov (NCT00107991) before any study procedures were performed. Patients were eligible if they were age 18 or older. Participants were required to have severe hidradenitis suppurativa clinically confirmed by the investigator and defined as Hurley stage II or III disease and have 4 or more lesions (e.g. nodules or abscesses) that had not responded to previous standard therapies such as topical or oral antibiotics, isotretinoin, or intralesional steroid injections[24]. Patients were required to use at least one form of effective contraception during the study period if female and of child bearing capacity or if male. Female patients who elected to use a hormonal form of contraception must have initiated the hormonal contraception at least 90 days prior to the start of the study drug and continued using this in the same form until the end of the study (week 18), or was otherwise excluded from the study. Patients were excluded if they had used oral or topical antibiotics, isotretinoin, or intralesional steroids within 30 days prior to or at any time during the study period. Patients who had used systemic immunosuppressants, an investigational medication, or a live vaccine 90 days prior to day 0 of this study were excluded. Patients were excluded if they experienced an active moderate to severe infection or an infection requiring treatment with antibiotics within 30 days of day 0 of the study, had a history of tuberculosis or positive screening visit PPD, or had a known history of an immune-suppressive disease. Patients who had clinically significant laboratory abnormalities, severe co-morbidities, history of alcohol or drug abuse within 12 months of the screening visit, were pregnant or lactating, or were using concurrent cyclophosphamide were also excluded.
Study design
This was a prospective, single-arm, single-dose, non-controlled, open-label, modified Simon’s two stage clinical trial of 50 mg etanercept/wk administered subcutaneously in patients with stage II or III hidradenitis suppurativa[25]. The study took place at the University of Pennsylvania Department of Dermatology. During an 18-week trial, etanercept 50 mg was administered subcutaneously once a week for 12 weeks. At week 12, etanercept was tapered to 25 mg/wk over a 2-week period in order to minimize the risk of a flare of their disease. The subjects were then followed for an additional 4 weeks after discontinuing the study drug on week 14. Subjects returned to clinic at baseline and weeks 2, 4, 8, 12, 14, and 18. The first participant was enrolled on May 26, 2005 and all visits were completed by August 7, 2008.
Efficacy end points
The primary efficacy measure was the proportion of patients classified as responders using the Physician’s Global Assessment (PGA) on a 100 mm visual analogue scale, with 0 mm corresponding to no active disease (only residual scars remain) and 100 mm corresponding to very severe disease with multiple, painful, inflammatory lesions. Visual analogue scales to assess global response in clinical trials of hidradenitis have been previously accepted[26]. A patient was classified as a responder if the individual achieved at least a 50% reduction on the PGA at week 12 compared to baseline. A single dermatologist, (RL), who was blinded to the definition of a responder as well as the starting and stopping rules of the study, graded all study patients except one (patient #11 who was graded by dermatologist JT).
Secondary outcome measures included number of lesions (nodules and abscesses), as well as patient reported outcomes including a patient’s global assessment rating, a patient self report of pain on a 100 mm visual scale (with 0 corresponding to no pain and 100mm corresponding to severe pain), and the Dermatology Life Quality Index (DLQI). All measures were compared from baseline to week 12, with responders defined as those achieving at least a 50% reduction in number of lesions, a 50% reduction in the pain score, a 50% improvement in the DLQI score, or a moderate or greater improvement in the patient’s global assessment.
Safety end points
All patients who received at least one dose of study drug were included in the safety analysis. Patients were carefully assessed at each visit for evidence of adverse events, including serious adverse events, malignancies, infections, and adverse events suggestive of hypersensitivity. A complete blood count, chemistry panel, renal and liver function tests were performed at baseline and at week 12.
Statistical analysis
The study was conducted using a modified Simon’s two-stage design to maximize the safety and efficiency of this early phase II clinical trial. We assumed that 50% of patients would be classified as responders on the Physician’s Global Assessment at week 12 as defined above. We determined that if 20% or fewer patients were classified as responders, then this agent would not be promising for future studies at the current dose. In addition, we assumed a type I error of 0.05 and a Type II error of 0.1. Thus, if the true responder rate is less than 20%, then the probability of this study recommending further investigation is 5%, and if the true response rate is 50% or more, then the probability of recommending the current dose of etanercept for further study is 90%. In the first stage of the study, 12 subjects were enrolled and their response status was determined at week 12. With the above assumptions, if 3 or more of the first 12 patients who received study drug were classified as responders at week 12, then this would justify treating an additional 9 patients in stage 2.
The primary analysis included all participants who were enrolled in the trial and received any treatment (intention to treat) with response rates reported using two methods. In the intention to treat analysis, all patients who did not reach week 12 were classified as non-responders. As a sensitivity analysis, we also performed an ITT analysis using the last outcome carried forward for all efficacy measures. The first 12 patients who entered the trial and received study drug were used to determine if an additional 9 patients would be enrolled based on the a priori rule described above. Additional subjects were enrolled in order to allow for an as treated (AT) analysis of up to 12 patients. The AT included all patients who completed treatment with Etanercept up to week 12 of the study, which provided the basis for an effectiveness analysis.
For the primary outcome, responders were classified based on PGA scores at week 12 compared to baseline and response rates were calculated with 95% confidence intervals (CI). Additionally, response rates with 95% confidence intervals were calculated for all secondary outcome measures. The median and interquartile range (IQR) values were calculated at baseline and at week 12 for the PGA, the number of hidradenitis lesions, the DLQI, and the patient report of pain. Comparisons were made using the Wilcoxon Signed Rank test. Data analysis was performed using STATA (Version10, StataCorp LP, College Station, Tex).
RESULTS
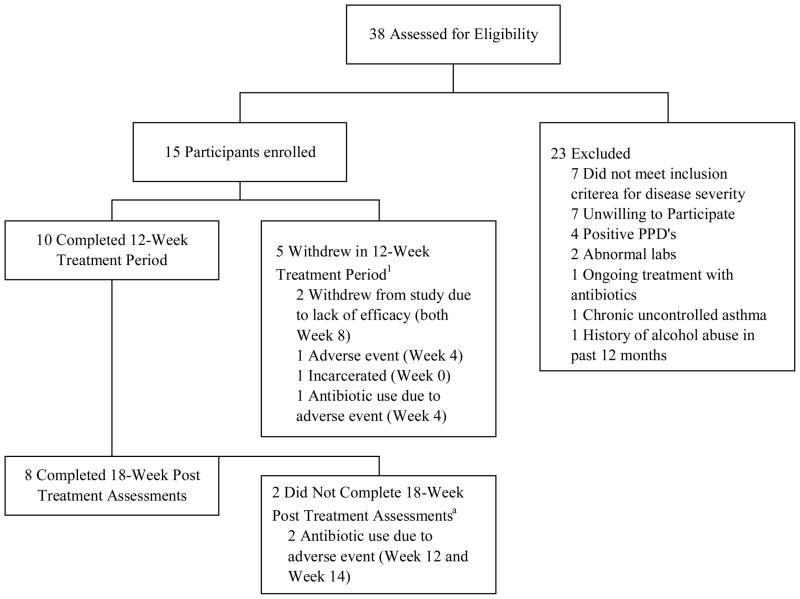
A total of 38 patients were assessed for eligibility (Fig. 1) and 15 patients were enrolled. Of these, 10 patients completed the 12 week treatment period and 5 patients withdrew during the treatment period. Of the 10 patients who completed the treatment period, 8 completed the post treatment assessment at 18 weeks. The characteristics of the study participants are shown in Table I and are further divided into an in intention to treat (ITT) population, and an as treated (AT) population. The ITT and AT populations were similar in terms of sex, age, race, duration of disease, prior therapies, body mass index (BMI) and baseline assessments and there were no significant differences in patients who completed 12 weeks of treatment compared to those who did not (data not shown).
Figure 1. Flowchart of participant enrollment.
1. (Week #) denotes the last patient visit before withdrawal from study.
Table I.
Baseline Patient Characteristics1
| Characteristics | Intention to Treat Analysis Population (n = 15) | As Treated Analysis Population (n = 10) |
|---|---|---|
| Sex | ||
| Male | 2 (13.33) | 1 (10) |
| Female | 13 (86.67) | 9 (90) |
| Age, y | ||
| Mean (median) [25th percentile, 75th percentile] | 42 (45) [30, 50] | 42.9 (46.5) [30, 53] |
| Race | ||
| White | 12 (80) | 9 (90) |
| Black | 3 (20) | 1 (10) |
| Baseline Assessments - Mean (median) [25th percentile, 75th percentile] | ||
| Physician’s Global Assessment (PGA) - | ||
| 10 cm Visual Analogue Scale Score | 4.17 (4.35) [3.1, 4.8] | 4.26 (4.55) [3.5, 4.8] |
| Number of Lesions | 14.07 (14) [8, 19] | 14.9 (14) [9, 19] |
| Patient Pain - | ||
| 10 cm Visual Analogue Scale Score | 6.19 (6.4) [4.5, 7.4] | 6.6 (7.25) [5.5, 7.9] |
| Dermatology Life Quality Index (DLQI)2 | 20.4 (19) [14, 29] | 21.5 (20.5) [16, 29] |
| Duration of Disease, y | ||
| Mean (median) [range] | 15.46 (12) [1–36] | 13 (11.5) [1–35] |
| Prior Systemic and Intralesional Therapies | 14 (93.3) | 9 (90) |
| Oral Antibiotics | 9 (60) | 6 (60) |
| Accutane | 8 (53.3) | 4 (40) |
| Surgery | 3 (20) | 3 (30) |
| Intralesional corticosteroids | 2 (13.3) | 0 |
| Oral contraceptives | 1 (6.7) | 1 (10) |
| Oral corticosteroids | 1 (6.7) | 0 |
| Injected antibiotics | 1 (6.7) | 1 (10) |
| Ketoconazole | 1 (6.7) | 1 (10) |
| Nicomide | 1 (6.7) | 1 (10) |
| Spironolactone | 1 (6.7) | 1 (10) |
| Cimetidine | ||
| Body Mass Index (BMI), kg/m2 | ||
| Mean (median) [range] | 35.1 (35.1) [17.9–47.5] | 37.03 (37.4) [25.2–47.5] |
Values expressed as number(percentage) unless otherwise indicated.
Meaning of DLQI Scores: 0–1 = no effect at all on patient’s life, 2–5 = small effect on patient’s life, 6–10 = moderate effect on patient’s life, 11–20 = very large effect on patient’s life, 21–30 = extremely large effect on patient’s life
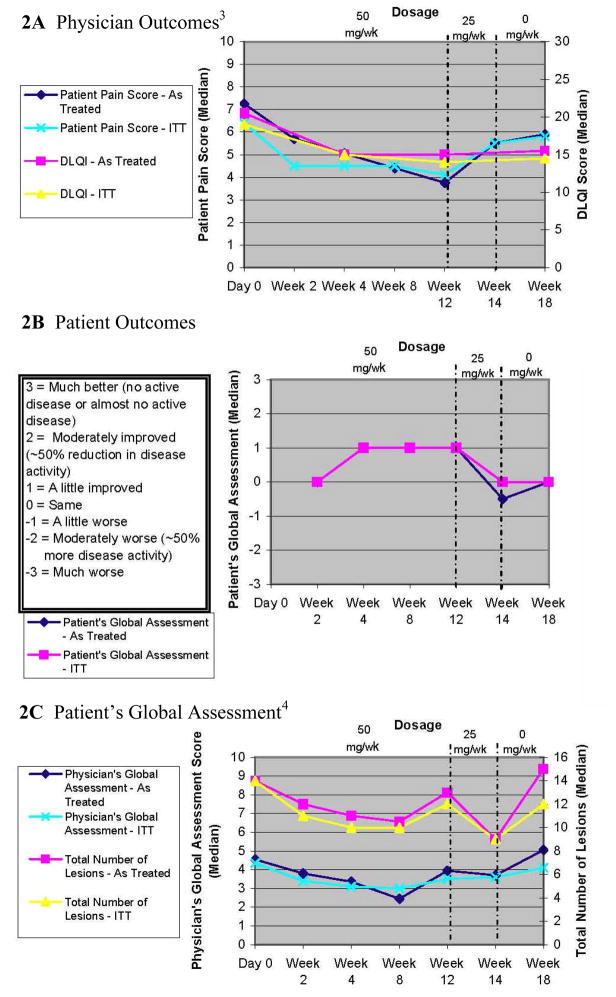
The physician observed outcomes are shown in Table II and Figure 2A. The ITT population median baseline PGA was 4.35 and lesion count was 14 and these scores decreased slightly after 12 weeks of treatment to 3.5 (P=0.14) and 12 (P=0.69), respectively. Only 2 of the first 12 patients were classified as responders based on our a priori definition (indicating a lack of efficacy) and therefore we did not enroll a complete second stage based on our a priori starting and stopping rule. The AT analysis yielded similar results for the physician observed outcomes (Table II and Figure 2A). Of note, the three patients who did achieve a clinical response had a lower median BMI (27.5) than those who did not achieve a clinical response (36); however, this was not statistically significant (P= 0.31).
Table II.
Physician Outcome Measures
| Intention to Treat Analysis (n = 15) |
As Treated Analysis (n =10) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | P Value1 | Response Rate - Percentage (95% CI) | Baseline | 12 Weeks | P Value1 | Response Rate -Percentage (95% CI) | |
|
Primary Outcome Measure | ||||||||
| Physician’s Global Assessment | ||||||||
| Median (25th percentile, 75th percentile) | 4.35 (3.1, 4.8) | 3.5 (2.2, 4.1) | 0.14 | 20.0 (4.3–48.1) | 4.55 (3.5, 4.8) | 3.95 (1.9, 4.8) | 0.31 | 30.0 (6.7–65.2) |
|
Secondary Outcome Measure | ||||||||
| Number of Lesions | ||||||||
| Median (25th percentile, 75th percentile) | 14 (8, 19) | 12 (9, 23) | 0.69 | 13.3 (1.66–40.5) | 14 (9, 19) | 13 (10, 18) | 0.92 | 20.0 (2.5–55.6) |
Calculated as Wilcoxon Signed Rank test
Figure 2. Outcome Measures1,2.
1. All outcome variables shown are continuous. The Patient’s Global Assessment was given at each visit, except at Day 0.
2. For all outcome variables under the intention to treat (ITT) analysis, median values were calculated with the last outcome carried forward for patients who withdrew from the study.
3. Under both the intention to treat (ITT) and as-treated analyses, blinded physician outcome measures were missing for 3 patients at week 2 and for 2 patients at week 14. In these instances, median values were calculated without those particular patients.
4. Under both the intention to treat (ITT) and as-treated analyses, the patient’s global assessment was missing for 1 patient at week 14. The median value was calculated without this patient.
The patient reported outcomes measures are shown in Table III and Figure 2A and B. In the ITT analysis the median pain score diminished from 6.4 to 4.1 (P=0.08) and the DLQI scores improved slightly from a median of 19 to 15 (P=0.02). Although DLQI scores improved statistically, the improvement was of minimal clinical significance based on the magnitude of the change[27]. While 57% of patients reported some improvement, no one reported complete remission at 12 weeks and only 29% of patients reported a moderate improvement in their disease. The results of the AT analysis were similar to the ITT analysis for patient reported outcomes. The time course of both physician and patient reported outcomes were similar in demonstrating some improvement by week 4. Both outcome measures seemed to show a maximum improvement by week 8 and a worsening once the treatment was stopped at week 14 (Fig. 2).
Table III.
Patient Outcome Measures
| Intention to Treat Analysis (n = 15) |
As Treated Analysis (n =10) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks | P Value1 | Response Rate - Percentage (95% CI) | Baseline | 12 Weeks | P Value1 | Response Rate - Percentage (95% CI) | |
|
Secondary Outcome Measures | ||||||||
| Patient’s Pain Score | ||||||||
| Median (25th percentile, 75th percentile) | 6.4 (4.5, 7.4) | 4.1 (1.4, 6.9) | 0.08 | 26.7 (7.8–55.1) | 7.25 (5.5, 7.9) | 3.75 (1.1, 8.3) | 0.06 | 40.0 (12.2–73.8) |
| DLQI | ||||||||
| Median (25th percentile, 75th percentile) | 19 (14, 29) | 15 (10, 22) | 0.02 | 20.0 (4.3–48.1) | 20.5 (16, 29) | 15 (6, 23) | 0.02 | 30.0 (6.7–65.2) |
| Patient’s Global Assessment2 - Number (Percentage) | NA | NA | 26.7 (7.8–55.1) | NA | NA | 40.0 (12.2–73.8) | ||
| Much worse | 0 | 0(0) | ||||||
| Moderately worse (~50% more disease activity) | 3 (21.4) | 2 (20) | ||||||
| A little worse | 1 (7.1) | 1 (10) | ||||||
| Same | 2 (14.3) | 1 (10) | ||||||
| A little improved | 4 (28.6) | 2 (20) | ||||||
| Moderately improved (~50% reduction in disease activity) | 4 (28.6) | 4 (40) | ||||||
| Much better (no active disease or almost no active disease) | 0 | 0.00 | ||||||
Calculated as Wilcoxon Signed Rank test
n=14 for Patient’s Global Assessment. One patient left the study after 1 visit. Response rate calculated with n=15 under intention to treat analysis.
The incidence of adverse events is shown in Table IV. In general, etanercept was well tolerated and there were no serious adverse events reported. Thirty eight adverse events were reported in 14 patients. Both ITT and AT populations had similar adverse event profiles with upper respiratory infections reported between 26–30% of the time. Other adverse events were uncommon and included nausea, paresthesias, skin infection, arthralgias and elevation of cholesterol. Of the patients who failed to complete the treatment period, two stopped because of skin infections at the site of HS lesions requiring oral antibiotics, one stopped because of worsening paresthesias due to carpal tunnel syndrome, and two stopped because of logistical issues (transportation issues or incarceration).
Table IV.
| Adverse Event | Intention to Treat Analysis n = 15 | As Treated Analysis n = 10 |
|---|---|---|
| Number of Patients Experiencing an Adverse Event | 14 | 10 |
| Total Number of Adverse Events | 38 | 28 |
| Upper Respiratory Tract Infection | 4 (26.7) | 3 (30.0) |
| Injection site erythema, bruising, or irritation | 2 (13.3) | 1 (10.0) |
| Nausea | 3 (20.0) | 2 (20.0) |
| Paresthesias | 2 (13.3) | 1 (10.0) |
| Cellulitis | 2 (13.3) | 1 (10.0) |
| Chest Pain | 1 (6.7) | 1 (10.0) |
| Flu-like symptoms | 1 (6.7) | 1 (10.0) |
| Muscle cramps | 1 (6.7) | 1 (10.0) |
| Hypertension | 1 (6.7) | 0 |
| Elevated cholesterol | 1 (6.7) | 1 (10.0) |
Values expressed as number of affected patients (percentage of affected patients) unless otherwise indicated. Some patients experienced multiple occurrences of the same adverse event.
No serious adverse events were reported.
DISCUSSION
Hidradenitis suppurativa is a devastating inflammatory disease for which no medical therapies are proven to be consistently efficacious. The unmet medical need for safe and efficacious treatments for HS is illustrated by the long distances patients traveled to enter this study (median distance > 150 miles), and the severe impairment in HrQOL at baseline as measured by the DLQI[27]. The results of our study demonstrate that etanercept 50mg SC once weekly is well tolerated in patients with HS, but has minimal evidence of clinically significant efficacy. Particular strengths of this study include its prospective nature, the systematic collection of safety and efficacy data, use of both physician and patient reported outcomes, and assessments by a physician who was blinded to the design of the trial and definitions of clinical response.
There have been at least two other prospective studies of etanercept for the treatment of hidradenitis. Giamarellos-Bourboulis et al. conducted an open label phase II trial of etanercept 50 mg once weekly for 12 weeks in 10 patients[28]. The primary outcome measure was a disease activity score which demonstrated that 6 out of 10 subjects had greater than 50% improvement. Similar findings were seen when using the Sartorius scale. When using a patient reported visual analogue scale to assess improvement, only 2 out of 10 patients had greater than 50% improvement at week 12, suggesting that the improvements may not have been clinically significant. Cusack et al. reported an open label study of 6 patients using etanercept 50 to 100 mg every week[29]. All of the patients improved, with 4 of 5 evaluable subjects showing greater than 50% improvement in DLQI score. In this study, 4 of six patients experienced disease flares requiring use of oral antibiotics during treatment with etanercept and therefore, some of the observed efficacy may have been due to concomitant antibiotic use.
At least two prospective studies have been performed using infliximab for hidradenitis suppurativa. Mekkes et al studied the long term benefit of infliximab by giving a standard week 0, 2, and 6 dosing schedule and evaluating the long term efficacy using the Sartorius score[30]. The average score diminished from 164+/−50 (mean+/−SD) before treatment to 89+/−49 after 1 year (P=0.002). DLQI scores showed a 50% or greater improvement at one year in 5 out of 10 patients. Fardet et al reported 7 patients prospectively treated with infliximab induction treatment of 5 mg/kg at weeks 0, 2, 6 and were evaluated at weeks 6 and 10[31]. The Sartorius score demonstrated improvement of greater than 50% in 2 of 7 patients. The same 2 patients reported “marked” improvement in both the physician and patient reported improvement scales as well as over 50% improvement on the Skindex-29 scale in at least one category (symptom, emotion, or function). Several patients experienced significant adverse reactions and the authors concluded that the efficacy of infliximab in patients with severe HS seems transient and is associated with significant side effects.
The existing prospective studies of TNF-α inhibitors for hidradenitis have varied in their outcome assessment, population studied, and dose and type of TNF-α inhibitor used. In particular, these studies tended to find higher responses when using the Sartorius score compared to using global assessment tools. Our study did not use the Sartorius score, however, our results clearly demonstrate a lack of significant clinical response based on the results of our patient reported and physician reported outcomes. Further studies are needed to determine the best outcome measurement for hidradenitis studies. Additionally, it is possible that differences in patient population could have affected the outcomes of the study. In particular, in psoriasis, it has been suggested that etanercept efficacy may be diminished in patients with an elevated body mass index[27, 32]. The median BMI of our study population was 35 and therefore it is possible that etanercept may be more effective in HS patients who have an ideal BMI. In fact, in our study, responders had a lower BMI than non responders; however, this finding was not statistically significant. Finally, none of these studies utilized a control group, and therefore, it is possible that observed benefits could be due to natural remissions, a placebo effect, or in some studies, the allowance of concomitant therapies which can improve HS.
In conclusion, our study demonstrated minimal evidence of clinically significant efficacy of etanercept 50mg SC once weekly in the treatment of hidradenitis. Future studies using higher doses of etanercept are indicated, however, patients need to be carefully monitored for infection and other adverse events. Ultimately, randomized, controlled trials will be necessary to demonstrate the risk to benefit ratio of TNF-α inhibitors in the treatment of hidradenitis.
Acknowledgments
Funding Source/Role of Sponsors: Supported by a grant an unrestricted grant from Amgen and grant K23AR051125 from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (JMG). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The authors greatly appreciate Ms. Deborah Leahy’s outstanding job in coordinating this study and thank the patients who participated in this trial.
ABBREVIATIONS
- PGA
Physician’s Global Assessment
- DLQI
Dermatology Life Quality Index
- HS
Hidradenitis suppurativa
- ITT
Intention to treat
- AT
As treated
- BMI
Body mass index
Footnotes
This work has not been previously published or presented.
Financial Disclosures/Conflicts of Interest (Potentially Relevant): JMG has received grant support from Amgen and Centocor, and is a consultant to Amgen, Abbott, Centocor, Genentech, and Astellas.
References
- 1.Lee RA, Yoon A, Kist J. Hidradenitis suppurativa: an update. Adv Dermatol. 2007;23:289–306. doi: 10.1016/j.yadr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Jemec GB. Medical treatment of hidradenitis suppurativa. Expert Opin Pharmacother. 2004;5(8):1767–70. doi: 10.1517/14656566.5.8.1767. [DOI] [PubMed] [Google Scholar]
- 3.Jemec GB, Heidenheim M, Nielsen NH. Hidradenitis suppurativa--characteristics and consequences. Clin Exp Dermatol. 1996;21(6):419–23. doi: 10.1111/j.1365-2230.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 4.Revuz JE, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35(2 Pt 1):191–4. doi: 10.1016/s0190-9622(96)90321-7. [DOI] [PubMed] [Google Scholar]
- 6.Revuz JE, et al. Prevalence and factors associated with hidradenitis suppurativa: Results from two case-control studies. Journal of the American Academy of Dermatology. 2008;59(4):596–601. doi: 10.1016/j.jaad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Wolkenstein P, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56(4):621–3. doi: 10.1016/j.jaad.2006.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Rosi YL, LL, Kang S. Treatment of hidradenitis suppurativa with infliximab in a patient with Crohn’s disease. J Dermatolog Treat. 2005;16(1):58–61. doi: 10.1080/09546630410024547. [DOI] [PubMed] [Google Scholar]
- 9.Trent JT, KF Tumor necrosis factor alpha inhibitors for the treatment of dermatologic diseases. Dermatol Nurs. 2005;17(2):97–107. [PubMed] [Google Scholar]
- 10.LaDuca J. Targeting tumor necrosis factor alpha. New drugs used to modulate inflammatory diseases. Dermatol Clin. 2001;19(4):617–35. doi: 10.1016/s0733-8635(05)70304-1. [DOI] [PubMed] [Google Scholar]
- 11.Adams DR, et al. Severe hidradenitis suppurativa treated with infliximab infusion. Arch Dermatol. 2003;139(12):1540–2. doi: 10.1001/archderm.139.12.1540. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan TP, et al. Infliximab for hidradenitis suppurativa. Br J Dermatol. 2003;149(5):1046–9. doi: 10.1111/j.1365-2133.2003.05663.x. [DOI] [PubMed] [Google Scholar]
- 13.Elkjaer M, DL, Benazzato L, Rodriguez J, Løgager V, Munkholm P. Efficacy of Infliximab treatment in patients with severe Fistulizing Hidradenitis. Journal of Crohn’s and Colitis. 2008 Sept;2(3):241–245. doi: 10.1016/j.crohns.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Antonucci A, NM, Negosanti L, Iozzo I, Varotti C. Acne inversa treated with infliximab: different outcomes in 2 patients. Acta Derm Venereol. 2008;88:274–275. doi: 10.2340/00015555-0397. [DOI] [PubMed] [Google Scholar]
- 15.Usmani N, CT, Everett S, Goodfield MD. Variable response of hidradenitis suppurativa to infliximab in four patients. Clin Exp Dermatol. 2007 Mar;32(2):204–205. doi: 10.1111/j.1365-2230.2006.02272.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi P. Adalimubab in the management of hidradenitis suppurativa. J Am Acad Dermatol. 2007 Feb;56(2):AB41. [Google Scholar]
- 17.Moschella S. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? A report of three treated cases. International Journal of Derm. 2007 Dec;46(12):1287–1291. doi: 10.1111/j.1365-4632.2007.03293.x. [DOI] [PubMed] [Google Scholar]
- 18.Laurence F, DA, et al. Infliximab for severe hidradenitis suppurativa: Transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007;56:624–628. doi: 10.1016/j.jaad.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Vozmediano JM, A-HJ Infliximab for the treatment of hidradenitis suppurativa. Dermatology. 2007;215(1):41–1. doi: 10.1159/000102032. [DOI] [PubMed] [Google Scholar]
- 20.Henderson RL., Jr Treatment of atypical hidradenitis suppurativa with the tumor necrosis factor receptor-Fc fusion protein etanercept.(CASE REPORTS) Journal of Drugs in Dermatology. 2006;5(10):1010(2). [PubMed] [Google Scholar]
- 21.Zangrilli AEM, Mio G, Mazzotta A, Chimenti S. Long-term efficacy of etanercept in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2008 doi: 10.1111/j.1468-3083.2008.02617.x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Moul DK, Korman NJ. Severe Hidradenitis Suppurativa Treated With Adalimumab. Arch Dermatol. 2006;142(9):1110–1112. doi: 10.1001/archderm.142.9.1110. [DOI] [PubMed] [Google Scholar]
- 23.Scheinfeld N. Treatment of coincident seronegative arthritis and hidradentis supprativa with adalimumab. Journal of the American Academy of Dermatology. 2006;55(1):163–164. doi: 10.1016/j.jaad.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Hurley H. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: RRK, RH Jr, editors. Dermatologic surgery: Principles and Practice. Marcel Dekker Inc; New York, NY: 1996. pp. 623–645. [Google Scholar]
- 25.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 26.Sachs DD, Gordon AT. Hidradenitis suppurativa of glands of Moll. Arch Ophthalmol. 1967;77(5):635–6. doi: 10.1001/archopht.1967.00980020637012. [DOI] [PubMed] [Google Scholar]
- 27.Hongbo Y, et al. Translating the Science of Quality of Life into Practice: What Do Dermatology Life Quality Index Scores Mean? J Investig Dermatol. 2005;125(4):659–664. doi: 10.1111/j.0022-202X.2005.23621.x. [DOI] [PubMed] [Google Scholar]
- 28.Giamarellos-Bourboulis EJ, et al. An open-label phase II study of the safety and efficacy of etanercept for the therapy of hidradenitis suppurativa. Br J Dermatol. 2008;158(3):567–72. doi: 10.1111/j.1365-2133.2007.08372.x. [DOI] [PubMed] [Google Scholar]
- 29.Cusack C, Buckley C. Etanercept: effective in the management of hidradenitis suppurativa. Br J Dermatol. 2006;154(4):726–9. doi: 10.1111/j.1365-2133.2005.07067.x. [DOI] [PubMed] [Google Scholar]
- 30.Mekkes JR, Bos JD. Long-term efficacy of a single course of infliximab in hidradenitis suppurativa. Br J Dermatol. 2008;158(2):370–4. doi: 10.1111/j.1365-2133.2007.08332.x. [DOI] [PubMed] [Google Scholar]
- 31.Fardet L, et al. Infliximab for severe hidradenitis suppurativa: Transient clinical efficacy in 7 consecutive patients. J Am Acad Dermatol. 2007 doi: 10.1016/j.jaad.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. Journal of the American Academy of Dermatology. 2008;58(3):443–446. doi: 10.1016/j.jaad.2007.11.011. [DOI] [PubMed] [Google Scholar]