Abstract
This project focused on the individual differences underlying observed variability in temporal processing among older listeners. Four measures of vowel temporal-order identification were completed by young (N=35; 18–31 years) and older (N=151; 60–88 years) listeners. Experiments used forced-choice, constant-stimuli methods to determine the smallest stimulus onset asynchrony (SOA) between brief (40 or 70 ms) vowels that enabled identification of a stimulus sequence. Four words (pit, pet, pot, and put) spoken by a male talker were processed to serve as vowel stimuli. All listeners identified the vowels in isolation with better than 90% accuracy. Vowel temporal-order tasks included the following: (1) monaural two-item identification, (2) monaural four-item identification, (3) dichotic two-item vowel identification, and (4) dichotic two-item ear identification. Results indicated that older listeners had more variability and performed poorer than young listeners on vowel-identification tasks, although a large overlap in distributions was observed. Both age groups performed similarly on the dichotic ear-identification task. For both groups, the monaural four-item and dichotic two-item tasks were significantly harder than the monaural two-item task. Older listeners’ SOA thresholds improved with additional stimulus exposure and shorter dichotic stimulus durations. Individual differences of temporal-order performance among the older listeners demonstrated the influence of cognitive measures, but not audibility or age.
INTRODUCTION
It is clear that as adults age, the likelihood that they encounter difficulties with speech understanding increases. While the concurrence of peripheral damage due to hearing loss plays the most significant role in decreased speech-understanding abilities in older adults (e.g., Dubno and Schaefer, 1992, 1995; Humes and Christopherson, 1991; Humes, 1996, 2002, 2005; van Rooij and Plomp, 1992), older adults have deficits that remain even after audibility of the speech message is restored (Humes, 2007). One form of processing that has been attributed to part of this continued impairment is temporal processing (e.g., Gordon-Salant and Fitzgibbons, 1993, 1999; Schneider and Pichora-Fuller, 2000; Pichora-Fuller, 2003). This is consistent with repeated findings that older adults perform more poorly on a variety of auditory temporal processing tasks, particularly involving complex sequences (Fitzgibbons and Gordon-Salant, 1996, 1998). However, it remains unclear the extent to which the speech-communication deficits of older adults are related to peripheral or central auditory deficits, or to more general cognitive declines, an area that has been identified as a priority in auditory aging research for 20 years (Working Group on Speech Understanding and Aging, 1988). The nature of the underlying impairment could have profound implications for the treatment of speech-understanding deficits in the elderly. In order to investigate this issue, Humes (2005) outlined two primary techniques to use for the delineation of the underlying impairment: (1) concurrent study in multiple modalities and (2) correlational analysis with cognitive functioning. This paper reports the results of an ongoing investigation that is examining the cognitive, sensory, and temporal processing abilities for a large group of older adults across multiple modalities (see Humes et al., 2009). In this report, we explored the auditory temporal-order processing abilities of older and younger adults for brief vowel sequences and analyzed individual differences to further identify related abilities in older adults.
There have been a number of age-related neurophysiological changes associated with aging, including declines in dopamine receptors, demyelination, cell loss, decreased cerebral blood flow, and increased inefficient dendritic branching which have been attributed to processing-speed deficits (e.g., Park et al., 2001). Changes in the auditory system due to insult or degeneration, combined with global neural changes associated with aging, may result in a generalized decreased ability to process rapid acoustic events. Indeed, deficits in some form of processing speed may underlie many age-related cognitive declines (Salthouse, 1996). Findings such as these suggest a “common cause hypothesis” of cognitive aging (Baltes and Lindenberger, 1997; Lindenberger and Baltes, 1994) which proposes that an underlying amodal neural change results in widespread cognitive declines (for a review, see Hofer et al., 2003) and concomitant sensory deficits. Processing time has been demonstrated as a crucial indicator of cognitive decline with aging and may be related to many of the persistent speech-understanding difficulties that older adults face, even with appropriate compensation for inaudibility associated with hearing loss. Therefore, in order to understand and potentially treat these lingering difficulties to improve communication and quality of life, it is essential to understand if and how auditory temporal processing deficits are manifest in older adult listeners.
Fluent speech is characterized by rapid acoustic changes that represent the information necessary to extract meaning. These dynamic acoustic changes likely play a facilitatory role (Dorman et al., 1975). However, most temporal-order research has used non-speech stimuli, such as tone sequences. Trainor and Trehub (1989) inferred that age-related temporal-order deficits might underlie some of the speech-understanding difficulties that older listeners face. WhileHumes and Christopherson (1991) concluded that audibility is the primary predictor of age-related differences across a variety of auditory processing tasks, the four tasks that discriminated between young and old listeners had a temporal component, two of which were the temporal-order discrimination of tones and of syllables. Indeed, older listeners are less able to distinguish tone sequences with contrasting order regardless of whether the task requires discrimination or identification (Trainor and Trehub, 1989). While “normal” performance is sometimes maintained for simple patterns, processing generally breaks down at faster processing rates and for more complex patterns (Fitzgibbons and Gordon-Salant, 1998). However, the effects of age remain regardless of various stimulus characteristics, such as stimulus duration, interval spacing, or sequence timing characteristics (Fitzgibbons et al., 2006; Shrivastav et al., 2008). Age-related differences have also been described as being resistant to practice effects (Trainor and Trehub, 1989). While age differences in performance have sometimes not been observed for temporal-order judgments between two stimuli presented to opposite ears (Szymaszek et al., 2006; Kołodziejczyk and Szelag, 2008), even here age differences become prominent for the very old (95–103 years) when compared to either young (19–25 years) or older (65–67 years) adults.
The auditory temporal-order processing deficits in older listeners appear to result from processing-speed declines. Fitzgibbons and Gordon-Salant (1998) suggested an auditory processing rate limitation underlies the deficits in temporal-order processing observed in older adults. These processing limitations appear to be associated with more general difficulties in higher-level perceptual and cognitive processing (Fitzgibbons et al., 2006). Humes et al. (2007) also observed modality-specific deficits in auditory processing speed in older adults, as measured with both time-compressed speech and an auditory speeded-spelling task. Modality specificity of this processing deficit was confirmed through the use of a parallel visual speeded-spelling task in these same listeners. In support of auditory speed-of-processing deficits, corresponding measures of auditory evoked potentials have suggested the involvement of the P2 component which also correlated with behavioral performance (Lewandowska et al., 2008). The results clearly demonstrate impaired temporal-order processing in older adults at higher central auditory levels that likely involve contributions from attentional (i.e., cognitive) resources (Szelag et al., 2009).
The current study explored temporal-order identification for vowel sequences using three stimulus conditions: (1) monaural two-item sequences, (2) monaural four-item sequences, and (3) dichotic two-item sequences. The dichotic two-item stimulus condition was used for two different tasks: vowel-sequence identification and ear-sequence identification. All told, there were four primary dependent measures of temporal-order identification in this study. These stimulus conditions and tasks were motivated by several considerations. Comparison of performance for the monaural two-item and monaural four-item sequences, for example, may shed light on the contributions of memory to the identification of brief word-length sequences. Comparison of performance on the monaural and dichotic two-item tasks, on the other hand, may provide insight into the limitations posed by peripheral sensory processes, such as forward masking, or central auditory factors involved with this task. Finally, it is important to note that these tasks required judgment of both temporal-order and vowel identifications; thus, these tasks require a higher cognitive load than temporal-order tasks that can be made on the bases of, for example, pitch ordering (e.g., Fitzgibbons and Gordon-Salant, 1998) or ear ordering (e.g., Kołodziejczyk and Szelag, 2008). The inclusion of the ear-sequence identification task provided the ability to examine temporal-order identification without requiring concurrent vowel identification. Thus, comparison of performance for the two dichotic tasks, vowel-sequence identification vs ear-sequence identification, enables examination of the role played by phonological processing (i.e., stimulus categorization).
Additional experiments reported here also explored the effect of stimulus duration, ear randomization, and stimulus exposure effects regarding the performance of older adults. Few studies have addressed individual differences among older adult listeners related to temporal processing, although several have discussed the large variability among older adults (Moore et al., 1992; Schneider et al., 1994; Snell, 1997; Snell and Frisina, 2000). A primary objective of this series of experiments was to obtain temporal-order data from a large group of older adults to examine individual differences in detail. In the analyses presented here, individual differences in performance were explored to determine correlations between different auditory temporal-order tasks, age, audibility, and general measures of cognitive functioning (WAIS-III; Weschsler, 1997). That is, aside from comparisons of the group data from young and older adults, this study also explored individual differences in temporal processing among older listeners and correlations with cognitive tasks.
EXPERIMENT 1: MONAURAL AND DICHOTIC TEMPORAL ORDER
This first experiment was designed to meet two specific aims. First, group differences in temporal-order identification for vowel sequences were examined between young and older adults. Second, with the substantial number of older listeners tested, individual differences in performance among the older individuals were explored.
Listeners
Two groups of listeners participated in this study. The first group consisted of 35 young adult listeners (11 males, 24 females) with a mean age of 23 years (range: 18–31 years). The second group was comprised of 151 older adults (68 males, 83 females) with a mean age of 71 years (range: 60–88 years). Participants were recruited through advertisements in the local newspaper, in flyers for local community centers, and posted at various locations on the Bloomington campus of Indiana University.
Selection criteria for this study included age (18–35 or 60–89 years), a Mini-Mental Status Exam (Folstein et al., 1975) score ≥25, and specific hearing sensitivity requirements. Maximum hearing thresholds for air conducted pure tones were not to exceed the following limits in at least one ear: 40 dB hearing loss (HL) (American National Standards Institute, 2004) at 250, 500, and 1000 Hz; 50 dB HL at 2 kHz; 65 dB HL at 4 kHz; and 80 dB HL at 6 and 8 kHz. It was also required that there be no evidence of middle ear pathology (air-bone gaps <10 dB and normal tympanograms). A pure-tone average (PTA) for the frequency range of the test stimuli used in this study was calcuated over 500, 1000, 1500, and 2000 Hz. Young listeners had mean PTA thresholds at 8 dB HL (SD=4 dB) and 8 dB HL (SD=5 dB) for right and left ears, respectively. Older listeners had mean PTA thresholds at 21 dB HL (SD=13 dB) and 20 dB HL (SD=11 dB) for right and left ears, respectively. Participants were paid for their participation. Due to the long testing duration of the entire multimodality project, there was some attrition across tasks resulting in a loss of approximately one participant in each age group for each task.
Stimuli
Stimuli were recorded by a male Midwestern talker in a sound-attenuating booth using an Audio-Technica AT2035 microphone. Four confusable vowel stimuli (i.e., from a restricted vowel space) ∕ɪ, ε, a, ʊ∕ were spoken rapidly in a ∕pVt∕ context in a carrier phrase “The first word is__now.” The words pit, pet, pot, and put were digitally edited to remove voiceless sounds, leaving only the voiced pitch pulses. Productions of four vowels that had the shortest duration, F2<1800 Hz, and good identification by two young normal-hearing listeners, one older normal-hearing listener, and one older hearing-impaired listener during final pilot testing were selected for stimuli. Stimuli were modified in MATLAB using STRAIGHT (Kawahara et al., 1999) to be 70 ms long with a fundamental frequency of 100 Hz. Stimuli were low-pass filtered at 1800 Hz and normalized to the same rms level. Low-pass filtering was used to minimize the influence of the high-frequency hearing loss of the older adults on their vowel-identification performance.
Calibration
Stimuli were presented via Tucker-Davis Technologies (TDT) System III hardware using 16 bit resolution at a sampling frequency of 48 828 Hz. The output of the TDT D∕A converter was passed through a programmable attenuator (PA-5), headphone buffer (HB-7), and then to an ER-3A insert earphone. The earphone was calibrated in a 2 cm3 coupler using a Larson Davis model 2800 sound level meter with linear weighting. The system was calibrated using a calibration vowel of the same rms amplitude as the test stimuli, but with a duration of 3 s. A single stimulus presentation measured 83 (±2) dB sound pressure level (SPL) and a presentation of two overlapping stimuli measured 86 (±2) dB SPL. Output levels were checked electrically at the beginning of each day of data collection and were verified acoustically with the sound level meter and 2 cm3 coupler at monthly intervals throughout the study.
Procedure
All participants passed an identification screening of the four vowel stimuli in isolation with at least 90% accuracy on one of no more than 20-trial blocks in their test ear. This was to ensure that listeners would be able to complete the subsequent auditory temporal-order measures which were targeting identification performance of either 50% or 75% correct (see below). If participants did not reach this 90% identification accuracy criterion during screening, they were rescreened on a separate day. Participants ultimately unable to reach this criterion were dismissed from further auditory testing. Only five older adults were excluded from the study because of their failure to identify the brief vowel stimuli in isolation with at least 90% accuracy. In addition, all participants completed a full cognitive assessment using the WAIS-III (Weschsler, 1997) prior to data collection.
At the first auditory temporal-order experimental session listeners completed, a stimulus familiarization task which consisted of listening to each vowel stimulus in isolation while receiving orthographic feedback as to which stimulus was presented. In addition, prior to each new experimental task, listeners completed a demonstration of that task for 12 different temporal-order sequences. Correct responses were displayed with no response required on behalf of the listeners. Demonstrations were repeated for a total of 24 stimulus presentations prior to each task. Listeners were offered additional repetitions of the demonstration, although only 8% of listeners requested additional presentations.
All participants completed four tasks in the following order: Monaural two-item Identification (Mono2), Monaural four-item Identification (Mono4), Dichotic two-item Vowel Identification (Dich2), and Dichotic two-item Location (or Ear) Identification (DLoc). The first task, Mono2, required participants to identify the order of two vowels presented monaurally to the test ear. The right ear was the test ear for all monaural measurements in this study, except for six older listeners who were tested using their left ear due to right ear thresholds exceeding the inclusion criteria. The second task, Mono4, presented a sequence of four vowels to the test ear. Two dichotic tasks were also completed. Dich2 was analogous to Mono2 with the exception that each of the two vowels was presented to a different ear, with the ear that was presented first randomized from trial to trial. DLoc used the same stimulus presentation as Dich2, except listeners were only required to identify the location (i.e., ear) that received the first stimulus. In this way, a direct comparison of performance between identification and location measurements could be made.
All auditory testing was completed in an IAC sound-attenuating booth with listeners seated in the sound booth either individually or in pairs. Each participant was seated comfortably in front of a touch-screen display (Elo Model 1915L). For all four tasks, the same vowel was never repeated twice in a row. The Mono4 task had the additional stipulation that each sequence must contain at least three of the four vowel stimuli. Thus, a single vowel could occur twice within a sequence, which was implemented to avoid the possibility of correctly guessing the identity of the fourth vowel based on the three preceding vowels. For the three vowel-identification tasks, responses were collected using columns consisting of four buttons labeled “pVt” on the screen, each column corresponding to the interval during which the stimulus was presented. Thus, for Mono2 and Dich2, there were two columns of four buttons displayed on the touch screen, and, for Mono4, there were four columns of four buttons displayed from left to right. Listeners were required to identify the correct vowel sequence exactly for the response to be judged correct. The location task, DLoc, only had two buttons (labeled “Right” and “Left”) for the listener to identify which ear was stimulated first. Orthographic feedback showing the correct sequence was provided on all trials.
The dependent variable measured was the stimulus onset asynchrony (SOA) between the presented vowels. The minimum SOA value was ⩾2 ms to ensure a sequential presentation for the stimuli. Given stimulus durations of 70 ms, SOA values less than 70 ms resulted in the stimuli overlapping in time. All tasks used the method of constant stimuli to measure the psychometric function relating percent-correct identification performance to SOA. For the three vowel-sequence identification tasks, threshold was defined as 50% correct, whereas 75% correct was defined as threshold for DLoc because chance performance was 50% for this ear-sequence task. Extensive piloting was completed to determine the appropriate starting SOA test intervals for young and older listeners in general. Due to the large variability of performance between older listeners, a single set of SOA values could not be used for all participants. Therefore, experimental testing was conducted in two phases. The first phase consisted of a preliminary wide-range estimate of SOA threshold (i.e., using a large step size, 25 ms), while the second phase consisted of narrow-range testing (i.e., using a smaller step size, 10 or 15 ms) to provide the actual SOA threshold estimates reported in the results. Table 1 lists the SOA test values used during initial wide-range testing across the four tasks for the young and older listeners. From this initial block of trials, the participant’s identification accuracy at each SOA value was fitted by a psychometric function (Weibull) and a preliminary threshold at 50% was estimated (75% for DLoc). Next, narrow-range testing for that same task was completed using an initial SOA that was 30 ms (40 ms for Dich2) less than the wide-range threshold estimate and a 10 ms (15 ms for Dich2) step size over six steps. This was designed to result in about three performance estimates below the targeted performance level and three estimates above this performance level.
Table 1.
Wide-range starting test values across the four experimental tasks. Values for older adult listeners are listed (values for young listeners are in parentheses if different).
| Mono2 | Mono4 | Dich2 | DLoc | |
|---|---|---|---|---|
| Starting | ||||
| SOA (ms) | 10 | 85 (35) | 45 | 100 (20) |
| No. of trials | 72 | 96 | 144 | 144 |
In the event that a threshold was not obtained by the wide-range task, a threshold was estimated based on visual inspection of the listener’s performance graph and the wide- range was rerun with a starting SOA 30 ms below the threshold estimated by visual inspection. Narrow-range estimates were only considered valid if the threshold could be estimated without extrapolation beyond the range of performance measured. If this was not the case, the results were considered invalid. In this case, the narrow-range was adjusted to include the new estimated threshold and the narrow-range measurements were repeated. In the end, each threshold estimate for each task was based on three valid narrow-range estimates for a total of 216 (Mono2), 288 (Mono4), or 432 (Dich2, DLoc) trials. Typically, all three narrow-range thresholds for a given task were obtained over the same SOA test range.
Breaks were always available to listeners between test blocks, were verbally offered after about 1 h of testing, and were prompted on screen for the two longer dichotic tasks midway through a test block to avoid possible fatigue. Test sessions were no longer than 2 h. Listeners returned for three to four testing sessions. A few listeners scheduled more sessions due to difficulty obtaining reliable threshold estimates to begin the task or a slower rate of completing the tasks.
Results
Data reliability
Across each of the four tasks, three threshold estimates were obtained. To test the reliability and stability of these measures, Pearson-r correlations across the three blocks for each of the four experimental tasks were completed separately for both age groups. All correlations were significant at p<0.01, thereby demonstrating similar performance across blocks, with one exception for the young group (block 1 vs block 3 of Dich2, r=0.33). As a test of the stability of the threshold estimates, eight repeated-measures general linear model analyses were completed, one for each dependent measure and each group. Results showed no significant main effect of block number for any dependent measure or group (p>0.01), thus demonstrating no learning effect across blocks. Based on these results, threshold estimates from all three blocks appear to be reliable and stable estimates of performance for both age groups. Therefore, the default calculation of final threshold estimates for each task and participant was the average of three (narrow-range) threshold estimates.
As a further check for within-subject outliers across blocks, the standard deviation was calculated across the three blocks for each listener. Any listener that had a standard deviation greater than three times the average three-block standard deviation for the respective age group was judged to have an unreliable threshold estimate. In these cases, the two closest threshold estimates were used to calculate the final threshold estimate. A few listeners did not have at least two threshold estimates that fell within three standard deviations, and were therefore treated as missing data (about 1 participant per group per task). Reliable data points having been identified, threshold estimates averaged across all three blocks (or two blocks, 6% of cases) were used in subsequent analyses.
Of particular interest in this study are not only the listeners who perform well enough on the experimental tasks to yield valid threshold estimates but also those who perform poorly or otherwise could not complete the experimental testing. Table 2 lists the number of participants unable to complete experimental testing for each task. Only six of these participants were unable to complete two tasks (typically the two dichotic tasks). Inspection of the data in Table 2 reveals that older adults were unable to complete the temporal-order identification tasks than younger adults, especially for Mono4. In order to include these individuals in subsequent analyses and not bias the data by excluding them, their performance was coded as extreme values (maximum recorded threshold plus one standard deviation) and medians were used for group analyses. Such nonparametric analyses are more resistant to violations of normality and allowed inclusion of all participants.
Table 2.
Number of participants unable to achieve a threshold for each experimental task.
| Mono2 | Mono4 | Dich2 | DLoc | |
|---|---|---|---|---|
| Young | 0 | 1 | 0 | 1 |
| Old | 1 | 21 | 6 | 6 |
Data analysis
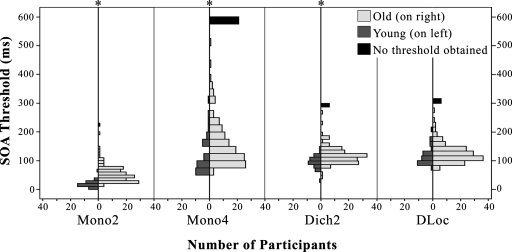
Table 3 displays the descriptive statistics for each group. In order to test the difference between groups for each experimental task, a Bonferroni-adjusted Mann–Whitney test was used for pairwise comparisons and demonstrated significant (p<0.001) between-group differences for all three vowel-sequence identification tasks, as shown in Fig. 1. However, no significant difference between age groups was achieved for the dichotic ear-sequence identification task (Bonferroni-adjusted p=0.032). A Wilcoxon Signed-Ranks Test was used to compare performance within a group across experimental tasks (see Table 4). For both age groups, SOA thresholds for Mono2 were significantly shorter than SOA thresholds for the other three tasks. For older adults, SOA thresholds for Mono4 differed significantly from SOA thresholds for both dichotic conditions. This was not the case, however, for younger adults. The performance of older adults did not differ significantly between the two dichotic tasks, whereas the performance of the young adults did differ significantly between these two tasks.
Table 3.
Descriptive statistics for experiment 1; IQ range=interquartile range.
| Older adult listeners | Young adult listeners | |||||
|---|---|---|---|---|---|---|
| N | Median | IQ range | N | Median | IQ range | |
| Mono2 | 150 | 49.1 | 35.4 | 35 | 15.8 | 15.7 |
| Mono4 | 129 | 154.1 | 142.5 | 33 | 90.3 | 90.54 |
| Dich2 | 143 | 116.0 | 37.4 | 34 | 96.5 | 28.8 |
| DLoc | 142 | 121.2 | 41.6 | 32 | 109.1 | 36.3 |
Figure 1.
Young and older adult performance distributions for the temporal-order tasks of experiment 1, *p<0.001.
Table 4.
Wilcoxon Signed-Ranks Test for comparison between tasks, Z scores listed.
| Group | Mono2 | Mono4 | Dich2 | |
|---|---|---|---|---|
| Mono4 | Old | −10.3a | ||
| Young | −5.1a | |||
| Dich2 | Old | −10.3a | −7.1a | |
| Young | −5.1a | −1.6 | ||
| DLoc | Old | −10.3a | −5.4a | −1.4 |
| Young | −5.1a | −0.9 | −4.0a |
p<0.001.
Discussion and extension of results
The significant intertask differences demonstrate that these four temporal-order identification tasks tap into different processing demands. Clearly, the shortest SOAs were observed for both age groups in the Mono2 stimulus condition, with threshold SOA values being about 80–100 ms shorter for this condition than the others. Apparently, it is easier for both age groups to perform this task when the vowel sequence is presented to the same ear and is comprised of only two stimuli. Keeping the stimuli in one ear but doubling the number of items in the sequence (Mono4) resulted in sizable increases in SOA thresholds (i.e., greater than a factor of 3) relative to the SOA thresholds for the Mono2 condition. The differences in chance performance between Mono2 (8%) and Mono4 (1%) do result in a difference in task difficulty. In order to equate d′ between the two tasks, new thresholds were calculated for the Mono4 task at a conservative 25% correct using conversion tables from Hacker and Ratcliff (1979). Significant differences remained for the Mono4 condition, both between young and older listeners (p<0.001) and between Mono2 and Mono4 tasks (p<0.001). However, Mono4 d′ adjusted thresholds were significantly better than Dich2 thresholds, further highlighting the additional demands of dichotic processing.
If limitations in vowel identification influenced performance on the monaural tasks (rather than some other factors related to temporal-order judgment), then, the longer sequence in Mono4 would compound this factor, thereby reducing the probability of correctly selecting the vowel sequence. However, while younger listeners did perform better overall when identifying vowels in isolation than older listeners (p<0.05), vowel-identification performance in isolation did not significantly correlate with performance on either the Mono2 (r=−0.05) or Mono4 (r=−0.07) tasks. This indicates that the sizable increases in threshold for Mono4 were not due to vowel-identification limitations, but more likely due to task (e.g., sequential processing) and∕or cognitive (e.g., memory) factors.
Considering dichotic presentations, separating the stimuli by presenting one to each ear resulted in considerably longer SOA thresholds relative to Mono2. No difference between dichotic tasks was found for older listeners. This provides further evidence that vowel identification was not a limiting factor for vowel-sequence tasks as no difference in performance was obtained for older listeners on the ear-identification task which did not involve vowel identification. Instead, it is likely that general temporal-order abilities limited performance on both vowel- and ear-identification tasks. However, one way of noting the processing demands of these tasks is by comparison to young performance. In agreement with previous studies of temporal-order for tones (Szymaszek et al., 2006; Kołodziejczyk and Szelag, 2008), no age differences were obtained for the dichotic ear-sequence identification task (DLoc), indicating that the processing requirements that underlie this task may be preserved with age. It may be that the simpler task requirements for the ear-sequence identification (DLoc) measures, as compared to the parallel vowel-sequence identification measures (Dich2), resulted in similar performance between groups, as it has been consistently found that age group differences in temporal processing often become apparent as the complexity of the task increases (Fitzgibbons and Gordon-Salant, 1995, 1998). The DLoc task is simpler than the Dich2 task, in part, because there is no need to identify the phonological composition of the stimuli, simply to determine their location or laterality. In addition, the DLoc task is simpler than the Dich2 task because there were only two response alternatives for DLoc (right vs left), whereas Dich2 measures were comprised of 12 possible response sequences. However, while the DLoc task complexity is less, the threshold SOA values measured indicate that the demands of the task were significantly greater for young adults. At least for younger listeners, phonetic labeling was easier than remembering to which ear the stimulus had been presented first. Vowel identification appears to have actually aided performance for these young listeners.
Regarding group differences, older listeners perform significantly poorer across all three vowel-sequence identification tasks. Given that all listeners had normal or near-normal hearing within the frequency range of the stimuli (below 1800 Hz) and that a high presentation level (83 dB SPL) was used, group differences cannot be accounted for in terms of differences in audibility. Indeed, the PTA did not correlate significantly with vowel identification (r=0.11), suggesting that simple audibility did not influence identification performance. Across all tasks, older listeners demonstrated large variability, yet many older listeners performed within the performance range of the young adults. This is consistent with other age group differences found for other measures of auditory temporal processing (Moore et al., 1992; Schneider et al., 1994; Snell, 1997; Snell and Frisina, 2000; Humes et al., 2009).
As noted, the large differences in SOA thresholds for both young and older listeners between the two-item monaural and two-item dichotic tasks were striking. This difference occurs even though the task requirements were identical: the only difference was presenting the sequence to one ear vs two ears, one stimulus per ear. Several possible factors could underlie this difference, including the obvious differences in physiological processing (i.e., central auditory processing) as well as cognitive explanations. First, Shrivastav et al. (2008) found that both presentation rate (i.e., SOA) and stimulus duration influence the performance of older listeners, with shorter tone durations leading to poorer discrimination performance. Given that experiment 1 only used one stimulus duration, it is not clear whether these performance patterns would persist with a different stimulus duration. Therefore, experiment 2 compared performance on these two-item identification tasks with 70 and 40 ms stimuli [40 ms stimuli were also used by Humes et al. (2010)]. Second, task performance differences may have been related to the relative demands due to divided-attention. Experiment 2 also examined how this factor might mediate monaural performance with 40 ms vowels. Finally, experiment 2 also examined test-retest learning, as it is not clear whether the performance observed in experiment 1 represents a maximal ability or an ability that can be enhanced with additional exposure to the stimuli and temporal-order tasks.
EXPERIMENT 2: ADDITIONAL FACTORS FOR OLDER LISTENERS
Experiment 2 used subsets of older listeners to investigate three additional phenomena: stimulus duration, stimulus-ear uncertainty (randomization), and test-retest learning. It has been reported that stimulus duration can have an impact on monaural temporal-order discrimination, most substantially for older adults (Shrivastav et al., 2008). As stimulus duration is an important dimension that can influence temporal-order performance, it is not clear whether the performance patterns observed in experiment 1 will persist for other stimulus durations. Therefore, experiment 2 compared the difference in performance for vowel-sequence identification tasks comprised of 40 ms or 70 ms stimuli. It is possible that dichotic thresholds would be more influenced by duration than monaural threshold due to the longer processing time required.
Second, experiment 1 demonstrated a large difference in SOAs between monaural and dichotic two-item sequences for both young and older listeners. The second purpose of experiment 2 was to explore one possible processing difference between the Mono2 and Dich2 tasks: divided attention. In the dichotic testing, listeners were required to switch attention between the two presentation ears, while listeners only needed to attend to one ear in the monaural task. That is, for the Dich2 task, the ear receiving the first stimulus of the two-item sequence varied randomly from trial-to-trial such that the listener did not know where to listen or where the sequence would be initiated. This was not the case for the Mono2 task. To examine the impact of this possible demand on attention, this experiment randomized the ear to which the two monaural stimuli were presented, such that on any given trial the two vowels could both be presented to either the right or left ear. This helped to match the level of location uncertainty across trials between the monaural and dichotic tasks.
Third, listeners in this study completed a large number of tasks throughout the course of the study in multiple modalities (55 h of testing). The conditions presented in this final experiment occurred after significant exposure to the stimulus materials in experiment 1, as well as completion of eight different temporal-masking conditions using the same 40 ms vowel stimuli (reported in Humes et al., 2010). All participants completed the same tasks and on average, participants completed about 12 h of testing with these vowel stimuli prior to participating in experiment 2. Thus, participants had considerable opportunity to learn stimulus and task-specific information prior to the measures completed in experiment 2. Perhaps some of the difference observed between age groups in experiment 1 reflects age-related differences in learning the task or the stimuli. The question explicitly tested here examined whether learning did occur for older adults. It may be that temporal-order processing abilities in older listeners are constrained by factors (e.g., physiological) not susceptible to learning.
Listeners
Two groups of older adult listeners from experiment 1 volunteered to complete a portion of the conditions from experiment 2. A subset (Group A) of 43 older listeners, 24 female and 19 male, ranging in age from 60–87 years (mean=70 years) from experiment 1 completed the measures investigating stimulus duration and test-retest learning. A second subset (Group B) of 24 older listeners, 16 females and 8 males, ranging in age from 62–83 years (mean=70 years) from experiment 1 completed the measures investigating the effect of ear randomization.
Methods
Five tasks were completed. Two tasks used the 70 ms vowels and were the same as those used in experiment 1: monaural two-item identification (Mono2_70) and dichotic two-item identification (Dich2_70). An additional three tasks used 40 ms versions of the stimuli used in experiment 1 excised from the middle portion of the 70 ms vowels. These three tasks were monaural two-item identification (Mono2_40), dichotic two-item identification (Dich2_40), and monaural two-item identification with randomization of the stimulus presentation ear across trials (M2Rand_40). Procedures were identical to those outlined in experiment 1 for the monaural and dichotic tasks. M2Rand_40 consisted of 144 trials, allowing for 72 trials to be presented to each ear. Group A completed shortened stimulus duration tasks Mono2_40 and Dich2_40 and the retest Mono2_70 and Dich2_70 tasks. Monaural versions were always tested prior to dichotic versions. Testing order of the two stimulus durations was counterbalanced across listeners. Group B completed the three 40 ms tasks (Mono2_40, Dich2_40, and M2Rand_40) to examine ear randomization as a possible factor in the larger dichotic thresholds.
Prior to completing the tasks in experiment 2, all older participants in both groups completed an additional screening task for the identification of these shortened vowels in isolation. Given the targeted threshold performance level of 50% for these two tasks, all participants were required to complete this screening with at least 60% accuracy in each ear to continue with experiment 2. Identification accuracy during screening actually ranged from 77.5%–100% with a mean of 95.2% (SD=6.2%) for the right ear and ranged from 62.5%–100% with a mean of 94.6% (SD=7.8%) for the left ear. Thus, most listeners (77%) identified the short 40 ms vowels in isolation with at least 90% accuracy, just as they had the longer 70 ms vowels used in experiment 1. Task demonstrations were provided prior to presenting any new experimental task and were analogous to those in experiment 1.
Results and discussion
The same data-reduction procedures as experiment 1 were used to obtain three-block averages to be used for an individual listener’s SOA threshold estimate. Four older participants were unable to complete one or two tasks. Mono2_70 and Dich2_40 each had two missing values, and Mono2_40 and Dich2_70 each had one missing value. The only participant in this data set who had an ear with hearing thresholds beyond the audiometric criteria for this study did not complete either dichotic test. Missing data for these four participants were entered as extreme value outliers. As a result, medians were computed for group values and nonparametric tests were again used for analysis. Pursing correlations between performance on each task with mid-frequency hearing thresholds (p>0.05) and vowel identification in isolation (p>0.05) were not significant, suggesting that individual differences in performance are not attributable to simple audibility or vowel-identification abilities.
Stimulus duration
Group A completed the tasks for the investigation of stimulus duration. A paired-sample Kruskal Wallis test was used to examine the effect of test order between stimulus durations (e.g., M2_70 first vs M2_40 first). Results did not differ significantly across the task orders (p>0.05). Therefore, data for counterbalanced conditions were pooled in subsequent analyses for stimulus duration.
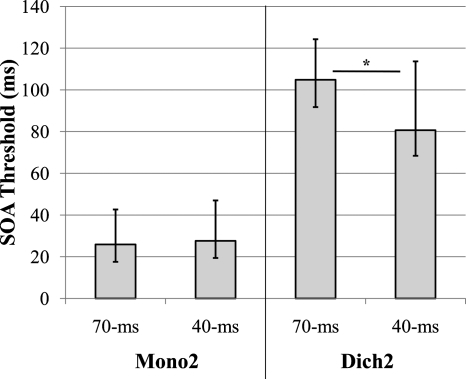
Figure 2 shows the median SOA values for the four stimulus conditions in this experiment. Comparisons between analogous tasks using different stimulus durations were tested using Wilcoxon Signed-Ranks tests. Results demonstrated no significant difference between the SOAs for the Mono2_70 (median=25.9 ms, IQ range=25.1 ms) and Mono2_40 (median=27.6 ms, IQ range=28.3 ms) tasks completed in experiment 2 (p>0.05). However, a significant effect of stimulus duration (p<0.001) was obtained for the dichotic task comparison of Dich2_70 (median=104.8 ms, IQ range=32.5 ms) and Dich2_40 (median=80.7 ms, IQ range=45.9 ms). This indicates that while no difference in SOA occurred between stimulus durations for monaural conditions, shorter stimulus durations actually lead to better SOA values for the older listeners in dichotic testing. However, while SOA thresholds decreased substantially for the shorter stimulus duration (40 ms) in the dichotic task, another measure, the interstimulus interval (ISI), was more similar (median ISI for Dich2_70 was 34.5 ms and for Dich2_40 was 40.4 ms). Nonetheless, all listeners had longer ISIs for the 40 ms stimuli (median difference=7.0 ms, IQ range=14.1 ms), which was significantly larger by means of a Wilcoxon Signed-Ranks test (Z=−3.7, p<0.001). Thus, using ISI as an index, it may be that 40 ms vowels are more difficult, requiring a larger temporal separation between the stimuli. Two older listeners had thresholds that led to a temporal overlap of the two 70 ms vowels (and therefore, no ISI or temporal separation), indicating that a silent interval is not required for successful performance on the dichotic task using 70 ms vowels. However, in general, it appears that additional processing for dichotic sequences occurs after the stimulus offset and that the processing requirements are somewhat greater for the shorter 40 ms stimuli.
Figure 2.
Effect of stimulus duration; SOA thresholds from older adults in experiment 2, error bars=interquartile range, *p<0.001.
Ear uncertainty
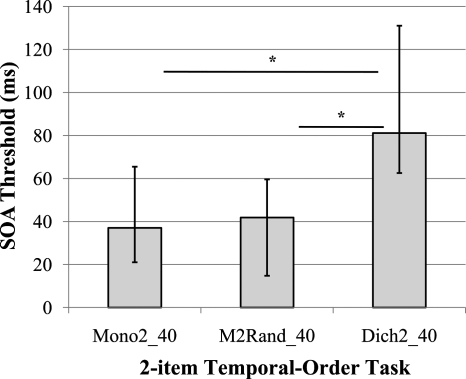
Group B completed these measures to determine if ear uncertainty resulted in the large differences between Mono2 and Dich2 tasks observed. Median SOA values for these 24 older adults appear in Fig. 3. Wilcoxon Signed-Ranks tests demonstrated significant (Bonferroni-adjusted p<0.001) differences between Dich2_40 (median=81.1 ms, IQ range=68.5 ms) and each monaural task (p<0.001), again demonstrating large differences between monaural and dichotic presentations, now for 40 ms stimuli. However, no differences were found between Mono2_40 (median=37.0 ms, IQ range=44.5 ms) and M2Rand_40 (median=41.9 ms, IQ range=44.8 ms), indicating that differences between the monaural and dichotic tasks for 40 ms vowels was not a result of attentional demands related to stimulus-ear uncertainty from trial-to-trial. Rather, differences appear to be related to inherent physiological (i.e., central auditory) differences between monaural and dichotic processing involved in the comparison of stimuli across ears.
Figure 3.
Effect of ear randomization; SOA thresholds from older adults in experiment 2, error bars=interquartile range, *p<0.001.
Test-retest learning
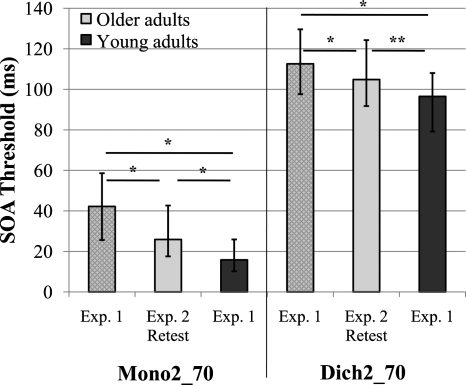
Group A also completed the retest 70 ms versions of experiment 1 to investigate if performance improved for older listeners with additional exposure. Figure 4 illustrates the effect of the repeated exposures to stimuli and tasks between experiments 1 and 2. Comparisons were again tested using Wilcoxon Signed-Ranks tests. The comparison of SOAs between the first and second completion of Mono2_70 indicated a significant 39% improvement (p<0.001). The SOA values for Dich2_70 also improved significantly following longer periods of exposure to the stimuli and tasks (p<0.001), but only with a 7% improvement. Thus, the older listeners were able to improve their performance on these vowel temporal-order identification tasks with increased exposure to the stimuli. Of particular interest is whether older listeners were able to approach the performance of young listeners after such exposures or whether older listener performance is limited by certain factors (i.e., physiological) and represents a maximal ability not susceptible to training. Therefore, comparisons between the SOAs from Mono2_70 and Dich2_70 conditions for older listeners obtained after repeated exposures in experiment 2 were compared to the results of the young listeners for the same tasks but from experiment 1 (Fig. 4). Results of the between groups Mann–Whitney tests still indicated a significant age group difference comparing the second testing of older listeners to the first testing of the young listeners for the two-item monaural task (Z=−4.02, p<0.001) and the two-item dichotic task (Z=−2.30, p=0.022). Thus, although the additional exposures to the brief vowel stimuli and the procedures in this study narrowed the gap (literally) in SOAs between the older adults and the young adults on these two measures of temporal-order identification, it was not enough to eliminate significant group differences. We are investigating whether young adults also improve over time, and currently partial results are available. Data from 42 young listeners indicate significant improvements with additional exposure (Bonferroni-adjusted p<0.025). Median SOAs went from 13.9 and 97.4 ms for the Mono2 and Dich2 tasks, respectively, to 7.3 and 85.7 ms following repeated exposures to the stimuli and tasks. Thus, it appears that the performance of young adults may also improve with exposure to a similar degree as older listeners (about 10–20 ms improvement across tasks and age groups).
Figure 4.
Effect of stimulus exposure: Older adult performance for experiment 1 (before exposure, cross-hatched light gray) and experiment 2 (after exposure, solid light gray) in comparison to young performance for experiment 1 (before exposure, solid dark gray). Error bars=interquartile range, *p<0.001, **p<0.025.
GENERAL DISCUSSION
Group differences
Overall, the results from this set of experiments demonstrate that older listeners have a significant difficulty identifying the order of rapid temporal events relative to young listeners. This difficulty becomes apparent and more pronounced as the cognitive complexity of the task increases, such as from identifying two-item sequences vs four-item sequences. One significant finding from experiment 1 was the substantially elevated SOA thresholds in the two-item dichotic tasks compared to the two-item monaural task. Differences between dichotic and diotic processing have also been noted previously (Szymaszek et al., 2006). Results from randomizing the presentation ear for monaural sequences in experiment 2 demonstrated that this difference could not be accounted for by increased demands on divided-attention resources, at least for 40 ms stimuli. Indeed, Tun et al. (1992) noted a speech rate by age interaction for recall of spoken passages that was independent of the attention processing demands of the task. Instead, performance differences appear related to the physiological processing of dichotic vs monaural sequences, where dichotic presentations require integration of temporal events across hemispheres.
Experiment 2 also highlighted two other substantial findings related to temporal-order identification for older listeners. These results refer to the effects of stimulus duration and stimulus exposure on the performance of older listeners. With regard to exposure, the initial measurements in experiment 1 do not represent best performance—older listeners were able to improve temporal-order identification performance with extended exposure to stimulus information. This is contradictory to previous results for the discrimination of tonal sequences that have suggested no learning effect for older listeners (Trainor and Trehub, 1989). However, Szymaszek et al. (2006) reported an effect of practice on temporal-order identification thresholds after one session. Although older listeners did improve performance on our tasks, they still did not reach the monaural performance of the young listeners from experiment 1. Comparison to a second set of young listeners with equivalent additional exposure demonstrates approximately equal improvements in SOA for both age groups. This either suggests that older listeners would require additional and more focused training to achieve performance thresholds near that of younger listeners or that older performance is somehow limited by a factor that maintains age group differences. It should also be noted that considerable care was taken in establishing the SOA thresholds in experiment 1, including the use of several demonstration trials to familiarize the listeners to the stimuli and tasks, use of initial wide-range constant-stimuli trial blocks that essentially served as additional practice, and then basing each threshold estimate (for most listeners) on three blocks of trials that did not significantly vary from each other. Despite these efforts to obtain stable performance estimates, older listeners clearly continued to improve their performance with repeated exposures to these brief vowel stimuli (i.e., 12 h of testing). It also is important to note that this additional exposure to the stimuli did not involve substantial additional exposure to temporal-order identification. Rather, as noted previously, extended exposure to these stimuli was in the form of identification of one of the four target vowels serving as the signal in various temporal-masking tasks. Thus, any learning that has taken place from repeated exposures to the stimuli is more of an implicit nature rather than explicit and certainly would not be considered to be any form of “training” temporal-order performance. For now, we can conclude that older adults do improve their thresholds with repeated exposures to the stimuli but not enough to eliminate differences in median SOA thresholds between the two age groups. Therefore, older adult abilities remain plastic and susceptible to learning, but significant age-related factors remain that inhibit performance.
The effect of stimulus duration also revealed an interesting result. Shorter stimulus durations did not adversely affect the performance of older adults. While resulting in no difference for monaural presentations (Fig. 2), shorter durations actually resulted in smaller SOA thresholds for dichotic sequences. On the surface this appears counterintuitive, as elevated thresholds for duration discrimination with shorter reference durations are well documented among older listeners (Fitzgibbons and Gordon-Salant, 1995) at least for tonal signals. Cullinan et al. (1977) presented diotic sequences of four vowels continuously to young listeners and found that performance on temporal-order identification improved as the stimulus length increased, although this was also confounded with slowing the rate of presentation. In contrast, for monaural presentations of three tone sequences, Fitzgibbons et al. (2006) reported an effect of rate with no contribution of stimulus duration. Shrivastav et al. (2008) found similar results for young normal-hearing participants listening to two- tone sequences with flanker tones using uniform duration and rate. Older listeners only demonstrated an effect of duration for stimuli less than 40 ms in that study. This is, in fact, what we found for our two-item monaural task: an effect of rate with no effect of duration (at least for the 70 and 40 ms vowels tested here).
However, it appears that stimulus duration does influence temporal-order identification performance for dichotic presentations. One possible reason for this advantage could be related to the larger silent interval between the offset of one stimulus and the onset of the next that shorter stimuli provide. This silent interval may be beneficial by providing more time for recovery (less masking) or processing; thereby, actually leading to shorter (better) SOA thresholds when shorter duration stimuli were used. Indeed, while SOA values improved, this silent duration, the ISI, significantly increased for shorter stimulus durations. It is important to note that the results for shorter stimulus durations were obtained only after significant exposure to the stimuli.
Individual differences among older listeners
This study was designed with a large sample size to explore individual differences among the older listeners, along with exploring a variety of task- and stimulus-specific variables that influence the temporal-order performance of older listeners in these experiments. First, correlations among the temporal-order tasks of experiment 1 were examined to determine if performance on one task was related to a listener’s performance on another task. Only older listeners with threshold estimates (N and correlations shown in Table 5) were included in these correlations. Results indicate significant Pearson correlations among all measures (p<0.01), with the highest agreement between the two-item identification measures (Mono2, Dich2; r=0.59).
Table 5.
Experiment 1 task correlations for data from older adults only, excluding participants who were unable to achieve a threshold value (N in parentheses).
| Mono2 | Mono4 | Dich2 | |
|---|---|---|---|
| Mono4 | 0.46a (128) | ||
| Dich2 | 0.59a (142) | 0.28a (121) | |
| DLoc | 0.30a (141) | 0.25a (120) | 0.34a (140) |
p<.01.
In order to determine if age, pure-tone audiometry, or cognitive measures were able to predict an older listener’s temporal-order identification performance, a second set of analyses were completed. Redundancy among the sets of audiometric and cognitive measures obtained for all participants was first reduced using principal component (PC) analysis with varimax rotation. Results of this analysis extracted three orthogonal PCs out of the 18 audiometric measures (Table 6), corresponding to low-frequency hearing thresholds in the right ear, the left ear, and bilateral high-frequency hearing thresholds. Four orthogonal PCs were extracted for the 15 cognitive measures (Table 7) based on the raw scores from the WAIS-III and corresponded roughly to verbal, performance, free recall∕pairing, and symbol-search measures. These seven audiometric and cognitive components were entered, along with age, as possible predictors in four multiple linear-regression analyses, one for each of the four temporal-order tasks [Table 8(a)]. Results indicate that cognitive measures of the WAIS, component 1 (verbal) and component 2 (performance), serve as the primary predictors of variance across the four experimental tasks. Therefore, cognitive status (i.e., the level of an individual’s cognitive skills) appears to account for between 8% and 29% of the variance in performance among the older participants. Additional variance was not accounted for by either audiometric status or age.
Table 6.
Audiogram principal component analysis: % variance accounted for in parentheses. Weights <0.4 removed for simplicity. R=right ear, L=left ear.
| Frequency (kHz) | Bilateral high-frequency (33.9%) | Right low-frequency (23.2%) | Left low-frequency (20.7%) |
|---|---|---|---|
| 0.25 R | 0.82 | ||
| 0.5 R | 0.89 | ||
| 1 R | 0.84 | ||
| 1.5 R | 0.77 | ||
| 2 R | 0.51 | 0.66 | |
| 3 R | 0.80 | 0.43 | |
| 4 R | 0.86 | ||
| 6 R | 0.84 | ||
| 8 R | |||
| 0.25 L | 0.81 | ||
| 0.5 L | 0.88 | ||
| 1 L | 0.79 | ||
| 1.5 L | 0.71 | ||
| 2 L | 0.58 | 0.53 | |
| 3 L | 0.81 | ||
| 4 L | 0.88 | ||
| 6 L | 0.87 | ||
| 8 L | 0.75 |
Table 7.
WAIS-III principal component analysis: % variance accounted for in parentheses. Weights <0.4 removed for simplicity.
| Variable | WAIS1 (25.5%) | WAIS2 (19.9%) | WAIS3 (13.8%) | WAIS4 (7.0%) |
|---|---|---|---|---|
| Information | 0.83 | |||
| Vocabulary | 0.80 | |||
| Similarities | 0.75 | |||
| Comprehension | 0.73 | |||
| Arithmetic | 0.61 | |||
| Letter-number sequencing | 0.51 | |||
| Picture arrangement | 0.52 | 0.54 | ||
| Matrix reasoning | 0.41 | 0.69 | ||
| Digit span | 0.46 | |||
| Picture completion | 0.66 | |||
| Block design | 0.66 | |||
| Digit-symbol coding | 0.75 | |||
| Pairing | 0.99 | |||
| Free recall | 0.99 | |||
| Symbol search | 0.95 |
Table 8.
Linear regression: (a) age, 4 WAIS-PCs, 3 audiogram-PCs as predictors; (b) with Mono2 also included as a predictor.
| Task variable | % total variance | Predictor variable | β coefficient | F (df) | p |
|---|---|---|---|---|---|
| (a) Mono2 | 14.5 | WAIS2 | −0.38 | 29.877 (2, 147) | <0.001 |
| 14.4 | WAIS1 | −0.38 | |||
| Mono4 | 3.4 | WAIS1 | −0.184 | 4.44 (1, 127) | 0.037 |
| Dich2 | 6.1 | WAIS1 | −0.247 | 9.674 (2, 140) | <0.001 |
| 6.0 | WAIS2 | −0.246 | |||
| DLoc | 8.3 | WAIS2 | −0.288 | 12.682 (1, 140) | 0.001 |
| (b) Mono4 | 20.9 | Mono2 | 0.457 | 33.224 (1, 126) | <0.001 |
| Dich2 | 34.3 | Mono2 | 0.586 | 73.207 (1, 140) | <0.001 |
| DLoc | 9.0 | Mono2 | 0.224 | 9.928 (2, 138) | <0.001 |
| 3.6 | WAIS2 | −0.203 | |||
Of interest is how much variance can be accounted for by a basic measure of temporal-order identification ability (Mono2). Running the regression analyses a second time to include Mono2 as a possible predictor [Table 8(b)] resulted in Mono2 becoming the primary predictor variable across all tasks, accounting for over 20% and 34% of variance for Mono4 and Dich2 tasks, with cognitive measures only entering into consideration for DLoc. This suggests that Mono4 and Dich2 tasks are not predicted by other WAIS-III cognitive measures not already accounted for by Mono2.
Overall, tests of individual differences among these older listeners suggest that cognitive measures are related to an older listener’s temporal-order identification ability for vowels, while audiometric status does not serve as a predictor. This latter finding is not surprising given that we low-pass filtered the vowel stimuli at 1800 Hz and used a relatively high presentation level to minimize the role of audibility. Thus, it appears plausible that general age-related cognitive declines may influence temporal processing abilities for vowels.
CONCLUSIONS
Four measures of temporal-order processing using speech stimuli were obtained among a large group of older adult listeners and compared to the performance of young listeners. As expected, results indicated age group differences for three measures of temporal-order identification with older listeners performing more poorly and with noticeably greater variability. However, the older group maintained a large overlap in performance with the young listeners. Increasing the length of the stimulus sequence from two to four vowels significantly degraded performance, possibly resulting from additional cognitive demands (i.e., memory). A large difference in performance between monaural and dichotic presentations occurred for both age groups and stimulus durations. This difference was not related to attentional demands created by stimulus presentation uncertainty across ears (at least for 40 ms vowels) and therefore appears to be specific to processing requirements. The manipulation of stimulus duration demonstrated that older listeners with substantial exposure to the stimuli had better SOA values with shorter stimuli for dichotic presentations; however, all listeners required a longer silent interval between stimuli to complete this task with shorter vowels, possibly indicating a need for more processing time. No difference in thresholds between vowel durations was obtained for monaural presentations. Older listeners also demonstrated improvement in temporal-order identification with significant additional exposure to the test stimuli, yet performance still did not match the performance of young listeners.
A major goal of this research project is to determine causes for individual differences in performance among the elderly, as large variability in performance among older listeners has been consistently noted in the literature. One notable result showed that verbal and performance measures of cognition predicted some of the variance associated with each of the four temporal-order vowel-identification measures. It should be noted that the stimuli here were speech, and perhaps verbal cognitive measures are more strongly associated with the temporal-order judgment of these vowels than might be found for analogous nonspeech stimuli. Nevertheless, the present results demonstrated that cognitive measures were able to account for some of the differences in temporal processing among older listeners, while no contributions of audibility or age were found among this group of older listeners.
ACKNOWLEDGMENTS
The authors would like to thank Thomas A. Busey and James C. Craig for their significant contributions to this project. They would also like to thank Dana Kinney as well as several graduate research assistants involved with this project. This work was supported, in part, by NIA Grant No. R01 AG022334 and by NIH-NIDCD Training Grant No. T32-DC00012.
Portions of the data were presented at the 153rd, 155th, and 157th Meetings of the Acoustical Society of America (J. Acoust. Soc. Am., 121, 3188; 123, 3716; 125, 2722) and at the 2007 Aging and Speech Communication: An International and Interdisciplinary Research Conference, Bloomington, IN.
References
- American National Standards Institute (2004). Specifications for audiometers (ANSI S3.6-2004) (ANSI, New York).
- Baltes, P. B., and Lindenberger, U. (1997). “Emergence of a powerful connection between sensory and cognitive functions across the adult lifespan: A new window to the study of cognitive aging?,” Psychol. Aging 12, 12–21. 10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- Cullinan, W. L., Erdos, E., Schaefer, R., and Tekieli, M. E. (1977). “Perception of temporal order of vowels and consonant-vowel syllables,” J. Speech Hear. Res. 20, 742–751. [DOI] [PubMed] [Google Scholar]
- Dorman, M. F., Cutting, J. E., and Raphael, L. (1975). “Perception of temporal order in vowel sequences with and without formant transitions,” J. Exp. Psychol. Hum. Percept. Perform. 1, 121–129. 10.1037/0096-1523.1.2.121 [DOI] [PubMed] [Google Scholar]
- Dubno, J. R., and Schaefer, A. B. (1992). “Comparison of frequency selectivity and consonant recognition among hearing-impaired and masked normal-hearing listeners,” J. Acoust. Soc. Am. 91, 2110–2121. 10.1121/1.403697 [DOI] [PubMed] [Google Scholar]
- Dubno, J. R., and Schaefer, A. B. (1995). “Frequency selectivity and consonant recognition for hearing-impaired and normal-hearing listeners with equivalent masked thresholds,” J. Acoust. Soc. Am. 97, 1165–1174. 10.1121/1.413057 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons, P. J., Gordon-Salant, S., and Friedman, S. (2006). “Effects of age and sequence presentation rate on temporal order recognition,” J. Acoust. Soc. Am. 120, 991–999. 10.1121/1.2214463 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons, P. J., and Gordon-Salant, S. (1995). “Age effects on duration discrimination with simple and complex stimuli,” J. Acoust. Soc. Am. 98, 3140–3145. 10.1121/1.413803 [DOI] [PubMed] [Google Scholar]
- Fitzgibbons, P. J., and Gordon-Salant, S. (1996). “Auditory temporal processing in elderly listeners,” J. Am. Acad. Audiol. 7, 183–189. [PubMed] [Google Scholar]
- Fitzgibbons, P. J., and Gordon-Salant, S. (1998). “Auditory temporal order perception in younger and older adults,” J. Speech Lang. Hear. Res. 41, 1052–1062. [DOI] [PubMed] [Google Scholar]
- Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state: A practical method for grading the cognitive status of patients for the clinician,” J. Psychiatr. Res. 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gordon-Salant, S., and Fitzgibbons, P. J. (1993). “Temporal factors and speech recognition performance in young and elderly listeners,” J. Speech Hear. Res. 36, 1276–1285. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant, S., and Fitzgibbons, P. J. (1999). “Profile of auditory temporal processing in older listeners,” J. Speech Hear. Res. 42, 300–311. [DOI] [PubMed] [Google Scholar]
- Hacker, M. J., and Ratcliff, R. (1979). “A revised table of d′ for M-alternative forced choice,” Percept. Psychophys. 26, 168–170. [Google Scholar]
- Hofer, S. M., Berg, S., and Era, P. (2003). “Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning,” Psychol. Aging 18, 285–305. 10.1037/0882-7974.18.2.285 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. (1996). “Speech understanding in the elderly,” J. Am. Acad. Audiol. 7, 161–167. [PubMed] [Google Scholar]
- Humes, L. E. (2002). “Factors underlying the speech-recognition performance of elderly hearing-aid wearers,” J. Acoust. Soc. Am. 112, 1112–1132. 10.1121/1.1499132 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. (2005). “Do ‘auditory processing’ tests measure auditory processing in the elderly?,” Ear Hear. 26, 109–119. 10.1097/00003446-200504000-00001 [DOI] [PubMed] [Google Scholar]
- Humes, L. E. (2007). “The contributions of audibility and cognitive factors to the benefit provided by amplified speech to older adults,” J. Am. Acad. Audiol. 18, 590–603. 10.3766/jaaa.18.7.6 [DOI] [PubMed] [Google Scholar]
- Humes, L. E., Burk, M. H., Coughlin, M. P., Busey, T. A., and Strauser, L. E. (2007). “Auditory speech recognition and visual text recognition in younger and older adults: Similarities and differences between modalities and the effects of presentation rate,” J. Speech Lang. Hear. Res. 50, 283–303. 10.1044/1092-4388(2007/021) [DOI] [PubMed] [Google Scholar]
- Humes, L. E., Busey, T. A., Craig, J. C., and Kewley-Port, D. (2009). “The effects of age on sensory thresholds and temporal gap detection in hearing, vision, and touch,” Percept. Psychophys. 71, 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E., and Christopherson, L. (1991). “Speech-identification difficulties of the hearing-impaired elderly: The contributions of auditory-processing deficits,” J. Speech Hear. Res. 34, 686–693. [DOI] [PubMed] [Google Scholar]
- Humes, L. E., Kewley-Port, D., Fogerty, D., and Kinney, D. (2010). “Measures of hearing threshold and temporal processing across the adult lifespan,” Hear. Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara, H., Masuda-Kastuse, I., and Cheveigne, A. (1999). “Restructuring speech representations using a pitch-adaptive time-frequency smoothing and an instantaneous frequency-based F0 extraction: Possible role of a repetitive structure in sounds,” Speech Commun. 27, 187–207. 10.1016/S0167-6393(98)00085-5 [DOI] [Google Scholar]
- Kołodziejczyk, I., and Szelag, E. (2008). “Auditory perception of temporal order in centenarians in comparison with young and elderly listeners,” Acta Neurobiol. Exp. (Warsz) 68, 373–381. [DOI] [PubMed] [Google Scholar]
- Lewandowska, M., Bekisz, M., Szymaszek, A., Wrobel, A., and Szelag, E. (2008). “Towards electrophysiological correlates of auditory perception of temporal order,” Neurosci. Lett. 437, 139–143. 10.1016/j.neulet.2008.03.085 [DOI] [PubMed] [Google Scholar]
- Lindenberger, U., and Baltes, P. B. (1994). “Sensory functioning and intelligence in old age: A strong connection,” Psychol. Aging 9, 339–355. 10.1037/0882-7974.9.3.339 [DOI] [PubMed] [Google Scholar]
- Moore, B. C. J., Peters, R. W., and Glasberg, B. R. (1992). “Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss,” J. Acoust. Soc. Am. 92, 1923–1932. 10.1121/1.405240 [DOI] [PubMed] [Google Scholar]
- Park, D. C., Polk, T. A., Mikels, J. A., Taylor, S. F., and Marshuetz, C. (2001). “Cerebral aging: Integration of brain and behavioral models of cognitive function,” Dialogues Clin. Neurosci. 3, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K. (2003). “Processing speed and timing in aging adults: Psychoacoustics, speech perception, and comprehension,” Int. J. Audiol. 42, 59–67. 10.3109/14992020309074625 [DOI] [PubMed] [Google Scholar]
- Salthouse, T. A. (1996). “The processing-speed theory of adult age differences in cognition,” Psychol. Rev. 103, 403–428. 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- Schneider, B. A., and Pichora-Fuller, M. K. (2000). “Implications of perceptual deterioration for cognitive aging research,” The Handbook of Aging and Cognition (Lawrence Erlbaum, Mahwah, NJ: ), pp. 155–219. [Google Scholar]
- Schneider, B. A., Pichora-Fuller, M. K., Kowalchuk, D., and Lamb, M. (1994). “Gap detection and the precedence effect in young and old adults,” J. Acoust. Soc. Am. 95, 980–991. 10.1121/1.408403 [DOI] [PubMed] [Google Scholar]
- Shrivastav, M. N., Humes, L. E., and Aylsworth, L. (2008). “Temporal order discrimination of tonal sequences by younger and older adults: The role of duration and rate,” J. Acoust. Soc. Am. 124, 462–471. 10.1121/1.2932089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell, K. B. (1997). “Age-related changes in temporal gap detection,” J. Acoust. Soc. Am. 101, 2214–2220. 10.1121/1.418205 [DOI] [PubMed] [Google Scholar]
- Snell, K. B., and Frisina, D. R. (2000). “Relationships among age-related differences in gap detection and word recognition,” J. Acoust. Soc. Am. 107, 1615–1626. 10.1121/1.428446 [DOI] [PubMed] [Google Scholar]
- Szelag, E., Dreszer, J., Lewandowska, M., and Szymaszek, A. (2009). “Neural representation of time and timing processes,” in Neural Correlates of Thinking, edited by Kraft E., Gulyás B., and Pöppel E. (Springer-Verlag, Berlin, Heidelberg: ), pp. 187–199. 10.1007/978-3-540-68044-4_12 [DOI] [Google Scholar]
- Szymaszek, A., Szelag, E., and Sliwowska, M. (2006). “Auditory perception of temporal order in humans: The effect of age, gender, listener practice and stimulus presentation mode,” Neurosci. Lett. 403, 190–194. 10.1016/j.neulet.2006.04.062 [DOI] [PubMed] [Google Scholar]
- Trainor, L. J., and Trehub, S. E. (1989). “Aging and auditory temporal sequencing: Ordering the elements of repeating tone patterns,” Percept. Psychophys. 45, 417–426. [DOI] [PubMed] [Google Scholar]
- Tun, P. A., Wingfield, A., Stine, E. A. L., and Mecsas, C. (1992). “Rapid speech processing and divided attention,” Psychol. Aging 7, 546–550. 10.1037/0882-7974.7.4.546 [DOI] [PubMed] [Google Scholar]
- van Rooij, J. C. G. M., and Plomp, R. (1992). “Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion,” J. Acoust. Soc. Am. 91, 1028–1033. 10.1121/1.402628 [DOI] [PubMed] [Google Scholar]
- Weschsler, D. (1997). Wechsler Adult Intelligence Scale, 3rd ed. (WAIS-III) (The Psychological Corporation, San Antonio, TX: ). [Google Scholar]
- Working Group on Speech Understanding and Aging (1988). “Speech understanding and aging,” J. Acoust. Soc. Am. 83, 859–895. 10.1121/1.395965 [DOI] [PubMed] [Google Scholar]