Abstract
A multiple sensor array was employed to identify the spatial locations of all vocalizing male bullfrogs (Rana catesbeiana) in five natural choruses. Patterns of vocal activity collected with this array were compared with computer simulations of chorus activity. Bullfrogs were not randomly spaced within choruses, but tended to cluster into closely spaced groups of two to five vocalizing males. There were nonrandom, differing patterns of vocal interactions within clusters of closely spaced males and between different clusters. Bullfrogs located within the same cluster tended to overlap or alternate call notes with two or more other males in that cluster. These near-simultaneous calling bouts produced advertisement calls with more pronounced amplitude modulation than occurred in nonoverlapping notes or calls. Bullfrogs located in different clusters more often alternated entire calls or overlapped only small segments of their calls. They also tended to respond sequentially to calls of their farther neighbors compared to their nearer neighbors. Results of computational analyses showed that the observed patterns of vocal interactions were significantly different than expected based on random activity. The use of a multiple sensor array provides a richer view of the dynamics of choruses than available based on single microphone techniques.
INTRODUCTION
Males of several different vertebrate groups, including anuran amphibians, orthopteran insects, and songbirds, aggregate into breeding choruses to vocally advertise their presence and willingness to mate (Gerhardt and Huber, 2002; Burt and Vehrencamp, 2005). Participation in a chorus can confer significant benefits on its residents, such as increased attraction of females and reduced predation risk; however, there are also disadvantages to such group mate attraction. Because males of many species are territorial, more physical competition may arise when males are closely spaced within chorusing groups. In addition, the high levels of noise generated in a chorus may mask the perception of an individual’s vocalizations by both receptive females and rival males. Both senders and receivers of acoustic signals in a dense, noisy chorus must therefore devise some perceptual strategy for discriminating and then localizing particular signals of interest against a complex acoustic background.
Field and laboratory observations of patterns of calling behavior in males of different chorusing species have attempted to identify such strategies (reviewed in Klump and Gerhardt, 1992; Gerhardt and Huber, 2002). One way to reduce masking of one’s own vocalizations by those of neighbors is to shift the timing of one’s signal (either individual notes or entire multinote calls) so as to minimize overlap with those emitted by other callers. This strategy can result in a pattern of note-by-note alternation or call alternation between several individual chorusing males (Schwartz, 1987, 1993; Grafe, 1996, 1999; Moore et al., 1989). As another strategy to reduce masking, males may physically space themselves within choruses so as to maintain some minimum distance between nearest neighbors (Brush and Narins, 1989; Schwartz and Gerhardt, 1989; Forrest and Green, 1991). Measurements of intermale distances and signal sound pressure levels suggest that call amplitude can mediate this spacing (Wilczynski and Brenowitz, 1988); visual cues and even physical interactions can also be important. These two general strategies may occur concurrently. In several species of chorusing insects and anurans, males time their own advertisement calls so as to actively avoid overlapping the calls of only their nearest or loudest neighbors (Brush and Narins, 1989; Schwartz, 1993; Minckley et al., 1995; Snedden et al., 1998; Greenfield and Rand, 2000; Schwartz et al., 2002). The temporal sequences of calling thus generated form rhythmic patterns of call alternation, or even call synchrony when local male density and calling rates are high. Such calling patterns have been modeled by the operation of an endogenous oscillator that can be reset by exogenous cues (Brush and Narins, 1989; Greenfield and Roizen, 1993; Greenfield and Schul, 2008). Presumably, acoustic interactions with chorus members outside of this local, restricted area occur randomly, such that the internal oscillator is not reset by the vocal activity of these far males.
Most of these studies of chorusing dynamics are based on vocal interactions occurring between small local groups of males located within a larger chorus. Very little is known about patterns of vocal interactions within a chorus as a whole (Grafe, 1997; Boatright-Horowitz et al., 2000). This is because of the difficulty in recording and analyzing chorus activity based on the single or paired microphone recording techniques most commonly used to assess calling patterns. The real challenge of studying vocal interactions in animal choruses is the difficulty in accurately identifying, discriminating, and localizing large numbers of calling individuals and their locations, especially in dense assemblages when considerable numbers of acoustic signals temporally and spatially overlap. This is unfortunate, because the biological task faced by females approaching an insect or anuran chorus is just that—discriminating individual acoustic elements within a large, complex auditory scene (Bee, 2007). Recently, several techniques based on the use of multiple microphone arrays have been introduced to enable large scale analysis and modeling of chorusing activity (McGregor et al., 1997; Hayes et al., 2000; D’Spain and Batchelor, 2006; Mennill et al., 2006; Mohan et al., 2008; Simmons et al., 2008; Jones and Ratnam, 2009). These techniques all attempt to automatically localize and discriminate many callers within a chorus based on both spatial location and the physical characteristics of their vocalizations. Although promising, these techniques have not been widely adopted for use on terrestrial choruses and so far have been tested on only a small number of animals within a larger chorus. Thus, they have not yet yielded a large amount of acoustic data that can elucidate biologically relevant patterns of natural vocal interactions in animal assemblages.
Previously, we described a technique for recording and analyzing chorus activity in the American bullfrog, Rana catesbeiana, using an array of multiple, closely spaced acoustic sensors (Simmons et al., 2008). This methodology is based on computing the differences in arrival time of sounds at the sensors using a cross-correlation algorithm, and using these time differences to then estimate sound source by vector triangulation. We initially tested this technique by identifying and localizing a small number of individual callers within a single larger bullfrog chorus. Here, we extend this initial analysis by examining the large scale spatial and temporal organization of two separate bullfrog choruses on five different recording nights. Individual callers were identified by the acoustic characteristics (fundamental and first harmonic frequencies) of their advertisement calls, and their spatial locations were estimated. This method enabled us to discriminate and localize both overlapping and nonoverlapping advertisement calls produced by vocalizing males located at different sites within the chorus. Our data highlight the different kinds of vocal interactions in bullfrog choruses, related to the spatial distances and local group behavior of individual males, which have not been readily available from data based on single microphone techniques (Boatright-Horowitz et al., 2000).
METHODS
Study sites and animals
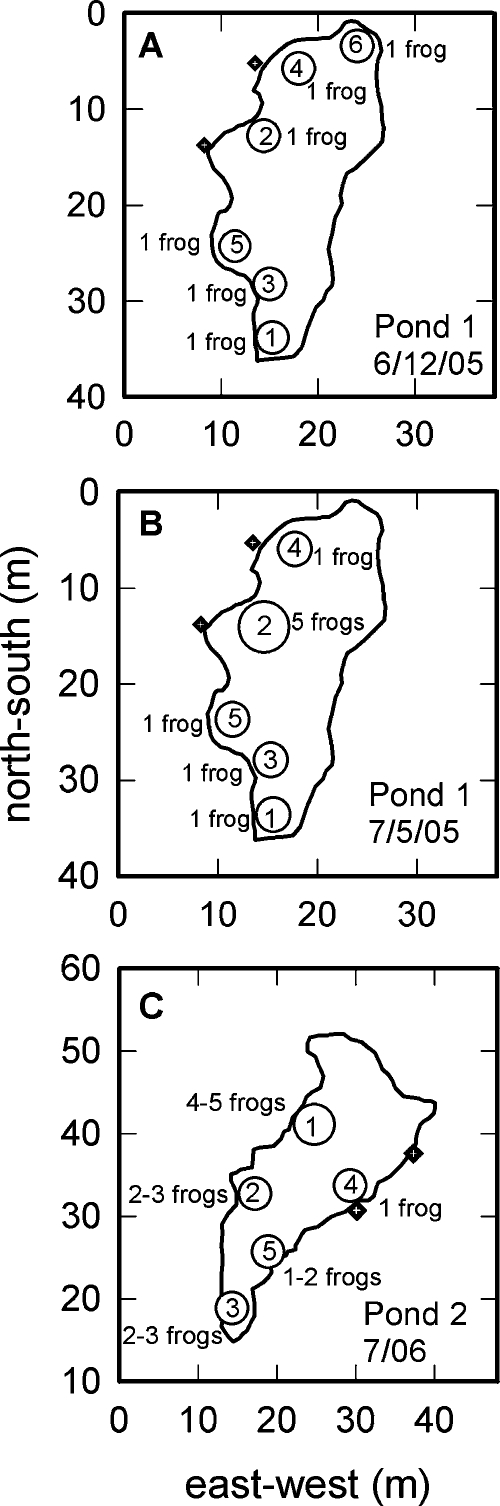
We performed acoustic recordings at two different ponds between 2100 and 2400 h on five nights in June and July of 2005 and 2006. On 6∕12∕05 and 7∕5∕05, recordings were made at a pond [pond 1; 42 m long by 20 m wide; outline and orientation in Figs. 1A, 1B] located in a suburban neighborhood in Rhode Island (described previously in Boatright-Horowitz et al., 2000). This pond was bordered on one side by thin, low vegetation and on three other sides by houses and a bike path. Due to development and to the construction of this bike path, the dimensions of this pond have changed since the site was first described in 2000. On 7∕5∕06, 7∕6∕06, and 7∕9∕06, we carried out recordings at another pond [pond 2; 40 m long by 15 m wide; outline and orientation in Fig. 1C] located on private property in central Massachusetts (described previously in Simmons et al., 2008). This pond was surrounded on three sides by heavy vegetation and woods, and on another side by a small clearing and house. Both ponds supported populations of bullfrogs (6–14 active callers on any given night) and green frogs (Rana clamitans; number not censused). Because green frogs were not very vocally active during our recording times and they appeared to be present in only small numbers, we did not undertake a quantitative analysis of any acoustic partitioning between these two species. Air temperatures (measured using a thermometer) on recording nights ranged between 17.8° and 23.3 °C, relative humidity (obtained from www.nws.noaa.gov) was between 76% and 87%, and there was no precipitation. Recording times at the different nights ranged between 85 and 172 min, with an average of 118 min.
Figure 1.
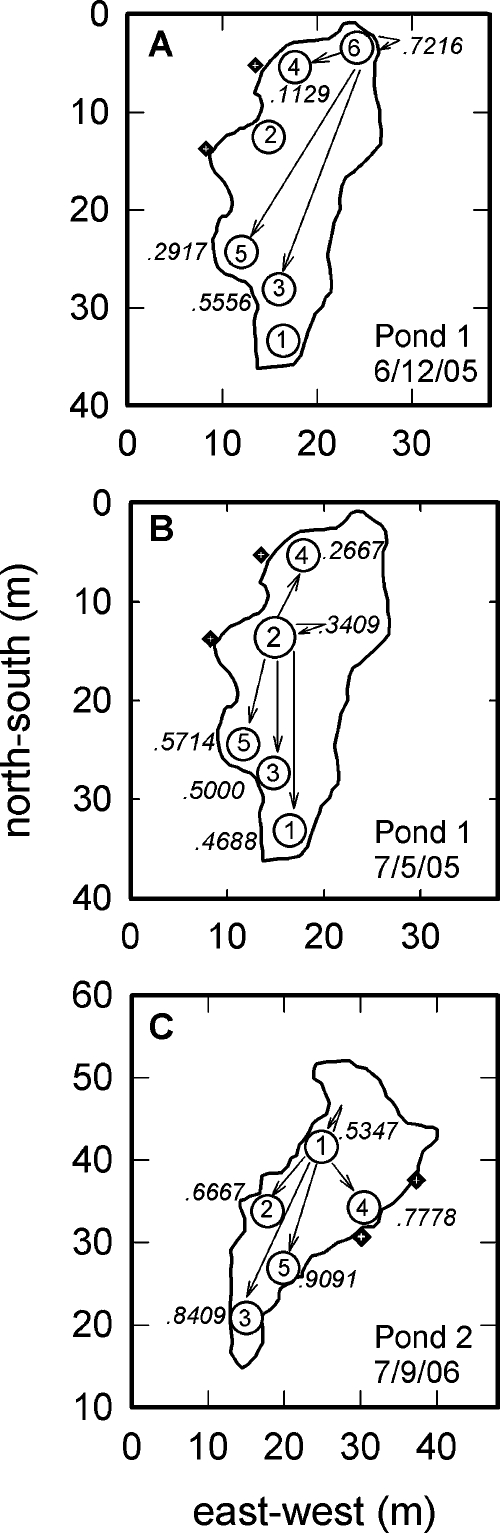
Diagrams of the two chorusing sites. On each diagram, the two black square∕white cross symbols outside of the pond outline indicate the position of the two acoustic sensors. (A) Map of pond 1 showing estimated locations and numbers of actively calling bullfrogs (designated by open circles with numbers) on the night of 6∕12∕05. Each circle is placed at the median of the vector intersection points derived from the computational model. Only one bullfrog was present at each indicated location. Locations are numbered in the order in which they were identified in the analysis. (B) Map of activity at pond 1 on the night of 7∕5∕05. Five vocalizing bullfrogs were now present at location 2. (C) Map of pond 2. The locations of bullfrogs were similar on all three nights (7∕5∕06, 7∕6∕06, and 7∕9∕06) at this site; the variability in numbers of animals at each location is indicated. On all three nights, location 4 contained only one bullfrog.
We set up the recording equipment approximately 30 min before the onset of any vocal activity by the bullfrogs. We did not capture or handle the animals for visual inspection or morphological measurements, and so did not disturb their natural behaviors. To correlate the directional estimates derived from the sensor array with actual spatial locations of individual bullfrogs, an observer periodically surveyed the pond during recordings and marked the location of each calling male on a scaled map. Spatial distances between these estimated locations were later verified by direct measurements using a surveying transit and with reference to aerial maps obtained using Google earth (see Simmons et al., 2008). Our research protocol was approved by the Brown University Institutional Animal Use and Care Committee.
Sensor array
We recorded the vocalizations of chorusing male bullfrogs using a sensor array previously described by Simmons et al. (2008). This array consists of two individual four-microphone sensors (“cubes,” 3.3 cm), each mounted on top of a vertical aluminum rod and held in a position 0.6 m above the surface of the ground by an adjustable survey tripod. A sensitive, calibrated, omnidirectional electret condenser microphone (Knowles Electronics, Model FG3329, Itasca, IL) was placed in the center of each of the four vertical faces of each sensor. When in use, each sensor was covered by a spherical black foam windscreen 10 cm in diameter. At each recording site, the two sensors on their tripods were placed on one side of the pond, separated by 10 m (square∕cross symbols alongside pond outlines in Figs. 12). They were set back about 0.5 m from the water’s edge, in an area clear of vegetation, rather than in the center of the pond, which the cabling system prevented. Because the pond was entirely on one side of the array, so that front-back localization was not needed, data were collected from only two of the four microphones in each sensor. In the leftmost sensor, channels 1 (to the left) and 3 (to the right) were used, while in the rightmost sensor, channels 5 (to the left) and 7 (to the right) were used.
Figure 2.
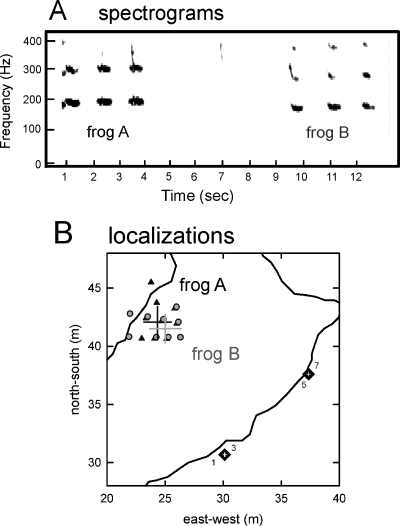
Estimates of source locations for two bullfrogs belonging to the same cluster to illustrate the effective accuracy of localization. (A) Spectrograms to 400 Hz of three note advertisement calls produced by two individual male bullfrogs (frog A and frog B), calling sequentially over a 14 s time interval. For calculation of these and other spectrograms by ADOBE AUDITION, sounds were downsampled to 1500 Hz (Blackmann-Harris window, 512 frequency bands). The first harmonic frequencies of the notes in each bullfrog’s advertisement call differ (190 Hz for frog A and 170 Hz for frog B). (B) Localization estimates derived from calls in A. The black square∕white cross symbols outside the pond’s outline show the locations of the two sensors. The active microphones (1, 3 and 5, 7) in each sensor are indicated. The symbols inside the pond outline show the points of vector intersection computed from the advertisement call notes of each individual male. Estimates for frog A are indicated by the black triangles, and estimates for frog B are indicated by the gray circles. The localization program provided 20 intersection points for the three notes emitted by frog A, and 28 intersection points for the three notes emitted by frog B. Although all of these points are plotted, not all are visible because many of their locations overlap. Variability is indicated by black and gray crossed lines for standard deviations in the east-west and north-south dimensions calculated from all location estimates for frog A and frog B, respectively. The intersection points of each cross designate the mean locations calculated from the intersection points for the two frogs. Frog A and frog B are separated by a mean diagonal distance of 1.68 m. The maximum dispersion between all intersection points for these two frogs, excluding the points outside the pond, is 3.5 m. Dispersion is due largely to effects of reverberation on each localization estimate.
A full description of data handling and display can be found in Simmons et al. (2008). Acoustic signals picked up by the two active microphones in each sensor cube were amplified (10× gain) and recorded on four channels of a Sony SIR-1000W wideband digital instrumentation recorder (Sony Precision Technology America Corp., Lake Forest, CA). The sounds were digitized simultaneously in each recorder channel at 48 kHz sampling rate (16-bit accuracy). Binary files containing the four channels of data [two from the 1,3 (left) sensor and two from the 5,7 (right) sensor] were subsequently downloaded into a Pentium-3 PC using Sony PCSCAN programs supplied with the Sony recorder. Each night thus produced four digitized data streams lasting for the entire recorded epoch. For processing, these files were broken into two “stereo” data streams corresponding to the two microphone channels in each cube sensor (microphones 1 and 3 or 5 and 7). Each stereo stream, which covered the entire night’s recording epoch for one sensor, then was subdivided into consecutive 10 s segments and low-pass filtered at 4 kHz to remove high frequency signals, using custom-written MATLAB routines (MathWorks, Natick, MA). For computational processing, these 10 s segments were further divided into shorter, overlapping 100 ms time segments (because overlap is 50%, there are 250 short segments in each longer 10 s stereo segment). Data were processed by a binaural computational model of the auditory system (available online at the Boston University EarLab website; Mountain et al., 2007), which operated by bandpass filtering the acoustic signals from each sensor into 32 parallel overlapping frequency bands (60 Hz–5 kHz). Time-of-arrival differences (from −180 to +180 μs) were calculated in each of these frequency bands separately. When running the model, a threshold is set to prevent background noise or very low-level sounds from entering into the processed data. Time difference estimates are then pooled across all 32 frequency channels to generate a histogram (bin width 10 μs) of time differences in each consecutive 100 ms segment of the signal. The final estimate of the time difference for any one 100 ms time segment is determined from the peak of this histogram. The model then plots the peaks derived from successive histograms to give a running history of arrival time differences across all 100 ms segments of each 10 s long stereo signal. The result is a stream of up to 250 separate time difference estimates between the left and right microphones. The threshold setting determines how many of the potentially 250 estimates actually appear in the model’s output.
All of the sequential arrival time difference streams for successive 10 s segments are concatenated to create a history of sound arrival time differences over the entire duration of the recorded session in each sensor cube (stereo pair). To locate the source of the sound on a map of the pond, the time difference estimates at each sensor are transformed into spatial angle estimates. Each sensor provides one directional estimate (vector) for each particular time difference in the data stream. Synchronized angle estimates from both sensors yield vector intersections that are used to estimate the location of the sound source. We computed the intersection point of the two vectors from each sensor using a custom-written MATLAB routine, and then plotted multiple intersection points obtained from consecutive segments of the recordings on a template of the pond. We calculated the median, mean, and standard deviations of these points to form an estimate of the location of the sound source. In the example in Fig. 2, two bullfrogs (frog A and frog B) are vocalizing one after the other during a 14 s segment of the chorus [spectrograms in Fig. 2A]. The diagram of the pond in Fig. 2B shows the position of the calculated points of intersection from the two sensors for the advertisement call notes of these two bullfrogs. The mean locations of the vector intersection points indicate that the two males are separated by a distance of 1.68 m. For bullfrog A, the standard deviation of its mean location is 1.36 m in the north-south dimension and 1.18 m in the east-west dimension. For bullfrog B, the standard deviation of its mean location is 1.21 m in the north-south dimension and 1.35 m in the east-west dimension. This example provides an indication of the accuracy of the localization program for processing real sound sources in a noisy natural environment.
There are several limitations of this sensor technique. One technical limitation is the relatively short aperture between the left and right microphones within each cube, which leads to higher variability in estimates of azimuth than if the aperture were larger. The size of each pond dictated a minimum spacing of 8–10 m between cubes to ensure that all of the bullfrogs were recorded, but the complexity of the natural situation, especially the presence of multiple sound-propagation paths due to irregular screens of vegetation around the ponds, caused decorrelation to occur in recordings of the same sounds at such widely spaced sensors. Use of two relatively small sensor units, each capable of estimating azimuth, combined with a wider spacing between sensors, proved to be an acceptable compromise. Another limitation is imposed by presence of vegetation around the recording sites, which was particularly thick and extended in depth on the far side of pond 2 [northwest side in Fig. 1C]. Sound sources emanating along this side of the pond were sometimes localized in the trees or outside the boundaries of the pond [Fig. 2B] because reverberation from the surrounding vegetation was especially strong from that direction. Another limitation emerged when two call notes arrived at the two sensors entirely simultaneously, with no brief interval of one note or the other being entirely separate from the other. Localization estimates for such completely overlapping notes could occur at places between the two actual calling bullfrogs. In cases of complete or near-complete note overlap, we used amplitude cues and knowledge of the first harmonic frequencies in the notes of particular known individuals when calling without interference, as well as visual inspections of the chorus site and field notes taken during recordings, to estimate the actual locations of these overlapping notes. This limitation means, however, that when two males are very closely spaced, as in Fig. 2B, the estimated locations of overlapping notes could be between their actual locations. This problem becomes more acute when it is realized that bullfrogs, particularly those who are closely spaced, do not stay stationary during chorusing activity, but move around and often engage in physical aggressive interactions with other males in their immediate vicinity. Movements of the sound source complicate the accuracy of localization. Finally, because the localization program also picked up and provided location estimates for advertisement calls of R. clamitans as well as for other sounds within the frequency range of bullfrog advertisement calls, we needed to identify these sounds by their spectrograms and then manually delete them from the processed data stream.
Acoustic analysis of bullfrog advertisement calls
Male bullfrogs emit complex advertisement calls consisting of 1–12 individual notes [croaks; Figs. 2A, 3]. Each note contains a number of harmonically related frequencies from about 200 to 2000 Hz, with a missing fundamental frequency around 80–125 Hz (Bee, 2004; Suggs and Simmons, 2005). The duration of the notes varies considerably between successive notes and between individuals, with the average duration about 550 ms and average internote interval around 530 ms (Simmons, 2004). The envelopes of these notes often contain amplitude modulations (AMs) that increase in number from one note to the next; these modulations are correlated with an increase in note duration (Suggs and Simmons, 2005). Bullfrogs exhibit relatively low calling rates, with intercall intervals as long as 16–36 s reported in some choruses (Emlen, 1976; Boatright-Horowitz et al., 2000; Bee, 2004).
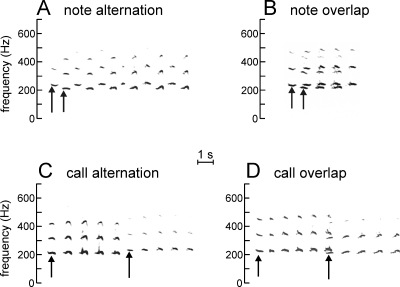
Figure 3.
Spectrogram examples of the four types of vocal interactions (bouts) identified in the recordings. Advertisement calls were low-pass filtered to show only the low frequency harmonics. Arrows indicate the first notes of each bullfrog’s individual call. The relative darkness of the spectrograms provides an indication of the relative amplitude of each individual’s call notes. (A) Note alternation, in which one bullfrog begins calling after the completion of the first note of another bullfrog’s call and the two continue with their notes alternating in time. (B) Note overlap, in which more than one-half of the notes in the first bullfrog’s call is overlapped by the call notes of another bullfrog. In this example, the second bullfrog does not begin calling until the completion of the first note in the leading bullfrog’s call, but subsequent notes of both bullfrogs overlap considerably. (C) Call alternation, in which one bullfrog begins calling within 2 s after the completion of another bullfrog’s call. (D) Call overlap, in which one bullfrog begins calling after the completion of more than one-half of the notes of another bullfrog’s call. In this example, only the last note of the first bullfrog’s call overlaps with the first note of the second bullfrog’s call.
To determine the spectral content of each recorded bullfrog advertisement call, the four-channel binary files of raw sensor recordings were separated into two stereo.wav files, one for channels 1 and 3 (left sensor) and the other for channels 5 and 7 (right sensor). General acoustic characteristics of the calls (duration, duty-cycle, harmonic frequencies, and onset time) were analyzed with custom-written MATLAB routines and then displayed as spectrograms and sound pressure waveforms using ADOBE AUDITION V 1.5 (Adobe Systems Inc., San Jose, CA).
We first isolated advertisement calls of bullfrogs vocalizing individually (without any overlap or interference from other frogs and when the calls of individual males were separated by at least 2 s), and then estimated, using the model output, the locations of these animals on a map of the pond (Fig. 1). The locations of individual males were cross referenced against locations estimated from visual sightings of calling bullfrogs at the time of data collection. We then identified four types of multiple bullfrog vocal interactions (bouts) from the recorded spectrograms (Fig. 3) and estimated their source locations. Note alternation [Fig. 3A] was defined as one bullfrog beginning his call after the completion of the first note of another male’s call, with the successive call notes of the two males distinctly alternating in time. Note overlap [Fig. 3B] occurred when more than one-half of the notes of the first caller were overlapped by the notes of another caller, resulting in overlap of successive notes. Several instances of note overlap consisted of bouts of three or more bullfrogs calling in near synchrony. In call alternation [Fig. 3C], one bullfrog began his call within 2 s of the completion of another male’s entire call. We chose 2 s as the cut-off point between call alternation and individual calling based on the analysis of intercall intervals between identified individuals (or groups of bullfrogs who overlapped calls) during the three nights from pond 2. Plotting the distribution of all intercall intervals showed two peaks, one at intervals less than 1 s in all data sets and a broader peak at intervals between 5 and 12 s, depending on chorus size. Dividing the overall distribution into two separate distributions, using a clear trough located at 2 s between the modes of each distribution as a cut-off point, minimized the standard deviations of both distributions in all three data sets. Successive calls with an intercall interval of 2 s or less were categorized as call alternations, while calls separated by more than 2 s were labeled individual calls. Finally, in call overlap [Fig. 3D], one bullfrog initiated calling after the completion of more than one-half of the notes of another male’s call, resulting in overlap of the last notes of the first caller and the first notes of the second caller. As with note overlap, some instances of call overlap reflected vocal responses of three or more bullfrogs calling in near synchrony. The sample spectrograms show that, even in cases of note overlap [Fig. 3B], at least one note from an individual and a portion of the note from the other individual (usually the first or last notes in each case) is free enough from interference that these portions can be analyzed to determine the first harmonic frequency of the note, as an indicator of individual identity (Bee, 2004). In practice, we first analyzed single, nonoverlapping call notes in multiple bullfrog interactions, and estimated the locations of these sound sources on the map. We found that, even in situations where notes overlapped completely in time, they differed enough in spectral frequency that individual males could be separated acoustically.
For statistical analysis, data were categorized as representing one of the five types of acoustic events (individual calling and the four types of vocal bouts) and as spatial location (within or between clusters). We defined a cluster as a location where two or more vocalizing males were very closely spaced (in practice, intracluster spacing ranged up to 6.5 m, depending on the numbers of bullfrogs within that local area). The program UNCERT (Hailman and Hailman, 1993) was used to compute the frequencies and probabilities of these types of vocal interactions (“events,” zero-order analysis) and the frequencies of transition from preceding to following events (first-order analysis) to determine the existence of any sequential calling patterns within a chorus. Chi-square tests were performed using SPSS V.16 (SPSS Inc., Chicago, IL) to analyze the relation between type of vocal interactions and spatial location of the callers, as well as to compare the frequency (rate) of observed calling events to the expected frequency (rate) if the bullfrogs were calling randomly. Due to the sometimes small number of call events, data were grouped together across recording sites to increase the power of analyses when appropriate.
Simulation of chorus activity
In order to model the calling behavior of the chorus residents, a custom-written MATLAB routine was used to simulate bullfrogs calling at the same rate as that observed in the natural choruses, but with each male vocalizing independently of its neighbors through a Poisson process. The simulation represented the bullfrogs as arrays of time bins in which silence was recorded as zeros and calls recorded as a series of ones. The initiation of calls was random, but each call lasted for the same duration as that observed in the natural chorus. The behavior of multiple bullfrogs could be assessed by summing the arrays together, and call interaction events were counted by logical filters that ran iteratively though the simulation epoch.
For each recording session, a simulation was run in which the same numbers of vocalizing bullfrogs were grouped into identical spatial locations (clusters) as determined from the empirical data, and each “male” generated calls at a rate that would produce an expected number of calls equal to that observed in the natural chorus. All bullfrogs in each simulation generated the same expected number of calls. Then, four different kinds of calling events were defined that corresponded to the five categories of events observed in the natural chorus. The events measured in the simulated data were individual calls, call alternation, and call overlap, which directly corresponded to the same categories in the empirical analysis, and note alternation∕overlap, which grouped together the two categories of note alternation and note overlap that were measured in the empirical data. In the simulation, bouts were identified and then classed as one of these four events based on a measure of the percentage of the bout epoch during which only one bullfrog was vocalizing (Table 1). These event definitions are mathematical approximations of the corresponding bout types observed in the empirical data based on the duration of overlap among calls from multiple bullfrogs. Although the simulated events were counted by logical filters whereas the empirical events were counted by observation, these definitions allow for a direct comparison between their occurrence in nature and at random.
Table 1.
Events measured in simulated and empirical data.
| Event type | Corresponding empirical category | Percentage of “individual call” |
|---|---|---|
| Individual call | Individual call | %=100 |
| Call alternation | Call alternation | 80≤%<100 |
| Call overlap | Call overlap | 50≤%<80 |
| Note alternation∕overlap | Note alternation and note overlap | 0≤%<50 |
Further, the identification and classification of bouts were performed both within spatial clusters (intracluster bouts) and between spatial clusters (intercluster bouts) as retrieved from the empirical calculations of location. Because a simulation was run to mirror the spatial arrangements observed in each of the recording sessions, direct comparison was possible between the intercluster and intracluster interactions observed in the natural chorus, and those that would occur if males were vocalizing at random. Because the quantity of each of these events in the simulation is a random variable, 50 trials of each simulation were run in order to generate a distribution of each output that could then be compared with the empirical data. Statistical differences between model output and empirical observations were analyzed by z-tests to generate the probability of each observation occurring in a population of randomly vocalizing bullfrogs.
RESULTS
Spatial location of vocalizing males
We analyzed five nights of chorus activity, two from pond 1 and three from pond 2. For the 6∕12∕05 recording from pond 1, bullfrogs were spaced out enough and calling activity was low enough that it was possible to clearly identify each active individual and his location based on both the field notes from that night and the localization program. In all the July recordings from both ponds, however, more vocalizing bullfrogs were present than on 6∕12∕05 and the close spacing of some of the males and the large number of overlapping calls made identification and localization of individuals more challenging. For these nights, clusters of closely spaced bullfrogs were identified, containing between two and five vocalizing males within a local area of the pond. Locations of individual calling bullfrogs at pond 1 on 6∕12∕05 and 7∕5∕05 are shown in Figs. 1A, 1B, respectively. Each circle on the map represents the median location estimate over the duration of each recording night; numbers within the circles are location∕cluster number. On 6∕12∕05, each numbered location represents a single vocalizing male. The closest distance between individual males at this site on this night was 5 m (locations 3 and 5, measured as the difference between the mean location estimates). The caller at location 6 was missing from the chorus when the next recording was made at this site on 7∕5∕05 [Fig. 1B]. On this date, location 2 now consisted of a cluster of five actively vocalizing males, four of whom were not recorded during the earlier night. Within location 2, these five males were separated by at most 4.2 m, the maximum dispersion of all vector intersection points for this location. They were separated by 13.9 m from their nearest neighbors at location 4. At pond 2, the estimated locations of bullfrogs were similar on the three recording nights [Fig. 1C], although numbers of vocalizing individuals in a particular local area varied across the three nights. Four of the identified sound source locations contained between two and five vocalizing bullfrogs, with only one location containing an isolated individual. Within a cluster, vocalizing males could be separated by as little as 1.68 m [Fig. 2B]. The maximum dispersion of all intersection points within this particular cluster was 6.5 m. The closest distance between different clusters was 7 m (locations 3 and 5, on opposite sides of the pond).
Overall activity and types of vocal events
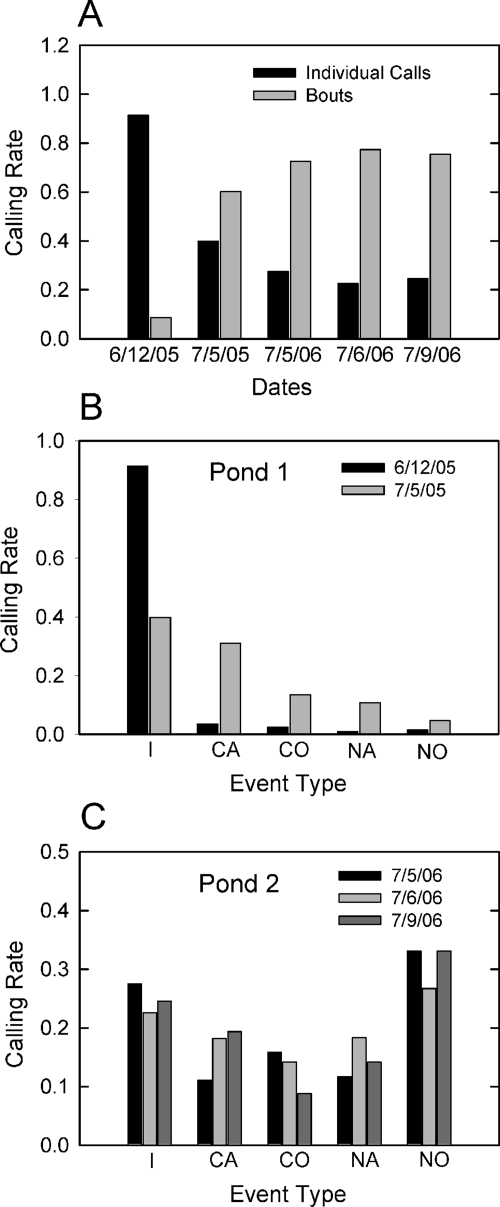
The relative amount of calling activity, expressed as calling rate (percent of call type over the length of that recording session), at the different sites is shown in Fig. 4. The relative rate of individual calling activity and of calling bouts (all acoustic interactions in which more than one bullfrog vocalized, including note overlap, note alternation, call alternation, and call overlap) varied between the June and the July nights [Fig. 4A]. On all four July nights, bullfrogs were significantly more likely to call in bouts than to call as individuals, while the opposite pattern holds for the night in June, X2(1)=339.8, N=1816, p<0.001. The rate of the different kinds of calling events at both ponds on all five nights also varied. At pond 1 [Fig. 4B], the most common calling event on both nights was individual calling, whether or not the animals were located within a local cluster with other calling males. On 6∕12∕05, the rate of other calling events was low. Conversely, on 7∕5∕05, call alternation was the most frequent event after individual calling, followed by call overlap, note alternation, and note overlap, respectively. The pattern of calling activity from the three nights at pond 2 differed from that at pond 1, and also showed some differences between individual nights [Fig. 4C]. When the pond as a whole was sampled, note overlap was the most frequent event at all three nights, followed by individual calling. The relative amounts of call alternation, note alternation, and note overlap varied between nights, indicating the dynamic nature of interactions in choruses.
Figure 4.
Calling rates (normalized for the total numbers of calling events per night) at the two different chorusing sites. (A) Rate (y axis) of individual calls (black bars) and multiple frog interactions (bouts; gray bars) across all five nights (x axis) of chorus activity. The rate of multiple frog bouts was significantly higher in July than in June. (B) Rate of calling events at pond 1 on 6∕12∕05 (black bars) and 7∕5∕05 (gray bars). I=individual call; CA=call alternation; CO=call overlap; NA=note alternation; NO=note overlap. (C) Rate of calling events at pond 2 on 7∕5∕06 (black bars), 7∕6∕06 (gray bars), and 7∕9∕06 (dark gray bars). Note overlap was the most common type of multiple frog interaction on all three nights, but there were many instances of all types of calls and variability between the three nights.
Within- and between-cluster interactions
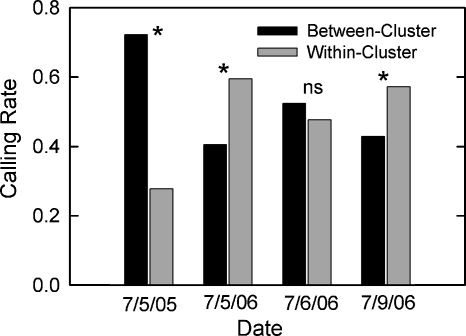
We next compared the frequency of the different kinds of bouts (regardless of the particular type of interaction) within and between identified local clusters of bullfrogs (Fig. 5). Data from 6∕12∕05 were eliminated from this analysis because there were no clusters of bullfrogs on this night. On 7∕5∕05, there were significantly more bouts between frogs in different clusters than between frogs within the same cluster, X2(1)=17.78, N=90, p<0.001. However, on both 7∕5∕06 and 7∕9∕06, there were significantly more within-cluster interactions than between-cluster interactions [7∕5∕06: X2(1)=9.87, N=274, p=0.002; 7∕9∕06: X2(1)=9.03, N=335, p=0.003]. The rate of within-cluster interactions and between-cluster interactions did not differ from chance on 7∕6∕06, X2(1)=1.77, N=508, p>0.05.
Figure 5.
Within-cluster and between-cluster calling rates on four nights at the two recording sites. Data show combined calling rates for all four types of acoustic interactions. Asterisks (∗) denote statistical significance of comparisons of between-cluster and within-cluster rates on a given chorusing night. ns=not statistically significant. There was a significantly higher number of between-cluster than within-cluster interactions at pond 1 on 7∕5∕05. At pond 2, there were significantly more within-cluster interactions on two of the nights (7∕5∕06 and 7∕9∕06), but no difference on the third night (7∕6∕06).
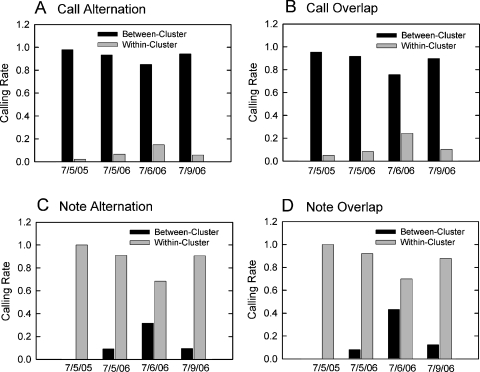
Different kinds of vocal bouts predominated within compared to between clusters. At both ponds, bullfrogs located in different clusters or spatial locations were more likely to participate in call alternation or call overlap than were bullfrogs located within the same cluster [Figs. 6A, 6B]. Again, data from the June recording night are not shown because no clusters were present. On all four July nights, call alternation occurred between clusters significantly more often than it occurred within clusters [7∕5∕05: X2(1)=42.09, N=46, p<0.001; 7∕5∕06: X2(1)=33.80, N=45, p<0.001; 7∕6∕06: X2(1)=59.71, N=121, p<0.001; 7∕9∕06: X2(1)=67.16, N=86, p<0.001]. At pond 1, there was only one location which contained a cluster of males (location 2), and males within this cluster alternated calls with each other only one time over the entire recording session. The data from pond 2 also show that more call alternation occurred between compared to within clusters on all three recording nights [Fig. 6A]. Call overlap on all four July nights [Fig. 6B] also occurred between clusters significantly more than it occurred within clusters [7∕5∕05: X2(1)=17.19, N=21, p<0.001; 7∕5∕06: X2(1)=41.67, N=60, p<0.001; 7∕6∕06: X2(1)=27.27, N=103, p<0.001; 7∕9∕06: X2(1)=24.64, N=39, p<0.001]. At pond 1 on 7∕5∕05, the cluster of males in location 2 engaged in call overlap with each other only one time over the entire recording session. Call overlap within clusters at pond 2 occurred much less often than it did between clusters on all three recording nights.
Figure 6.
Calling rates for the four different kinds of bouts for the four July chorus nights at the two chorus sites. (A) Rate of call alternation events. Call alternation was significantly more likely to occur between clusters compared to within clusters on all four nights. (B) Rate of call overlap events. The pattern of call overlap differed significantly between clusters compared to within clusters in all choruses. Call overlap was more likely to occur between clusters. (C) Rate of note alternation events. Note alternation occurred exclusively within clusters at pond 1 (7∕5∕05), so no statistical test could be performed on these data. At the other three choruses, note alternation was significantly more likely to occur within clusters than between clusters. (D) Rate of note overlap events. Note overlap was observed only within clusters and never between clusters at pond 1. At the other choruses, note overlap occurred significantly more often within clusters than between clusters.
While call alternation and call overlap were more common between bullfrogs in different clusters, note alternation and note overlap were more likely to occur between bullfrogs located within the same cluster [Figs. 6C, 6D]. Statistical analyses could not be performed on data from 7∕5∕05 (pond 1) because note alternation and note overlap occurred exclusively within clusters on this night. For all three nights at pond 2, note alternation was significantly more frequent within clusters than between clusters [7∕5∕06: X2(1)=29.45, N=44, p<0.001; 7∕6∕06: X2(1)=16.13, N=120, p<0.001; 7∕9∕06: X2(1)=41.29, N=63, p<0.001]. Note overlap was significantly more likely to occur within rather than between clusters at this location as well [7∕5∕06: X2(1)=88.20, N=125, p<0.001; 7∕6∕06: X2(1)=26.24, N=166, p<0.001; 7∕9∕06: X2(1)=83.82, N=147, p<0.001].
Patterns of sequential interactions
Patterns of sequential calling between males in different spatial locations were calculated using the UNCERT program. This analysis is based only on numbers of acoustic events, and not on their type (that is, individual calling and the four different kinds of calling bouts were not distinguished in the analysis). At pond 1 on 6∕12∕05, the most active callers were the bullfrogs at locations 3 and 6, and these two were also the most widely spaced [Fig. 7A]. Bullfrogs at locations 1 and 2 each called only one time during that recording session, so data from these males were not included in the sequential analysis. The probabilities of the male at location 6 vocalizing directly after its farthest vocalizing neighbors were 0.5556 (location 3) and 0.2917 (location 5), while the probability of vocalizing directly after its nearest neighbor, at location 4, was only 0.1129 [Fig. 7A]. The bullfrog at location 5 never vocalized directly after its nearest neighbor, location 3, but vocalized directly after the male at location 6 (probability of 0.1031) and location 4 (probability of 0.1774).
Figure 7.
Patterns of sequential calling in choruses, derived from first-order analysis in UNCERT. The numbers in italics on each pond diagram are the probability of that location (cluster) responding immediately after the most active location (cluster). Arrows represent the direction of interaction. The arrow that goes back onto itself shows the probability of the most active cluster (location) vocalizing immediately after itself. (A) Pond 1 on 6∕12∕05. The most active location on this night is location 6. The probability of the bullfrog at location 6 vocalizing immediately after itself is 0.7216. The bullfrog in location 6 was most likely to vocalize after the bullfrog in location 3 (0.5556), its farthest neighbor. Locations 1 and 2 were not included in the analysis, because the animals at these locations called only once during the recording session. (B) Pond 1 on 7∕5∕05. The chorus organization differed on this night than on the earlier night shown in (A). The animal in location 6 was absent from the chorus, and the most active location was now location 2. Numbers in italics show the probabilities that any bullfrog at location 2 followed the calls of any individuals at the other locations. The sequential probabilities of calling were similar to the three farthest neighbors (locations 5, 3, and 1) and lowest to the nearest neighbor at location 4. The bullfrogs in location 2 followed themselves with a probability of 0.3409. (C) Pond 2 on 7∕9∕06. The most active location at this night was location 1. Bullfrogs in this location followed themselves with a probability of 0.5347. The highest sequential probabilities of calling were to the farthest neighbors at locations 3 and 5.
At pond 1 on 7∕5∕05, five bullfrogs aggregated into a cluster (location 2), and this cluster overall produced the most calls. Figure 7B shows the transition probabilities for this chorus. Location 2 showed the highest probabilities of calling directly after its three farthest neighbors, location 1 (0.4688), location 3 (0.5000), and location 5 (0.5714), and its lowest probability of calling after its nearest neighbor, location 4 (0.2667). Bullfrogs in cluster 2 vocalized after other bullfrogs in that same cluster with a probability of 0.3409.
Sequential probabilities of calling from one night (7∕9∕06) at pond 2 are shown in Fig. 7C. For this analysis, each cluster is considered as a unit, and no attempt was made to separately identify the separate probabilities of vocalizations of the individual frogs within each cluster. Location 1, with four bullfrogs on this night, was the most active location. Animals at this location vocalized after another animal at the same location with a probability of 0.5347. When examining transitional probabilities of calling from location 1 to the other locations, bullfrogs in location 1 were found to vocalize least often after their closest neighbors, at location 4 (one bullfrog; probability of 0.7778) and location 2 (two bullfrogs, 0.6667). The probabilities of bullfrogs in cluster 1 vocalizing after their farthest neighbors, at location 3 (two bullfrogs, 0.8409) and location 5 (two bullfrogs, 0.9091), were higher. Data from the other two nights at this pond show similar trends, with the higher probabilities of sequential calling between locations occurring to farther, rather than nearer, clusters.
Comparison to random simulation
A normal distribution was used to calculate the probability of each of the empirical results occurring under the random conditions of the MATLAB simulation. When comparing the observed frequency of individual calls to the expected (chance) frequency (correcting for the length of the actual recording session), significantly fewer individual calls were observed than would be expected if the males were calling randomly and independently of each other (6∕12∕05: z=19.42, p<0.0001, one-tailed; 7∕5∕∕05: z=99.18, p<0.0001, one-tailed; 7∕5∕06: z=190.25, p<0.0001, one-tailed; 7∕6∕06: z=213.01, p<0.0001, one-tailed; 7∕9∕06: z=50.41, p<0.0001, one-tailed; all p values are adjusted according to the Bonferroni correction).
On all four July nights (excluding 6∕12∕05 because there were no clusters of bullfrogs at this night), call alternation within clusters occurred significantly less frequently than would be expected from chance (7∕5∕05: z=21.48, p<0.0001, one-tailed; 7∕5∕06: z=31.39, p<0.0001, one-tailed; 7∕6∕06: z=26.46, p<0.0001, one-tailed; 7∕9∕06: z=32.95, p<0.0001, one-tailed; all p values according to the Bonferroni correction). Call overlap was also less frequent within clusters than was predicted by the random model (7∕5∕05: z=−32.67, p<0.0001, one-tailed; 7∕5∕06: z=−238.42, p<0.0001, one-tailed; 7∕6∕06: z=−184.08, p<0.0001, one-tailed; 7∕9∕06: z=−249.79, p<0.0001, one-tailed; all p values according to the Bonferroni correction).
The MATLAB simulation grouped note overlap and note alternation events together. The random simulation predicted significantly fewer note overlaps and note alternations occurring within clusters than what was observed in the empirical data (7∕5∕05: z=−32.67, p<0.0001, one-tailed; 7∕5∕06: z=−238.42, p<0.0001, one-tailed; 7∕6∕06: z=−184.08, p<0.0001, one-tailed; 7∕9∕06: z=−249.79, p<0.0001, one-tailed; all p values according to the Bonferroni correction).
DISCUSSION
Using a novel multiple sensor recording technique, we analyzed the acoustic and spatial patterns of vocalizations between groups of male bullfrogs at two natural chorusing sites. This technique has the advantages of being able to simultaneously record sounds of all vocalizing bullfrogs within the chorus and provides estimates of their individual locations and the acoustic characteristics of their calls. Disadvantages of the technique include its computational complexity and inaccuracies in its location estimates. In particular, spatial position of individual bullfrogs within a cluster can show considerable scatter, resulting from movements of the animals during chorusing activity, reverberations produced by the heavy vegetation surrounding some areas of the pond, and inclusion of sounds other than bullfrog advertisement calls in the recorded data. Addition of more sensors to the array should alleviate these problems.
Our data reveal both individual calling behavior and complex vocal interactions between males at these sites. First, we show individual bullfrogs are not evenly spaced throughout the chorus, but often aggregate into local clusters where they are in close spatial proximity with other calling males. Second, we show that aggregation into clusters affects the types of vocal interactions in which the animals engage. In particular, we show that the rates of note and call overlap differ in relation to the spatial distances between the callers. Third, we show that aggregation into clusters modifies, but does not eliminate, the near-far sequential pattern of advertisement calling described previously based on single microphone techniques (Boatright-Horowitz et al., 2000). Clustering thus does not prevent animals from “paying attention to” the calls of noncluster residents. Fourth, we show that these patterns of vocal interactions differ from what is expected from a model in which advertisement calling occurs randomly and independently from that of other chorus residents. Finally, we show that the numbers of vocalizing male bullfrogs in a particular chorus is not stable, even over consecutive nights. Together, these results extend data from previous studies, based on visual observations and single microphone techniques, suggesting that choruses are dynamic, rather than static, assemblages (Emlen, 1976) in which individuals can change their calling strategies under particular circumstances and according to particular behavioral rules (Boatright-Horowitz et al., 2000; Greenfield and Rand, 2000; Freeberg and Harvey, 2008).
Variability in bullfrog spacing
Spacing of males within chorusing assemblages can be highly variable between species (Gerhardt and Huber, 2002) and over the course of a breeding season within the same species (Emlen, 1976). Previous observations of bullfrog chorusing behavior (Emlen, 1976; Boatright-Horowitz et al., 2000) indicated that males are highly territorial and individually spaced, with distances between nearest neighbors ranging widely, from as little as 3 to as many as 17 m, depending on chorus density and availability of calling sites. The data collected here show that vocalizing male bullfrogs, rather than maintaining distinct individual locations throughout the breeding season, often organize into smaller groups or clusters within the larger chorus. At both chorus sites, individuals within clusters are more closely spaced than single individuals in different locations, and intercluster distance is larger than spacing within a cluster. This spatial organization is defined based solely on data from vocalizing males; other studies based on visual surveys (Emlen, 1976) included nonvocalizing males in their location estimates. It is possible that nonvocalizing males are individually spaced and not part of a local cluster, but, given that these males are not vocalizing, they are not contributing to the acoustic scene of the chorus and have not been considered as part of these data. We did observe aggressive interactions (physical contact and aggressive vocalizations) between vocalizing bullfrogs within, but not between, clusters. Aggressive interactions did not appear to influence the overall chorus organization, at least over the length of our recording sessions, but, along with movements of individuals seemingly unrelated to aggressive encounters, they did complicate the accuracy of the program in pinpointing a unique location for each individual male within a cluster.
With an overall low density of chorus members (9–14 at the two ponds where clusters were observed), why would males space themselves close together, rather than making use of the entire chorusing site? One possibility is that clusters may form in especially attractive spatial locations. Since female bullfrogs oviposit in the territory of their mates, several males may stake claim to a patch of high quality territory in order to increase their chances of breeding success. Similarly, there may be some local geographic or environmental characteristic, unrelated to female choice behavior, which makes some spots more attractive to vocalizing males than other spots within the same chorus. We currently have no data with which to assess either of these possibilities. Clustering may also arise due to spatial constraints at the chorusing site. Boatright-Horowitz et al. (2000) conducted a field experiment at pond 1 six years prior to the data collection for the present study and found a similar number of bullfrogs, but all individually located and separated by a minimum of 4 m (mean separation 28 m). They did not observe any clustering of vocalizing males at that site at any time during that field season. Six years later, human encroachment onto the chorusing site had grown: most noticeably, a bike path was constructed along one margin of the pond, and changes in drainage also served to shrink the pond dimensions. It is possible that the animals may now have responded to the reduced habitat by tolerating closer neighbors out of necessity, particularly during the height of the breeding season when more bullfrogs were present. It is also possible that some of the variability in spacing we observed is related to seasonal effects. At pond 1, two nights of chorusing activity were recorded approximately 1 month apart. The first night was in early June, at the very beginning of the breeding season, and the second was in early July, when the season was well underway and more animals were present. In June, each location contained just one bullfrog, while in July a cluster of five closely spaced males was present. We currently have no geographic explanation for the clustering behavior at pond 2, except to note that location 4, where only one bullfrog was found, is the location at this pond closest to a cleared backyard where children congregated.
Local clustering of males in a larger chorus could provide some biological benefits to the vocalizing males themselves, benefits to approaching females, or to both. If males are gathered into such local aggregations, then the cost of mate assessment by females could be lower, by attracting them to a more restricted area from which several potential mates are advertising. Females would have to expend more energy traveling to different sites within the chorus in order to evaluate males close-up if those males were widely dispersed (Gerhardt and Huber, 2002). Traveling to several different sites within the chorus could also make the females more susceptible to predation. Evidence for this explanation, summarized from results of female choice behavior in different species of chorusing anurans and insects, is equivocal (Gerhardt and Huber, 2002). Aggregations of males into smaller local areas could facilitate the production of synchronous calls, which might produce a more salient acoustic stimulus (Klump and Gerhardt, 1992).
Conversely, small spatial separations between vocalizing males could lead to masking of an individual’s own advertisement call by the calls of the other males within that cluster. This in turn might negatively affect the female bullfrogs’ ability to detect and localize specific individuals, by minimizing any spatial release from masking allowed by larger spatial separations (Schwartz and Gerhardt, 1989; Bee, 2007). We argue below that the clustering of males into local calling spots increases the temporal cues in a complex signal and thus indirectly can aid in sound localization by approaching females and by far chorus residents.
Variability in calling patterns
Besides the clustering of bullfrogs into closely spaced locations, our acoustic sensor technique introduces another novel finding, differences in the kinds of acoustic interactions between and within clusters. Our earlier data on the organization of bullfrog choruses (Boatright-Horowitz et al., 2000), based on single microphone recordings, could not reliably distinguish between patterns of note overlap and call overlap, and so those kinds of complex vocal interactions were not identified or localized in that study. We show here that the aggregation of males into local clusters within a larger chorus affects the type of vocal interactions in which the males engage. In particular, alternation of entire advertisement calls and overlap between small proportions of calls are more common patterns of vocal interactions between farther-spaced males located in different clusters, while note alternation and note overlap are more commonly observed between closely spaced males located within the same cluster. The high occurrences of note alternation and note overlap within clusters suggest that male bullfrogs, like males of other anuran species (Brush and Narins, 1989; Schwartz, 1993; Greenfield and Rand, 2000; Schwartz et al., 2002), do vocalize in response to their closest neighbors. But, in some other anuran species (Schwartz, 1993; Greenfield and Rand, 2000), vocalizing males are more likely to avoid overlap with near neighbors than with farther neighbors. Some of these differences in calling patterns may be related to the different acoustic structure of advertisement calls in different species. Bullfrog advertisement calls are long duration, multiple harmonic signals emitted at relatively slow rates, while advertisement calls of neotropical frogs such as Hyla microcephala (Schwartz, 1993) are pulselike in structure and are emitted at relatively high rates.
Our data also show that, when examining between-cluster vocal bouts made up of call alternation or call overlap, bullfrogs located within clusters preferentially respond to their farther neighbors [Figs. 7B, 7C], although the pattern is not as strong as that identified earlier in a chorus where clusters of vocalizing males were not present [Boatright-Horowitz et al., 2000; see also Fig. 7A]. These comparisons again highlight the complex, dynamic patterns of vocal interactions that occur in natural choruses. As previously discussed (Boatright-Horowitz et al., 2000), near-far sequential patterns of calling might provide approaching listeners with salient, easily localizable acoustic cues. If this is so, then it raises the question of why anurans often call in close temporal and spatial proximities in patterns that lead to substantial overlap of vocalizations.
Many species that rely on vocalizations to communicate actively avoid overlapping their signals with those of conspecifics (Gerhardt and Huber, 2002). Overlapping calls may increase the difficulty faced by females in detecting and∕or localizing individual males within a chorus, perhaps by disrupting or destroying fine temporal cues in the males’ calls that are important for discrimination (Schwartz, 1987, 1993; Grafe, 1996). Calling as individuals and call alternation are common strategies to maintain acoustic space between advertising male frogs and to countervail these deleterious effects of overlap (Schwartz, 1987, 1993; Grafe, 1996). The distinction between these two different calling strategies (individual calling and call alternation) is a matter of definition (we used a 2 s intercall interval to distinguish them), but both result in one male’s call having no acoustic interference from another male’s call. Our data show a predominance of individual calls on all five nights analyzed, suggesting that bullfrogs, even those located in clusters, require some of their calls to be free from interference. Even when animals are localized into local clusters, call alternation and call overlap (defined here as an interaction when most of the notes in each male’s call occur without interference) are the most common types of acoustic interaction occurring between these clusters. Together, these calling patterns may facilitate the female’s ability to localize males by eliminating any masking produced by high amplitude signals spaced close together.
Choruses of orthopteran insects exhibit patterns of synchronous or near-synchronous calling in which calls or notes of neighbors can completely overlap (Greenfield and Roizen, 1993). No species of anuran amphibian exhibits the extreme synchrony of calling found in some of these insect choruses, but some species call in rhythmic bursts of activity in which one male’s calls seem to stimulate calling by other males, resulting in extensive call or note overlap. The benefits of call synchrony include the maintenance and amplification of species-specific temporal patterns, facilitation of the detection of female acoustic replies, and reduction in the detectability or locatability of signalers by predators (Gerhardt and Huber, 2002). Call synchrony may emerge from the operation of an inhibitory-resetting pacemaker that generates a temporal rhythm and that assumes that males pay attention to acoustic cues (i.e., each other; Greenfield and Roizen, 1993; Greenfield and Schul, 2008). It may be the result of a strategy in which each male vies to be the leader in a calling bout, either because females prefer leading calls (Minckley et al., 1995; Grafe, 1996) or in order to mask the calls of neighboring males (Grafe, 1999).
Our data show instances of note overlap, in which most of the notes of one bullfrog’s call overlapped partially or completely with the notes of another bullfrog’s call. Note overlap commonly occurred in the acoustic interactions of males located within the same cluster. The least common type of acoustic interaction we observed was note alternation, in which one bullfrog seemingly timed his notes to fall between the notes of another bullfrog. Although this could also be a strategy for avoiding masking, it may not be an efficient one for this species. Bullfrog calls consist of several notes that usually increase in duration from first to last note (Suggs and Simmons, 2005). Additionally, there is a great deal of individual variability associated with note duration and internote interval. One study reported individual note durations from nine male bullfrogs ranging from 370 to 970 ms, with a similar range for internote intervals (Simmons, 2004). The combination of variable note durations between bullfrogs and increasing note durations within each male’s call may make timing notes to precisely alternate with those of another bullfrog very difficult. Analysis of spectrograms showed that instances of note alternation were likely to merge into patterns of note overlap as number of call notes and the number of vocalizing males increased. Thus, our data suggest that note overlap and note alternation may be part of the same general strategy, that of attempting near-synchronous calling patterns within a local cluster.
Spatial organization and vocal interactions may vary seasonally. In addition to changes in the individuals making up the chorus and their relative locations, the early and midseason recordings from pond 1 also differed substantially in call activity. Early in the season, bullfrogs were much more likely to call individually than they were to engage in any type of multiple-male acoustic interaction. The opposite pattern held for the recording done in July, in which multiple-male vocal activity (across all four types of interactions) was greater than individual calling. This latter pattern was also observed on the three nights in July at pond 2, suggesting that individual calling may be more common in the early breeding season, but bouts of two or more bullfrogs calling simultaneously or in response to one another become more numerous as the season progresses. More data on seasonal variability in choruses are needed to address this issue.
Increased temporal cues in near-synchronous signals
Consistent with the hypothesis of Gerhardt and Huber (2002) regarding one function of call synchrony, note overlap (leading to note synchrony) could be a strategy for increasing the salience of species-specific temporal patterns of calling. Overlap could also increase the amplitude of the combined signal, providing another cue allowing increased salience. We examined these two possibilities by quantifying the relative amplitudes of the first harmonic frequency in notes made by individual identified bullfrogs (location 1, pond 2), both when these males called individually and when they called in note overlap with other males in that cluster. The calls showing note overlap between calls of different males were greater in amplitude, by a maximum of 3 dB, than calls by the same bullfrogs when they were calling individually. This 3 dB value is within the range of spatial release from masking observed in one study of Hyla cinerea (Schwartz and Gerhardt, 1989), but below that needed to produce the effect in Hyla chrysoscelis (Bee, 2007). In a field situation, the 3 dB increase in amplitude produced by note overlap may not be a reliable enough cue on which to base a perceptual decision.
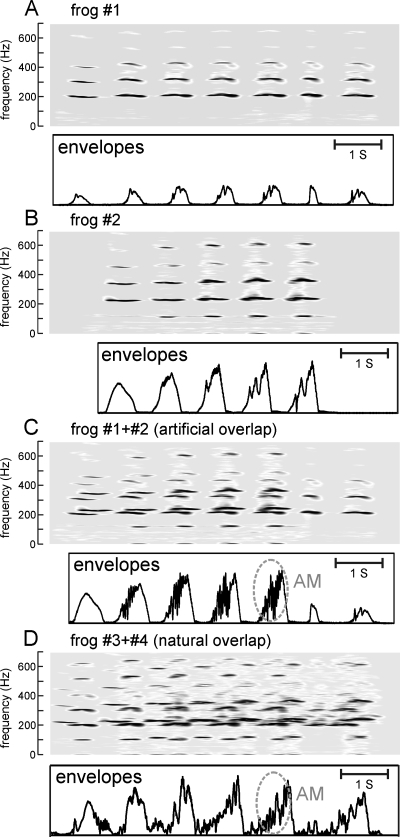
Besides producing a signal greater in amplitude, the occurrence of note overlap also altered the time domain waveforms of the resultant combined signals (Fig. 8). As previously described (Suggs and Simmons, 2005), the envelopes of successive notes in advertisement calls of individual male bullfrogs progressively increase in the rate of slow AMs. We examined the changes in the AM produced in natural call notes when these notes overlap. Figures 8A, 8B show spectrograms and envelopes of advertisement call notes from two bullfrogs (frog 1 and frog 2) calling individually. The envelopes of these notes are amplitude modulated, and the rate of AM is higher in later than in earlier notes. We then artificially overlapped these notes, producing the spectrograms and envelopes shown in Fig. 8C. The overlap produced higher AM in the envelopes of the overlapped signal than seen in the envelopes of the nonoverlapped notes. Figure 8D shows an example of actual note overlap in the vocalizations of two other bullfrogs, frog 3 and frog 4. Again, more rapid AM is added to the envelopes of the overlapped notes, due to reinforcement and cancellation from interference caused by their frequency differences. Thus, overlapping of notes produces more AM. Physiological studies of the bullfrog’s auditory system (Simmons et al., 1996, 2000) show that AM rates of 10–100 Hz, which are within the AM range present in both individual and overlapped call notes, are robustly coded by phase-locked discharges in both the eighth nerve and the auditory midbrain. Phase locking is a reliable cue for coding and discriminating the periodicities of complex signals, and the increased AM of overlapping notes could expand the strength of phase locking of these signals. This pronounced AM might serve perceptually as a “supranormal” stimulus that attracts the female’s attention to a general location in the chorus, even if it masks the unique location of specific individuals within that particular cluster. Once a female is attracted to the general vicinity of the cluster, males within that cluster may then compete for her with physical contests or by calling individually. Field studies of female bullfrog choice behavior would be necessary to determine the value of bouts between near neighbors and the perceptual salience of overlapped notes.
Figure 8.
Demonstration of how overlap of call notes results in increased AM due to interference between signals. (A) Spectrograms to 700 Hz and envelopes of the seven notes in an advertisement call of an individual bullfrog, frog 1, calling alone. (B) Spectrograms and envelopes of the five notes in an advertisement call of another bullfrog, frog 2, also vocalizing alone. (C) Spectrograms and envelopes of call notes from frog 1 and frog 2, which were artificially superimposed by aligning and mixing the calls to overlap their individual notes. (D) Spectrogram and envelopes of call notes from two other bullfrogs, frog 3 and frog 4, which the frogs themselves produced in an overlapping pattern. This is an example of actual note overlap occurring naturally. The overlapping advertisement call notes in (C) and (D) show more complex envelopes than the nonoverlapped call notes in (A) and (B). Most of the envelopes for the overlapped notes, whether artificial (C) or real (D), show roughly 10–30 cycles of AM (gray ovals) on top of the smoother envelope for the notes by themselves.
Overall, the dynamics of calling behavior that we observed suggest that chorusing bullfrogs may balance out their need to be individually heard by potential mates with the increased ease of detection that local aggregations within a chorus allows. Males thus may not only tolerate but also cooperate with very close neighbors in regulating calling activity. These data also show the importance of developing, perfecting, and implementing microphone array techniques for gathering a more comprehensive view of the strategies chorusing animals use to parse out a complex acoustic stream.
ACKNOWLEDGMENTS
Data collection and analyses were supported by the NIH Grant No. R01 DC05257 to Andrea M. Simmons and the ONR Contract No. N00014-04-1-0415 to James A. Simmons. M.G. was supported by the LEARN Program, Lafayette College. Preliminary versions of these results were presented at the Second International Conference on Acoustic Communication by Animals, Corvallis OR, Aug. 12–15, 2008, and at the 158th Meeting of the Acoustical Society of America [J. Acoust. Soc. Am. 126, 2270 (2009)].
References
- Bee, M. A. (2004). “Within-individual variation in bullfrog vocalizations: Implications for a vocally mediated social recognition system,” J. Acoust. Soc. Am. 116, 3770–3781. 10.1121/1.1784445 [DOI] [PubMed] [Google Scholar]
- Bee, M. A. (2007). “Sound source segregation in grey treefrogs: Spatial release from masking by the sound of a chorus,” Anim. Behav. 74, 549–558. 10.1016/j.anbehav.2006.12.012 [DOI] [Google Scholar]
- Boatright-Horowitz, S. L., Horowitz, S. S., and Simmons, A. M. (2000). “Patterns of vocal interaction in a bullfrog (Rana catesbeiana) chorus: Preferential responding to far neighbors,” Ethology 106, 701–712. 10.1046/j.1439-0310.2000.00580.x [DOI] [Google Scholar]
- Brush, J. S., and Narins, P. M. (1989). “Chorus dynamics of a neotropical amphibian assemblage: Comparison of computer simulation and natural behavior,” Anim. Behav. 37, 33–44. 10.1016/0003-3472(89)90004-3 [DOI] [Google Scholar]
- Burt, J. M., and Vehrencamp, S. L. (2005). “Dawn chorus as an interactive communication network,” in Animal Communication Networks, edited by McGregor P. K. (Cambridge University Press, Cambridge, UK: ), pp. 320–343. [Google Scholar]
- D’Spain, G. L., and Batchelor, H. H. (2006). “Observations of biological choruses in the Southern California Bight: A chorus at midfrequencies,” J. Acoust. Soc. Am. 120, 1942–1955. 10.1121/1.2338802 [DOI] [PubMed] [Google Scholar]
- Emlen, S. T. (1976). “Lek organization and mating strategies in the bullfrog,” Behav. Ecol. Sociobiol. 1, 283–313. 10.1007/BF00300069 [DOI] [Google Scholar]
- Forrest, T. G., and Green, D. M. (1991). “Sexual selection and female choice in mole crickets (Scapteriscus: Gryllotalpidae): Modeling the effects of intensity and male spacing,” Bioacoustics 3, 93–109. [Google Scholar]
- Freeberg, T. M., and Harvey, E. M. (2008). “Group size and social interactions are associated with calling behavior in Carolina chickadees (Poecile carolinensis),” J. Comp. Psychol. 122, 312–318. 10.1037/0735-7036.122.3.312 [DOI] [PubMed] [Google Scholar]
- Gerhardt, H. C., and Huber, F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions (University of Chicago Press, Chicago, IL: ). [Google Scholar]
- Grafe, T. U. (1996). “The function of call alternation in the African reed frog (Hyperolius marmoratus): Precise call timing prevents auditory masking,” Behav. Ecol. Sociobiol. 38, 149–158. 10.1007/s002650050227 [DOI] [Google Scholar]
- Grafe, T. U. (1997). “Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus,” Anim. Behav. 53, 1103–1117. 10.1006/anbe.1996.0427 [DOI] [Google Scholar]
- Grafe, T. U. (1999). “A function of synchronous chorusing and a novel female preference shift in an anuran,” Proc. Biol. Sci. 266, 2331–2336. 10.1098/rspb.1999.0927 [DOI] [Google Scholar]
- Greenfield, M. D., and Rand, A. S. (2000). “Frogs have rules: Selective attention algorithms regulate chorusing in Physalaemus pustulosus (Leptodactylidae),” Ethology 106, 331–347. 10.1046/j.1439-0310.2000.00525.x [DOI] [Google Scholar]
- Greenfield, M. D., and Roizen, I. (1993). “Katydid synchronous chorusing is an evolutionarily stable outcome of female choice,” Nature (London) 364, 618–620. 10.1038/364618a0 [DOI] [Google Scholar]
- Greenfield, M. D., and Schul, J. (2008). “Mechanisms and evolution of synchronous chorusing: Emergent properties and adaptive functions in Neoconocephalus katydids (Orthoptera: Telligoniidae),” J. Comp. Psychol. 122, 289–297. 10.1037/0735-7036.122.3.289 [DOI] [PubMed] [Google Scholar]
- Hailman, E. D., and Hailman, J. P. (1993). UNCERT User’s Guide, Zoology Department, University of Wisconsin, Madison, WI. Available at http://www.animalbehavior.org/Resources/CSASAB/#UNCERT (Last viewed 12/15/09).
- Hayes, S. A., Mellinger, D. K., Croll, D. A., Costa, D. P., and Borsani, J. F. (2000). “An inexpensive passive acoustic system for recording and localizing wild animal sounds,” J. Acoust. Soc. Am. 107, 3552–3555. 10.1121/1.429424 [DOI] [PubMed] [Google Scholar]
- Jones, D. L., and Ratnam, R. (2009). “Blind location and separation of callers in a natural chorus using a microphone array,” J. Acoust. Soc. Am. 126, 895–910. 10.1121/1.3158924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump, G. M., and Gerhardt, H. C. (1992). “Mechanisms and function of call-timing in male-male interaction in frogs,” in Playback and Studies of Animal Communication, edited by McGregor P. K., (Plenum, New York: ), pp. 153–174. [Google Scholar]
- McGregor, P. K., Dabelsteen, T., Clark, C. W., Bower, J. L., Tavares, J. P., and Holland, J. (1997). “Accuracy of a passive acoustic location system: empirical studies in terrestrial habitats,” Ethol. Ecol. Evol. 9, 269–286. [Google Scholar]
- Mennill, D. J., Burt, J. M., Fristrup, K. M., and Vehrencamp, S. L. (2006). “Accuracy of an acoustic location system for monitoring the position of duetting songbirds in tropical forest,” J. Acoust. Soc. Am. 119, 2832–2839. 10.1121/1.2184988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minckley, R. L., Greenfield, M. D., and Tourtellot, M. K. (1995). “Chorus structure in tarbrush grasshoppers: Inhibition, selective phonoresponse and signal competition,” Anim. Behav. 50, 579–594. 10.1016/0003-3472(95)80121-9 [DOI] [Google Scholar]
- Mohan, S., Lockwood, M. E., Kramer, M. L., and Jones, D. L. (2008). “Localization of multiple acoustic sources with small arrays using a coherence test,” J. Acoust. Soc. Am. 123, 2136–2147. 10.1121/1.2871597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, S. W., Lewis, E. R., Narins, P. M., and Lopez, P. T. (1989). “The call-timing algorithm of the white-lipped frog, Leptodactylus albilabris,” J. Comp. Physiol. [A] 164, 309–319. 10.1007/BF00612991 [DOI] [Google Scholar]
- Mountain, D., Anderson, D., Bresnahan, G., Brughera, A., Deligeorges, S., Hubbard, A.Lancia, D. and Vajda, V. (2007). EarLab: A virtual laboratory for auditory experimentation. Available at http://scv.bu.edu/SCV/vizgal/earlabnew/earlab.html (Last viewed 2/11/10).
- Schwartz, J. J. (1987). “The function of call alternation in anuran amphibians: A test of three hypotheses,” Evolution 41, 461–471. 10.2307/2409249 [DOI] [PubMed] [Google Scholar]
- Schwartz, J. J. (1993). “Male calling behavior, female discrimination and acoustic interference in the Neotropical treefrog Hyla microcephala under realistic acoustic conditions,” Behav. Ecol. Sociobiol. 32, 401–414. 10.1007/BF00168824 [DOI] [Google Scholar]
- Schwartz, J. J., Buchanan, B. W., and Gerhardt, H. C. (2002). “Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting,” Behav. Ecol. Sociobiol. 53, 9–19. 10.1007/s00265-002-0542-7 [DOI] [Google Scholar]
- Schwartz, J. J., and Gerhardt, H. C. (1989). “Spatially mediated release from auditory masking in an anuran amphibian,” J. Comp. Physiol. [A] 166, 37–41. 10.1007/BF00190207 [DOI] [Google Scholar]
- Simmons, A. M. (2004). “Call recognition in the bullfrog, Rana catesbeiana: Generalization along the duration continuum,” J. Acoust. Soc. Am. 115, 1345–1355. 10.1121/1.1643366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, A. M., Sanderson, M. I., and Garabedian, C. E. (2000). “Representation of waveform periodicity in the auditory midbrain of the bullfrog, Rana catesbeiana,” J. Assoc. Res. Otolaryngol. 1, 2–24. 10.1007/s101620010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, A. M., Shen, Y., and Sanderson, M. I. (1996). “Neural and computational basis for periodicity extraction in frog peripheral auditory system,” Aud. Neurosci. 2, 109–133. [Google Scholar]
- Simmons, A. M., Simmons, J. A., and Bates, M. E. (2008). “Analyzing acoustic interactions in natural bullfrog choruses,” J. Comp. Psychol. 122, 274–282. 10.1037/0735-7036.122.3.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedden, W. A., Greenfield, M. D., and Jang, Y. (1998). “Mechanisms of selective attention in grasshopper choruses: Who listens to whom?” Behav. Ecol. Sociobiol. 43, 59–66. 10.1007/s002650050466 [DOI] [Google Scholar]
- Suggs, D. N., and Simmons, A. M. (2005). “Information theory analysis of patterns of modulation in the advertisement call of the male bullfrog, Rana catesbeiana,” J. Acoust. Soc. Am. 117, 2330–2337. 10.1121/1.1863693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski, W., and Brenowitz, E. A. (1988). “Acoustic cues mediate inter-male spacing in a neotropical frog,” Anim. Behav. 36, 1054–1063. 10.1016/S0003-3472(88)80065-4 [DOI] [Google Scholar]