Abstract
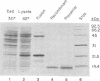
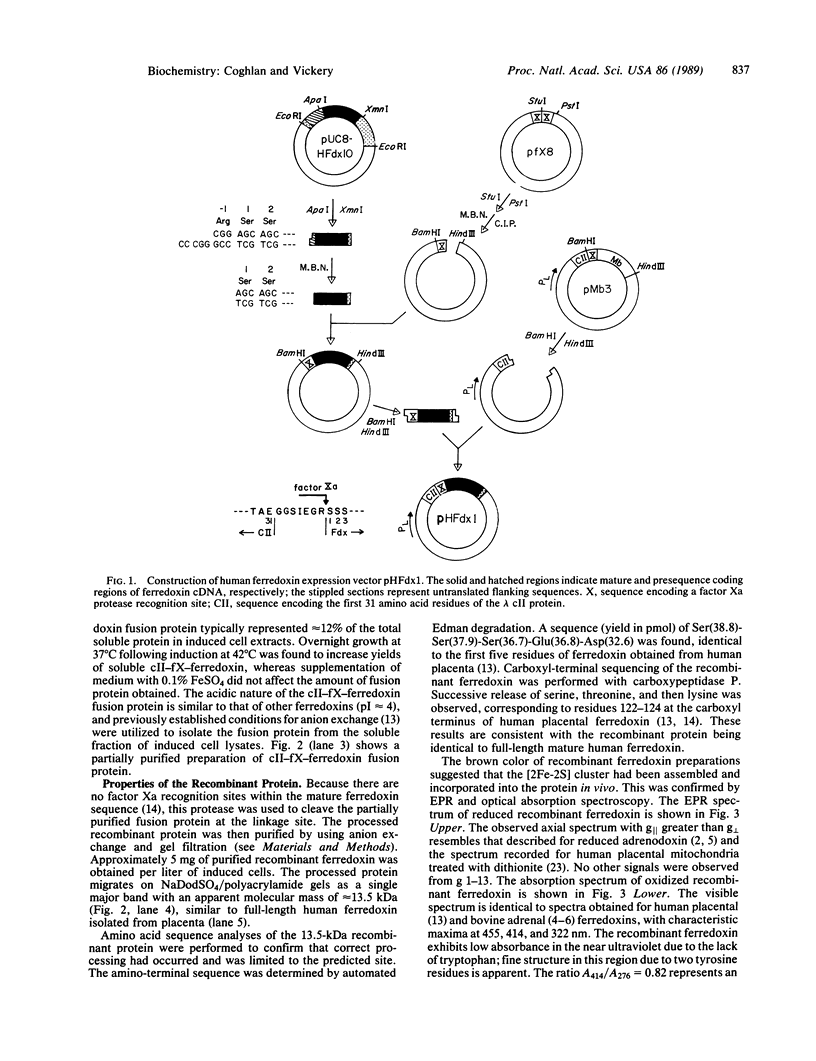
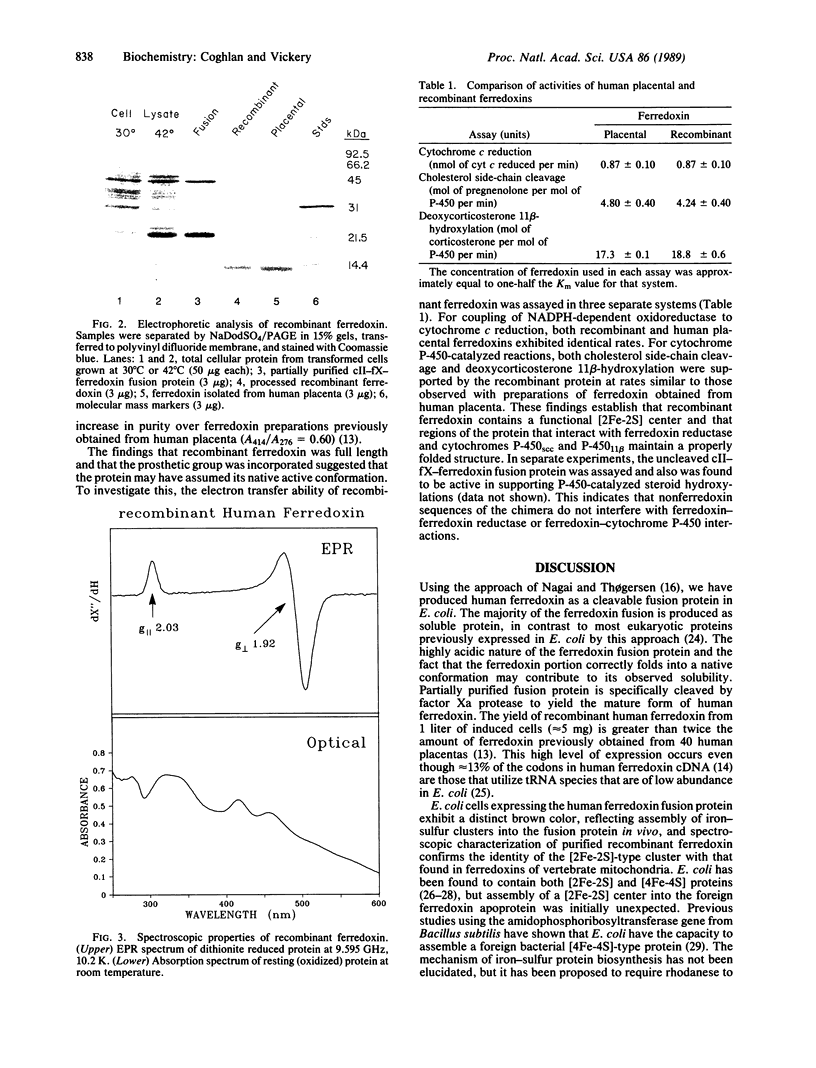
A cDNA fragment encoding human ferredoxin, a mitochondrial [2Fe-2S] protein, was introduced into Escherichia coli by using an expression vector based on the approach of Nagai and Thøgersen [Nagai, K. & Thøgersen, M. C. (1984) Nature (London) 309, 810-812]. Expression was under control of the lambda PL promoter and resulted in production of ferredoxin as a cleavable fusion protein with an amino-terminal fragment derived from bacteriophage lambda cII protein. The fusion protein was isolated from the soluble fraction of induced cells and was specifically cleaved to yield mature recombinant ferredoxin. The recombinant protein was shown to be identical in size to ferredoxin isolated from human placenta (13,546 Da) by NaDodSO4/PAGE and partial amino acid sequencing. E. coli cells expressing human ferredoxin were brown in color, and absorbance and electron paramagnetic resonance spectra of the purified recombinant protein established that the [2Fe-2S] center was assembled and incorporated into ferredoxin in vivo. Recombinant ferredoxin was active in steroid hydroxylations when reconstituted with cytochromes P-450scc and P-450(11) beta and exhibited rates comparable to those observed for ferredoxin isolated from human placenta. This expression system should be useful in production of native and structurally altered forms of human ferredoxin for studies of ferredoxin structure and function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander K., Volini M. Properties of an Escherichia coli rhodanese. J Biol Chem. 1987 May 15;262(14):6595–6604. [PubMed] [Google Scholar]
- Blum H., Poole R. K., Ohnishi T. The orientation of iron-sulphur clusters in membrane multilayers prepared from aerobically-grown Escherichia coli K12 and a cytochrome-deficient mutant. Biochem J. 1980 Aug 15;190(2):385–393. doi: 10.1042/bj1900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan V. M., Cupp J. R., Vickery L. E. Purification and characterization of human placental ferredoxin. Arch Biochem Biophys. 1988 Aug 1;264(2):376–382. doi: 10.1016/0003-9861(88)90302-5. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Parcells J. H., Wang H. P. Purification of adrenodoxin reductase, adrenodoxin, and cytochrome P-450 from adrenal cortex. Methods Enzymol. 1978;52:132–142. doi: 10.1016/s0076-6879(78)52015-6. [DOI] [PubMed] [Google Scholar]
- Kimura T., Suzuki K. Components of the electron transport system in adrenal steroid hydroxylase. Isolation and properties of non-heme iron protein (adrenodoxin). J Biol Chem. 1967 Feb 10;242(3):485–491. [PubMed] [Google Scholar]
- Knoell H. E., Knappe J. Escherichia coli ferredoxin, an iron-sulfur protein of the adrenodoxin type. Eur J Biochem. 1974 Dec 16;50(1):245–252. doi: 10.1111/j.1432-1033.1974.tb03893.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D., Seybert D. W., Lancaster J. R., Jr, Salerno J. C., Kamin H. Steroidogenic electron transport in adrenal cortex mitochondria. Mol Cell Biochem. 1982 May 28;45(1):13–31. doi: 10.1007/BF01283159. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Zalkin H., Switzer R. L., Vollmer S. J. Cloning of the Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase gene in Escherichia coli. Nucleotide sequence determination and properties of the plasmid-encoded enzyme. J Biol Chem. 1983 Sep 10;258(17):10586–10593. [PubMed] [Google Scholar]
- Matocha M. F., Waterman M. R. Discriminatory processing of the precursor forms of cytochrome P-450scc and adrenodoxin by adrenocortical and heart mitochondria. J Biol Chem. 1984 Jul 10;259(13):8672–8678. [PubMed] [Google Scholar]
- Matocha M. F., Waterman M. R. Synthesis and processing of mitochondrial steroid hydroxylases. In vivo maturation of the precursor forms of cytochrome P-450scc, cytochrome P-450(11)beta, and adrenodoxin. J Biol Chem. 1985 Oct 5;260(22):12259–12265. [PubMed] [Google Scholar]
- Meyer J., Bruschi M. H., Bonicel J. J., Bovier-Lapierre G. E. Amino acid sequence of [2Fe-2S] ferredoxin from Clostridium pasteurianum. Biochemistry. 1986 Oct 7;25(20):6054–6061. doi: 10.1021/bi00368a033. [DOI] [PubMed] [Google Scholar]
- Mittal S., Zhu Y. Z., Vickery L. E. Molecular cloning and sequence analysis of human placental ferredoxin. Arch Biochem Biophys. 1988 Aug 1;264(2):383–391. doi: 10.1016/0003-9861(88)90303-7. [DOI] [PubMed] [Google Scholar]
- Nabi N., Omura T. In vitro synthesis of NADPH-adrenodoxin reductase and adrenodoxin of bovine adrenal cortex, and the existence of a large precursor of adrenodoxin. J Biochem. 1983 Nov;94(5):1529–1538. [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Otsuka H., Tamaoki B. I. Requirement of a new flavoprotein and a non-heme iron-containing protein in the steroid 11-beta- and 18-hydroxylase system. Biochim Biophys Acta. 1966 Jul 6;122(1):34–42. doi: 10.1016/0926-6593(66)90089-0. [DOI] [PubMed] [Google Scholar]
- Palmer G., Brintzinger H., Estabrook R. W. Spectroscopic studies on spinach ferredoxin and adrenodoxin. Biochemistry. 1967 Jun;6(6):1658–1664. doi: 10.1021/bi00858a012. [DOI] [PubMed] [Google Scholar]
- Picado-Leonard J., Voutilainen R., Kao L. C., Chung B. C., Strauss J. F., 3rd, Miller W. L. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem. 1988 Mar 5;263(7):3240–3244. [PubMed] [Google Scholar]
- Sagara Y., Ito A., Omura T. Partial purification of a metalloprotease catalyzing the processing of adrenodoxin precursor in bovine adrenal cortex mitochondria. J Biochem. 1984 Dec;96(6):1743–1752. doi: 10.1093/oxfordjournals.jbchem.a135007. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Miller D. A. Cholesterol side-chain cleavage, cytochrome P450, and iron-sulfur protein in human placental mitochondria. Arch Biochem Biophys. 1978 Oct;190(2):800–808. doi: 10.1016/0003-9861(78)90340-5. [DOI] [PubMed] [Google Scholar]
- Springer B. A., Sligar S. G. High-level expression of sperm whale myoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8961–8965. doi: 10.1073/pnas.84.24.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. Improved purification of bovine adrenal iron-sulfur protein. Biochim Biophys Acta. 1972 Apr 15;263(2):272–278. doi: 10.1016/0005-2795(72)90079-7. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Szabo A., Boxer S. G. Cloning, expression in Escherichia coli, and reconstitution of human myoglobin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5681–5684. doi: 10.1073/pnas.82.17.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari H., Kimura T. Study of the adrenal non-heme iron protein (adrenodoxin) by electron spin resonance. Biochem Biophys Res Commun. 1966 Jul 6;24(1):106–112. doi: 10.1016/0006-291x(66)90417-7. [DOI] [PubMed] [Google Scholar]