Abstract
Objective:
To clinically characterize the temporal relationship between dyskinesia and the antiparkinsonian response when dyskinesia first emerges during long-term levodopa therapy and to determine if it is consistent with the hypothesized mechanism by which dyskinesia develops.
Methods:
Dyskinesia and the antiparkinsonian response to levodopa during 2-hour levodopa infusions were monitored at intervals through the first 4 years of long-term levodopa therapy in 20 subjects with idiopathic Parkinson disease (PD) and previously untreated with levodopa. The onset and offset of the antiparkinsonian response and dyskinesia were compared when dyskinesia first appeared during the 4 years. The findings were compared to 20 subjects with PD on long-term levodopa with dyskinesia and motor fluctuations.
Results:
The onset and offset of the antiparkinsonian response and dyskinesia generally coincided when dyskinesia first appeared during the 4 years and did not suggest any temporal dissociation of the 2 responses. Further, the latency to the onsets of dyskinesia and the antiparkinsonian response tended to shorten during long-term levodopa therapy, suggesting that both responses were sensitized by long-term levodopa.
Conclusions:
The similar onsets and offsets of the antiparkinsonian response and dyskinesia when dyskinesia first appears are not consistent with the postulated progressive decrease in threshold for dyskinesia during long-term levodopa therapy. Other mechanisms for the development of dyskinesia need to be considered.
GLOSSARY
- GCRC
= General Clinical Research Center;
- OHSU
= Oregon Health & Science University;
- PD
= Parkinson disease.

e–Pub ahead of print
Levodopa-induced dyskinesia is postulated to result from repeated pulsatile dopaminergic stimulation which progressively lowers the threshold for dyskinesia by sensitization.1 At the beginning of levodopa therapy, the threshold for dyskinesia is much higher than the peak plasma levodopa concentrations. Conversely, the threshold for the antiparkinsonian response is envisioned to be lower than that for dyskinesia. The hypothesized different thresholds allow dissociation of the antiparkinsonian response and dyskinesia. Continued levodopa therapy lowers the threshold for dyskinesia and dyskinesia occurs briefly at peak concentrations. Eventually, similar concentrations produce dyskinesia and the antiparkinsonian response, making the responses inseparable (figure 1A). This hypothesis assumes 1) the antiparkinsonian response and dyskinesia have distinctly different dose responses and 2) they are altered differently during long-term levodopa therapy.
Figure 1 Concepts of how dyskinesia emerges during long-term levodopa therapy
(A) Diagram illustrating how dyskinesia emerges during long-term levodopa therapy according to the hypothesis that it is related to increasing sensitivity (a lowering of the threshold) for levodopa-induced dyskinesia. Panels illustrate early (a), mid (b), and late (c) stages of long-term levodopa therapy. The key feature is the progressive lowering of the threshold for dyskinesia with the first appearance of dyskinesia only at peak dose and dissociated from the antiparkinsonian actions gradually progressing to simultaneous appearance of dyskinesia and antiparkinsonian response. The lines above the panels illustrate when the antiparkinsonian response (ANTIPD) and dyskinesia (DYS) occur during the dose cycle. (B) Modification of (A) to make it consistent with our observations. The threshold for dyskinesia and the antiparkinsonian response are identical when dyskinesia first appears and both progressively decrease during long-duration levodopa therapy.
Evidence for this hypothesized scenario is from temporal extremes. Initial levodopa therapy rarely produces dyskinesia, consistent with a high threshold for dyskinesia.2,3 After years of levodopa therapy, dyskinesia and the antiparkinsonian response occur together,2,3 indicating similar sensitivities to levodopa. However, little data exist for the intermediate period, when the antiparkinsonian response and dyskinesia are supposedly dissociated.2,3
We investigated the onset and offset of dyskinesia and the antiparkinsonian response as measures of sensitivity to levodopa when dyskinesia first appeared during long-term therapy to examine the temporal dissociation of the 2 responses. Further, we examined the changes in latencies and magnitudes of responses, indices of sensitization, during the first 4 years of therapy to determine if the evolution of the 2 responses would suggest that they originated from different mechanisms.
METHODS
Subjects.
This is a retrospective study using data generated in 3 previous Oregon Health & Science University (OHSU) institutional review board–approved studies using identical experimental paradigms, 2-hour levodopa infusions after overnight without antiparkinsonian medication in subjects with idiopathic Parkinson disease (PD).4–6 All subjects gave informed consent. The first study was a longitudinal study that followed PD subjects for 4 years from their first ever dose of levodopa. For the 2 other double-blind, intervention studies in subjects with PD with motor fluctuations and dyskinesia, the response to placebo was used for the analyses described below.
Protocol.
The protocol, conducted as an inpatient study in the OHSU General Clinical Research Center (GCRC), used 2-hour 1 mg/kg/hour levodopa infusions running between 9 am and 11 am after the subjects had been without antiparkinsonian medications overnight. Tapping speed and tremor, indices of the antiparkinsonian response, and dyskinesia were monitored at half-hour intervals from 8 am to 2 pm. A fuller description of the subjects and methods is available in the articles referenced above.
Analysis.
Onset of dyskinesia was defined by the first time that dyskinesia was scored during or up to 2 hours after the levodopa infusion and offset was defined by the last time that dyskinesia was present. The onset and offset of antiparkinsonian action was determined by the time that the tapping score in the more affected arm was 10% or greater than the mean of the 8, 8:30, and 9 am baseline tapping rates. In the Early LD Therapy group, the increase in tapping speeds was sometimes modest, and a reduction of tremor score by 2 or more points was used in 7 subjects rather than a 10% increase in tapping to determine antiparkinsonian response onset and offset.
Statistics.
The onset and offset times were not normally distributed and for that reason these data are presented as medians with 25% and 75% tails and the comparisons are with Wilcoxon signed rank tests. The changes in onset of dyskinesia and peak severity of dyskinesia during the 4 years were normally distributed and tested with paired t tests.
RESULTS
Dyskinesia and antiparkinsonian response with first dose of levodopa.
Twenty subjects received 2-hour levodopa infusions just prior to starting long-term levodopa therapy as part of the longitudinal study of the response to levodopa over the first 4 years of long-term levodopa therapy. There are 2 more subjects in addition to those described in a previous report4 because 1 subject who developed dyskinesia within the first year of the study was subsequently lost to follow-up and a second subject completed the protocol after the publication referenced above. The demographics of this study population are presented in table 1.
Table 1 Subject demographics
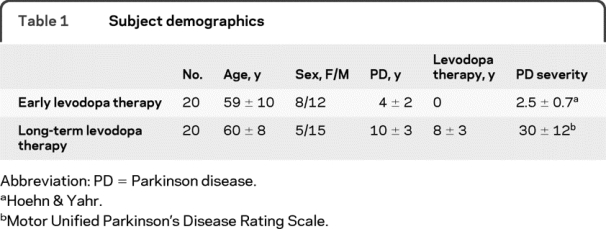
No dyskinesia was observed with the first levodopa infusion, consistent with clinical wisdom and the hypothesis that dyskinesia emerges during long-term levodopa therapy (table 2). An antiparkinsonian response was detectable in 17 of the 20 subjects.
Table 2 Onset and offset of antiparkinsonian actions and dyskinesia measured in hours from beginning of 2-hour levodopa infusion

Dyskinesia and antiparkinsonian response in subjects with motor fluctuations and dyskinesia.
The onset of increased tapping speed and of dyskinesia as well as the offset of increased tapping and dyskinesia with 2-hour levodopa infusions were compared in 20 subjects selected for having motor complications of long-term levodopa therapy and participating in 2 other protocols.5,6 The number of subjects is less than the total subjects in the 2 trials because 3 subjects participated in both studies and 3 subjects did not have a 10% increase in tapping speed to allow measurement of onset and offset of the antiparkinsonian response. Demographics of this population are presented in table 1.
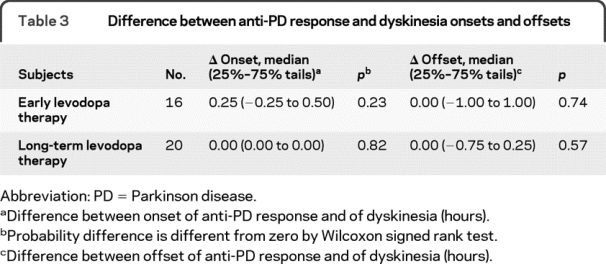
The median onset and offset of increases in tapping speed and dyskinesia were essentially identical (table 2). The median difference between the onset of the antiparkinsonian response and dyskinesia was zero as was the median difference between offsets (table 3). These findings are also consistent with clinical wisdom and the hypothesized model that in advanced subjects antiparkinsonian response and dyskinesia are generally inseparable.
Table 3 Difference between anti-PD response and dyskinesia onsets and offsets
Development of dyskinesia during the longitudinal study.
At the end of the 4-year longitudinal study, 16 of the 20 de novo subjects had developed dyskinesia. The cumulative number of patients who had exhibited dyskinesia was 8 after 6 months of levodopa therapy, 12 after 12 months, 15 after 24 months, and 16 after 48 months. The one subject in whom dyskinesia first appeared at 4 years was not studied after 2 years of long-term levodopa therapy. Dyskinesia was not observed in 2 subjects at subsequent CRC admissions after it was first seen. These 2 subjects were the 2 subjects with onset of dyskinesia at 3.5 hours after the start of the levodopa infusion in figure 2A.
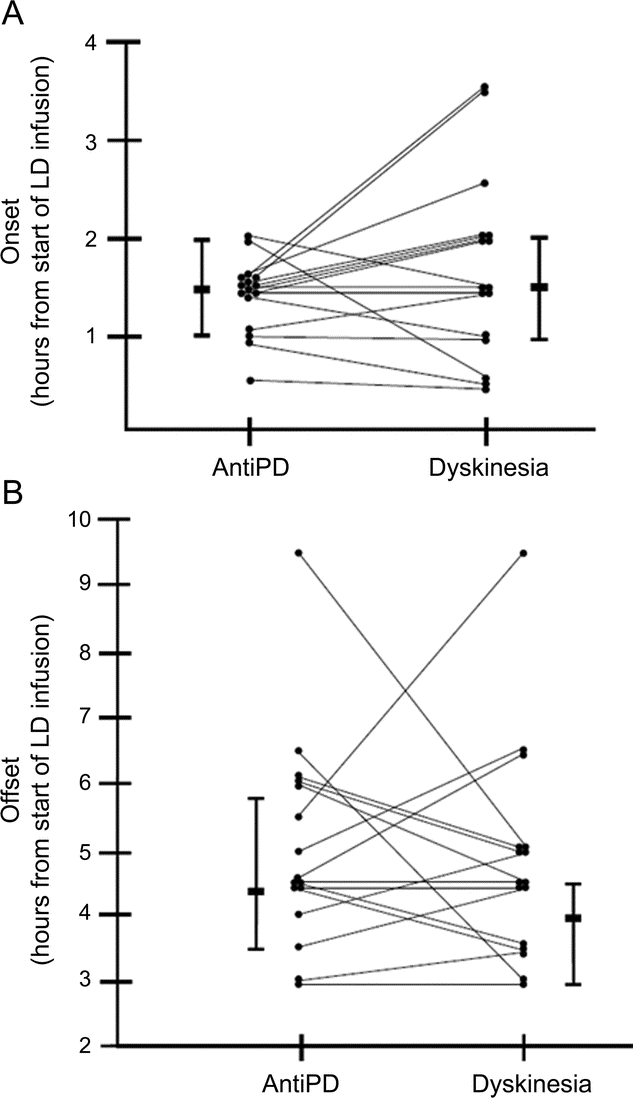
Figure 2 Onset and offset of dyskinesia and antiparkinsonian responses when dyskinesia first emerged
(A) The time of onset of the antiparkinsonian response (AntiPD) and dyskinesia after starting the 2-hour levodopa (LD) infusions on the first admission to the clinical research center when dyskinesia was observed. (B) The time of offset of the antiparkinsonian response (AntiPD) and dyskinesia after starting the 2-hour LD infusions on the first admission to the clinical research center when dyskinesia was observed. The medians and 25% and 75% tails are illustrated.
Onset and offset of antiparkinsonian response and dyskinesia when dyskinesia was first observed during the longitudinal study.
The median onset and offset times of dyskinesia when dyskinesia first appeared did not differ significantly from those of the antiparkinsonian response (table 2). In 4 subjects, dyskinesia appeared before the antiparkinsonian response; in 4 subjects, dyskinesia and antiparkinsonian response began simultaneously; and in 8 subjects, dyskinesia began after the antiparkinsonian response (figure 2A). The median difference between time of onset of the antiparkinsonian response and dyskinesia was 0.25 hour and the median difference of offset time of the antiparkinsonian response and dyskinesia was zero (table 3 and figure 2, A and B).
Change in onset and peak severity of dyskinesia.
Comparing the onset and the peak severity of dyskinesia in the 13 subjects in whom we had dyskinesia measures at 2 time points during the 4 years showed a reduced latency to onset of dyskinesia from 1.9 ± 0.9 to 1.2 ± 0.6 hours (p = 0.03) and an increased severity of dyskinesia from 2.0 ± 0.8 to 4.2 ± 3.1 (p = 0.02). We previously showed that the latency to the antiparkinsonian response shortened and the magnitude of the tapping response increased during this study.4 Further evidence for an earlier onset of antiparkinsonian response and dyskinesia with long-term levodopa therapy is also seen in the times of onset for the subjects with long-term levodopa therapy vs the subjects in the first 4 years of levodopa therapy in table 2.
DISCUSSION
Our concept of the relationship between levodopa-induced antiparkinsonian response and dyskinesia influences the manner in which we prescribe levodopa and guides research into better strategies to treat PD. Thus, characterizing this relationship between dyskinesia and the antiparkinsonian response is important.
The most important observation of this study is that the onset and offset of the antiparkinsonian response and dyskinesia were similar when dyskinesia first emerged. This observation indicates that the sensitivity or threshold for dyskinesia and for the antiparkinsonian response are similar from early in long-term therapy; as opposed to the hypothesis that the sensitivity to levodopa for the antiparkinsonian response and for dyskinesia are very different initially and only approach one another with years of levodopa therapy.1 An implication of our observation is that a therapeutic window between the antiparkinsonian response and dyskinesia does not exist except before dyskinesia emerges. Figure 1B illustrates the scenario that best fits our observations.
As sensitivity is a property of the dose-response curve, this observation also offers no evidence that the dose-response curves are different for antiparkinsonian responses and for dyskinesia. If the dose-response curves are not different, it indicates that manipulation of levodopa doses will not allow dissociation of the antiparkinsonian response and dyskinesia. Similar dose response curves could also indicate that the antiparkinsonian response and dyskinesia result from the same pharmacologic effects on the basal ganglia circuitry. That is, dyskinesia may be an integral part of the antiparkinsonian response. The fact that anti-bradykinesia effects and dyskinesia were elicited together with microstimulation of the dorsal globus pallidus interna sites would support this reasoning.7,8
A widely accepted observation is that sensitivity for the antiparkinsonian response and for dyskinesia are similar in more advanced patients2,3 and in nonhuman primates.9 This observation of identical sensitivities for the desired motor effects and for dyskinesia in more advanced patients would have to be considered a coincidence if there were different dose response curves for the antiparkinsonian response and for dyskinesia. The fact that the sensitivities for the antiparkinsonian response and for dyskinesia are identical in advanced disease is consistent with our observation that the dose-response curves (thresholds) are the same when dyskinesia first appears and are presumably the same throughout the course of the disease when levodopa induces an antiparkinsonian response and dyskinesia.
What does explain the emergence of dyskinesia if a progressive increase in sensitivity (progressive decrease in threshold) for dyskinesia does not account for the fact that dyskinesia is not present with initial treatment with levodopa but appears months or years later? An alternate hypothesis is that the magnitude of the dyskinesia response progressively increases from an effect that is so subtle as to not be recognized in early treatment, to minimal fidgeting or extraneous movements that escape the subject's attention, to full-blown involuntary movements of levodopa-induced dyskinesia. The fact that dyskinesia can emerge very quickly in severely affected or young people with PD is consistent with this argument,10,11 as is the observation that dyskinesia can be seen with the first dose of levodopa in rodents and monkeys with toxin-induced parkinsonism.12,13
The second important observation of this study is that the antiparkinsonian responses and the dyskinesia response evolved in a similar manner during the first 4 years of levodopa therapy. With time, both the increase in tapping speed and dyskinesia began with a shorter latency during the 2-hour levodopa infusions. This observation is consistent with a number of previous studies.14–17 Peak dyskinesia severity increased and the peak tapping speed also increased, most clearly seen if the long-duration response was partially removed by withholding levodopa for 3 days. A larger magnitude response has also been suggested in earlier studies.2,3,16 These apparent changes in sensitivity are incorporated in figure 1B. The shorter latency and increased magnitude of responses to a drug are characteristics of behavioral supersensitivity.18 Thus there are similar changes in the antiparkinsonian and dyskinesia responses to levodopa during the first 4 years of levodopa therapy, consistent with the concept of sensitization, but offering no evidence that the 2 responses are mediated by different pharmacologic mechanisms.
The coupling of dyskinesia and the antiparkinsonian response is also suggested by large randomized clinical trials of various pharmacologic interventions in patients with PD with motor fluctuations and dyskinesia; increased “on” time is generally accompanied by increased dyskinesia.19 The one apparent exception to the parallel effects on antiparkinsonian and dyskinesia responses is amantadine. Amantadine suppresses dyskinesia without worsening parkinsonism, as shown in several small clinical trials.20,21 But even here there is a hint that amantadine may blunt the antiparkinsonian response in primates,22 as is true with another proposed antidyskinetic drug, sarizotan.23
It is important to point out some caveats about this study. First, it is retrospective, examining studies done in our clinical research center using identical protocols but for other purposes. Secondly, the studies are acute, examining the responses to 2-hour levodopa infusions. Constant rate, 2-hour IV infusions are used to produce smooth, nearly identical time-concentration plasma levodopa curves but the difference from plasma levodopa profiles of orally administered drug may be important in some unappreciated manner. Also, avoidance of oral absorption may eliminate delayed absorption, which may be an issue in some advanced patients.24 Finally, we are primarily using finger tapping as an index of the antiparkinsonian response. Finger tapping has generally served as a drug-responsive marker for bradykinesia, the key feature of parkinsonism.25–27 In some subjects beginning levodopa therapy, we used tremor scores in lieu of tapping scores because the subjects did not have a 10% increase in tapping rate.
Our observations of the emergence and progression of dyskinesia during the first 4 years of levodopa indicate that the antiparkinsonian and dyskinesia responses to levodopa are very similar and do not suggest any obvious pharmacologic differences to allow dissociation of the 2 responses. However, absence of evidence for differences in the antiparkinsonian response and the dyskinesia response does not prove that the responses are the same. Further, some patients obtain motor benefit from levodopa without developing dyskinesia for long periods of time, proving that the antiparkinsonian response and dyskinesia do not always coexist. On the other hand, since the introduction of dl-dopa and subsequently levodopa, it has been observed that dyskinesia is a marker for a good response to the drug.28
Our modification of the current model for development of dyskinesia can be seen as a discouraging conclusion because so many therapeutic strategies seek to dissociate the dyskinesia and antiparkinsonian responses to levodopa with the belief that they are 2 different clinical responses. But our conclusions may also be a stimulus to “think outside the box.” Other pharmacologic or physiologic methods to manipulate the disordered basal ganglia circuits may be more fruitful. Another possibility is to explore the long-duration response and methods to enhance this motor response that is not associated with rapid motor fluctuations or dyskinesia.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. John G. Nutt.
ACKNOWLEDGMENT
The authors thank the subjects who participated in the protocols, the nursing staff who monitored the subjects in the Clinical Transitional Research Center, and Amy Achterman for assistance in preparation of the manuscript and figures. Byung S. Park, PhD, and Jean O'Malley, MPH, of Biostatistics Design Program in Oregon Clinical Translational Research Institute, provided statistical advice.
DISCLOSURE
Dr. Nutt has received funding for travel from Novartis and Teva Pharmaceutical Industries Ltd.; has received speaker honoraria from Novartis; has served as a consultant for XenoPort Inc., IMPAX Laboratories, Inc., Neurogen Inc., Synosia Therapeutics, and NeuroDerm, Ltd.; and has received research support from Schering-Plough Corp, the NIH (NINDS R01 NS 21062 [PI] and UL1-RR024140 [PI]), the Veterans Administration (PADRECC [Co-PI]), and the National Parkinson Foundation. Dr. Chung received a Veterans Administration Career Development Award. Dr. Holford has received consulting fees from Allergan. Dr. Holford serves as an Associate Editor of the Journal of Biopharmaceutical Statistics and on the editorial boards of Drug Metabolism and Pharmacokinetics, International Journal of Medicine and Complementary Medicine, Biopharmaceutics & Drug Disposition, Pharmacokinetics & Pharmacodynamics, Pharmaceutical Research, and the European Journal of Clinical Pharmacology; receives royalties from the publication of Basic and Clinical Pharmacology, 11th Edition (McGraw-Hill Medical, 2009); has received honoraria for lectures not sponsored by industry; serves as a consultant for Allergan, Inc., Amgen, Johnson & Johnson, Merck Serono, Eli Lilly and Company, Pfizer Inc., and Vision7; and receives research support from the Michael J. Fox Foundation.
Address correspondence to Dr. John G. Nutt, Department of Neurology, Oregon Health and Science University, Portland, OR 97239 nuttj@ohsu.edu
Editorial, page 1169
e-Pub ahead of print on March 10, 2010, at www.neurology.org.
Study funding: Supported by The Veterans Administration PD Research, Education and Clinical Center (PADRECC), the National Parkinson Foundation, and the NIH (NIH National Institute of Neurological Disorders and Stroke RO1-NS 21062 and the General Clinical Research Center RR000334, and the Oregon Clinical and Translational Research Institute UL1-RR024140). The study of the NR2B NMDA antagonist was also supported by a research grant from Pfizer Global Research and Development.
Disclosure: Author disclosures are provided at the end of the article.
Received October 1, 2009. Accepted in final form December 14, 2009.
REFERENCES
- 1.Olanow CW, Obeso JA, Stocchi F. Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nat Clin Pract Neurol 2006;2:382–392. [DOI] [PubMed] [Google Scholar]
- 2.Mouradian MM, Juncos JL, Fabbrini G, Schlegel J, Bartko JJ, Chase TN. Motor fluctuations in Parkinson's disease: central pathophysiological mechanisms, part II. Ann Neurol 1988;24:372–378. [DOI] [PubMed] [Google Scholar]
- 3.Nutt JG, Woodward WR, Carter JH, Gancher ST. Effect of long-term therapy on the pharmacodynamics of levodopa: relation to on-off phenomenon. Arch Neurol 1992;49:1123–1130. [DOI] [PubMed] [Google Scholar]
- 4.Nutt JG, Carter JH, Lea ES, Sexton GJ. Evolution of the response to levodopa during the first 4 years of therapy. Ann Neurol 2002;51:686–693. [DOI] [PubMed] [Google Scholar]
- 5.Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson's disease. Ann Neurol 2004;55:766–773. [DOI] [PubMed] [Google Scholar]
- 6.Nutt JG, Hogarth P, Weaver JJ, et al. Effects of the NR2B subunit selective NMDA receptor antagonist CP-101, 606 on motor disability in Parkinson's Disease. Mov Disord 2008;23:1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejjani B, Damier P, Arnulf I, et al. Pallidal stimulation for Parkinson's disease: two targets? Neurology 1997;49:1564–1569. [DOI] [PubMed] [Google Scholar]
- 8.Krack P, Pollak P, Limousin P, et al. Opposite motor effects of pallidal stimulation in Parkinson's disease. Ann Neurol 1998;43:180–192. [DOI] [PubMed] [Google Scholar]
- 9.Kuoppamaki M, Al-Barghouthy G, Jackson MJ, Smith LA, Quinn N, Jenner P. l-dopa dose and the duration and severity of dyskinesia in primed MPTP-treated primates. J Neural Transm 2007;14:1147–1153. [DOI] [PubMed] [Google Scholar]
- 10.Langston WJ, Ballard P. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP): implications for treatment and the pathogenesis of Parkinson's disease. Can J Neurol Sci 1984;11:160–165. [DOI] [PubMed] [Google Scholar]
- 11.Quinn N, Critchley P, Marsden CD. Young onset Parkinson's disease. Mov Disord 1987;2:73–91. [DOI] [PubMed] [Google Scholar]
- 12.Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson's disease. J Pharmacol Exp Ther 2007;323:277–284. [DOI] [PubMed] [Google Scholar]
- 13.Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson's disease: a feature inherent to the treatment or the disease? Prog Neurobiol 2009;87:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A. Pharmacodynamic modeling of oral levodopa: clinical application in Parkinson's disease. Neurology 1993;43:367–371. [DOI] [PubMed] [Google Scholar]
- 15.Colosimo C, Merello M, Hughes AJ, Sieradzan K, Lees AJ. Motor response to acute dopaminergic challenge with apomorphine and levodopa in Parkinson's disease: implications for the pathogenesis of the on-off phenomenon. J Neurol Neurosurg Psychiatry 1996;61:634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harder S, Baas H. Concentration-response relationship of levodopa in patients at different stages of Parkinson's disease. Clin Pharmacol Ther 1998;64:183–191. [DOI] [PubMed] [Google Scholar]
- 17.Sohn YH, Metman LV, Bravi D, et al. Levodopa peak response time reflects severity of dopamine neuron loss in Parkinson's disease. Neurology 1994;44:755–757. [DOI] [PubMed] [Google Scholar]
- 18.Kuczenski R, Segal DS. Psychomotor stimulant-induced sensitization: behavior and neurochemical correlates. In: Kalivas PW, Barnes CD, eds. Sensitization in the Nervous System. Caldwell, NJ: Telford Press; 1988:177–205. [Google Scholar]
- 19.Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983–995. [DOI] [PubMed] [Google Scholar]
- 20.Verhagen L, Del Dotto P, van den Mundkhof P, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease. Neurology 1998;50:1323–1326. [DOI] [PubMed] [Google Scholar]
- 21.Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson's disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000;23:82–85. [DOI] [PubMed] [Google Scholar]
- 22.Blanchet PJ, Konitsiotis S, Chase TN. Amantadine reduces levodopa-induced dyskinesia in parkinsonian monkeys. Mov Disord 1998;13:798–802. [DOI] [PubMed] [Google Scholar]
- 23.Olanow CW, Damier P, Goetz CG, et al. Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study). Clin Neuropharmacol 2004;27:58–62. [DOI] [PubMed] [Google Scholar]
- 24.Melamed E, Bitton V, Zelig O. Delayed onset of responses to single doses of l-dopa in parkinsonian fluctuators on long-term l-dopa therapy. Clin Neuropharmacol 1986;9:182–188. [DOI] [PubMed] [Google Scholar]
- 25.van Laar T, Jansen EN, Essink AW, Neef C, Dosterloo S, Roos RA. A double-blind study of the efficacy of apomorphine and its assessment in ‘off’-periods in Parkinson's disease. Clin Neurol Neurosurg 1993;95:231–235. [DOI] [PubMed] [Google Scholar]
- 26.van Hilten JJ, Wagemans EAH, Ghafoerkhan SF, van Laar T. Movement characteristics in Parkinson's disease: determination of dopaminergic responsiveness and threshold. Clin Neuropharmacol 1997;20:402–408. [PubMed] [Google Scholar]
- 27.Homann CK, Suppan K, Wenzel K, et al. The bradykinesia akinesia incoordination test (BRAIN Test), an objective and user-friendly means to evaluate patients with parkinsonism. Mov Disord 2000;15:641–647. [DOI] [PubMed] [Google Scholar]
- 28.Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med 1967;276:374–379. [DOI] [PubMed] [Google Scholar]



