Abstract
Background:
Antiretroviral medications have been shown to benefit neurocognition in HIV/AIDS, and neurocognitive deficits are a risk factor for poor adherence to these medications. However, little is known about the predictive pathways linking medication adherence with cognitive ability.
Methods:
In the current 6-month cohort study, antiretroviral medication adherence was tracked prospectively among 91 HIV-positive adults using electronic monitoring. Comprehensive neuropsychological evaluations were performed at baseline and 6 months.
Results:
Multivariate path analyses provided evidence that antiretroviral adherence and cognitive ability are reciprocally related, although the neurocognitive pathways of this relationship appear to vary by predictive direction. Executive function and learning/memory were most strongly predictive of levels of medication adherence achieved, whereas higher levels of adherence were predictive of relative improvements in a wide range of frontostriatal brain functions including processing speed, attention, executive functions, and motor functioning.
Conclusions:
These data provide evidence that cognition and adherence are reciprocally related in HIV/AIDS. In particular, executive dysfunction may play a key role in this relationship. Interventions aimed at improving or preserving executive functions could hold promise for interrupting progressive declines in adherence and neurocognitive ability in HIV/AIDS.
GLOSSARY
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders, 4th edition;
- HAART
= highly active antiretroviral therapy;
- MEMS
= Medication Event Monitoring System;
- PI
= protease inhibitor.
Antiretroviral treatment regimens have demonstrated effectiveness in preventing or remediating HIV-related cognitive impairments.1,2 Unfortunately, only 50%–60% of HIV-positive patients achieve the consistently high levels of antiretroviral medication adherence (e.g., >90%) required for sustained viral suppression and immune reconstitution.3–6 To the degree that meticulous medication adherence results in the improvement or protection of cognitive ability, adherence and cognition should covary over time. Consistent with this expectation, the cognitive domains that have been found to be most frequently impacted by HIV-related neurodegeneration are also among the domains most consistently associated with adherence.6–8 Likewise, individuals with cognitive deficits (particularly in retrospective memory, prospective memory, or executive functions) are at increased risk for poor medication adherence.9–11
Considering this literature collectively, it would be reasonable to infer that a bidirectional relationship may exist between cognition and antiretroviral medication adherence among individuals with HIV/AIDS. This could potentially involve a recursive feedback loop whereby disease-related cognitive decline could be progressively amplified through corresponding decrements in medication adherence. However, this issue has not yet been examined directly. Guided by the pattern of previous findings, in the current 6-month observational study we hypothesized that higher levels of baseline ability in the domains of executive functions and learning/memory would predict better prospective medication adherence,9,11 and that greater levels of adherence would predict better functioning at 6-month follow-up across all cognitive domains measured,12,13 independently of the effect of baseline neurocognitive performance.
METHODS
Standard protocol approvals, registrations, and patient consents.
All research methods and procedures were approved by institutional review board panels at UCLA and the West Los Angeles VA Medical Center. Written informed consent was obtained from all participants.
Participants.
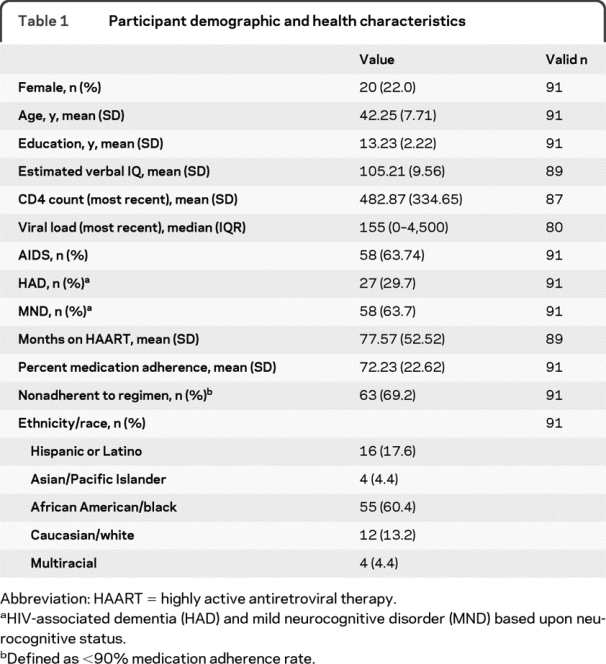
Recruitment of HIV-positive adults was conducted from community agencies in the Los Angeles area that specialize in providing services to HIV-infected individuals, and using fliers posted in infectious disease clinics at university-affiliated medical centers. All individuals were prescribed self-administered highly active antiretroviral therapy (HAART) during their period of participation. All visits took place between November 2001 and November 2005. The initial participant sample included 276 individuals examined for eligibility. A total of 157 individuals were excluded based upon criteria including current alcohol or drug dependence (109 individuals), history of stroke (14 individuals), seizure disorder (22 individuals), anoxic injury (3 individuals), traumatic brain injury with loss of consciousness greater than 30 minutes (26 individuals), or other non-HIV-related neurologic illnesses (9 individuals). Six follow-up visits were scheduled at intervals of approximately 1 month. A total of 28 individuals missed the sixth visit (thereby missing the “time 2” neuropsychological re-evaluation) and were excluded from analysis. Demographic and health characteristics for the final analytic sample of 91 participants are presented in table 1. For this final sample, first visit to final visit duration was M = 6.24 (SD = 0.55) months. Participants who were included, those who were excluded, and those lost to follow-up did not differ significantly on any demographic, health, or neurocognitive characteristics of interest (see table e-1 on the Neurology® Web site at www.neurology.org).
Table 1 Participant demographic and health characteristics
Measures.
Medication adherence.
Adherence to an antiretroviral medication was tracked prospectively over approximately 6 months using the Medication Event Monitoring System (MEMS [Aprex, Union City, CA]), which employs a pressure-activated microprocessor in the medication bottle cap to record the date, time, and duration of dosing events. These data were later downloaded from the bottle cap using a personal computer. Priority of the medication chosen for MEMS monitoring was in the following order: protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, nucleotide reverse transcriptase inhibitors, and other classes of medication. In order to maximize MEMS data accuracy, participants were instructed not to use pill organizers or take “pocket doses” (e.g., removing multiple pills for later use), and to open the MEMS cap only when taking a dose. Additionally, the following corrections were made to MEMS data in order to account for potentially superfluous bottle openings: only one MEMS cap opening was counted per 2-hour period, number of MEMS cap openings per day was truncated to the total number of prescribed doses, and MEMS cap openings performed by the research team were removed from the data. MEMS adherence was calculated as [(doses taken)/(doses prescribed)] for each of the 6 monthly monitoring intervals. For use in data analyses, total MEMS adherence was calculated as the mean of monthly adherence rates.
Neurocognition.
Participants completed a fixed neuropsychological test battery (see appendix e-1) at baseline and during the final follow-up visit. Raw test scores were converted to demographically corrected t scores using published normative data. Individual test t scores were grouped into 1 of 6 cognitive domains (attention, information processing speed, learning/memory, verbal fluency, motor functioning, and executive functioning) and averaged to establish domain t scores for use in primary data analyses.
Additional measures.
Modified substance use modules from the Structured Clinical Interview for DSM-IV14 were administered to evaluate criteria related to alcohol and substance use disorders. Verbal IQ was estimated using the American version of the National Adult Reading Test.15 Immunologic information including CD4 count and HIV viral load were provided by participants via medical records. Total CNS Penetration Effectiveness rank for each participant's antiretroviral regimen was calculated using previously described methods.16
Procedure.
After providing written informed consent, participants completed a detailed demographic questionnaire and structured psychiatric interview under the supervision of a licensed clinical psychologist. Participants received instruction in how to use the MEMS caps and were scheduled for 6 follow-up visits, during which data were downloaded from the device. At both the baseline and 6-month follow-up visits, participants completed fixed neuropsychological evaluations administered by trained psychometrists under the supervision of a board-certified neuropsychologist.
Analysis.
The SPSS 16.0 statistical package was used for all data analyses, with pairwise exclusions for missing values (2.1% of all data points). Descriptive statistics were conducted on all variables to summarize the data. Path analyses were then conducted using a series of regressions to examine the relationships of medication adherence to neurocognition in each domain at time 1 and time 2. This process was repeated for each of the cognitive domains examined (global cognition, processing speed, attention, executive function, learning/memory, motor function, and verbal fluency).
RESULTS
Mean cognitive performance at time 1 and time 2 is presented in table 2 by cognitive domain. As shown, mean performance at all time points in this HIV-positive sample was below the average score of t = 50 by comparison to population norms (global cognition M ranged from 42.53 to 43.56; domain Ms ranged from 38.31 to 45.15). Dependent samples t tests demonstrated small but significant increases in performance from time 1 to time 2 in global cognition as well as the individual domains of processing speed and executive function, with a trend for an increase in learning/memory performance (p = 0.053). No change over time was found for the domains of attention (p = 0.74), motor function (p = 0.71), or verbal fluency (p = 0.45).
Table 2 Mean cognitive performance at time 1 and time 2 by domain
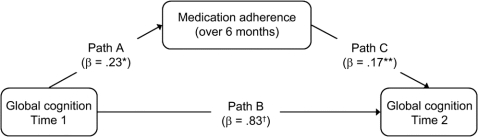
A series of path analyses were then conducted to examine reciprocal relationships between global cognition and antiretroviral medication adherence, as depicted in the figure. First, estimates were obtained for relationships between time 1 cognition and medication adherence (path A) using regression. Next, path B (the relationship between time 1 cognition and time 2 cognition) and path C (the relationship between adherence and time 2 cognition) were evaluated simultaneously, providing multivariate estimates of these relationships, free from potential bias related to the strong autocorrelation of cognition over time. Whereas primary hypotheses were evaluated based upon the significance of regression weights in each stage of analysis (for cognition predicting adherence, or adherence predicting cognition), R2 statistics were also examined in order to evaluate the total effect size for each relationship. In order to provide multivariate estimates of paths B and C under the assumption that predictive variance shared by these paths should be conservatively attributed to path B, partial R2 was examined for path B, and semi-partial R2 was examined for path C. Parameters of interest obtained from path analyses are presented in table 3 for each neurocognitive domain.
Figure Model of longitudinal path analysis of global cognition and medication adherence
N = 91; values for paths B and C represent multivariate results; *p < 0.05, **p < 0.01, †p < 0.001.
Table 3 Results of longitudinal path analysis of cognition and medication adherence
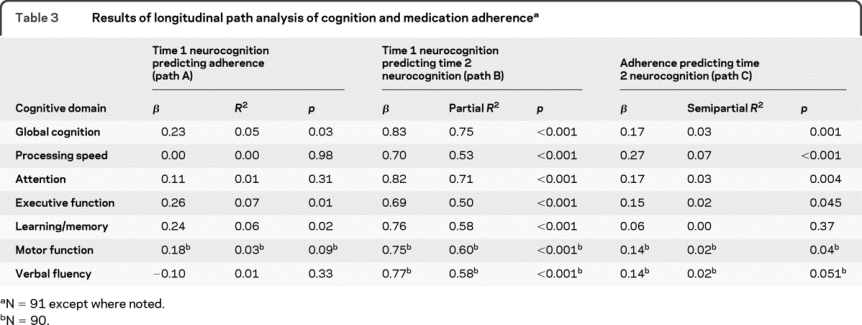
As shown for path A, better global cognition at time 1 predicted higher levels of total medication adherence over the subsequent 6-month period (β = 0.23, R2 = 0.05, p = 0.03). This global effect appears to be primarily related to the individual domain effects of executive function (β = 0.26, R2 = 0.07, p = 0.01) and learning/memory (β = 0.24, R2 = 0.06, p = 0.02). Processing speed, attention, and verbal fluency were not significant predictors of medication adherence. As would be expected for path B, neurocognition at time 1 was strongly predictive of neurocognition at time 2 (global cognition β = 0.83, partial R2 = 0.75, p < 0.001; domain-level partial R2 ranged from 0.50 to 0.71), highlighting the importance of removing this potential source of bias from estimates of path C. Evaluating path C, higher levels of medication adherence were predictive of better global cognition at time 2 (β = 0.17, semipartial R2 = 0.03, p < 0.001), driven by domain-level associations with processing speed (β = 0.27, semipartial R2 = 0.07, p < 0.001), attention (β = 0.17, semipartial R2 = 0.03, p = 0.004), executive function (β = 0.15, semipartial R2 = 0.02, p = 0.045), and motor function (β = 0.14, semipartial R2 = 0.02, p = 0.04), whereas the effect of adherence on learning/memory was nonsignificant (β = 0.06, semipartial R2 = 0.00). Notably, a fully reciprocal adherence–cognition relationship was demonstrated by global cognition and the domain of executive function, which were significant as both predictors and dependent variables in all primary analyses.
Follow-up control analyses were then conducted to evaluate the possible influence of premorbid intellectual abilities, differences in the CNS penetration of participants' medication regimens, and changes in substance or alcohol abuse status between time 1 and time 2. Relevant path analytic relationships were reevaluated after the inclusion of each of these variables individually. Controlling for estimated premorbid verbal IQ, the strength of path A was somewhat attenuated for global cognition (β = 0.15, p = 0.21) and executive function (β = 0.16, p = 0.18) relative to primary findings, but path A was only minimally attenuated for learning/memory (β = 0.21, p = 0.047). These control findings were consistent with the strong univariate associations verbal IQ shared with baseline global cognition (r = 0.50, p < 0.001) and executive function (r = 0.46, p < 0.001) in comparison to the more modest association between verbal IQ and learning/memory (r = 0.22, p = 0.04). Primary findings for Paths B and C were minimally impacted by premorbid IQ control analyses. Overall, these follow-up analyses suggested that some psychometric overlap existed between premorbid intellect and the cognitive abilities predictive of adherence, such as executive function and global cognition, but differences in premorbid IQ did not account for a meaningful proportion of the prospective relationship between medication adherence and cognitive abilities. Likewise, in a parallel series of control analyses, primary path analytic results were not meaningfully impacted by differences in the CNS penetration of participants' antiretroviral regimens, or by changes in participants' substance or alcohol abuse status (incident/relapsing or remitting diagnoses) from time 1 to time 2.
DISCUSSION
Findings from this study provide evidence that neurocognition and antiretroviral medication adherence are reciprocally related among individuals with HIV/AIDS. Although the proportion of adherence that could be predicted by global cognition (R2 = 0.05) and the proportion of global cognition that could be uniquely predicted by adherence (semipartial R2 = 0.03) were each relatively small, the clinical significance of these findings may be magnified by the potential for such effects (examined here for a 6-month period) to continue over the much longer time frames of treatment required for most individuals with HIV/AIDS. These findings highlight the importance of obtaining information on patients' neurocognitive functioning, as both a predictor of possible challenges in achieving good medication adherence, and a potential reflection of relative adherence and treatment outcome.
Additionally, examination of individual cognitive subdomains demonstrated some divergence between the aspects of cognition most strongly predictive of adherence to one's medication regimen and the aspects of cognition most strongly predicted by levels of adherence achieved. Consistent with previous findings using a laboratory-based medication management task,11 executive function and learning/memory were found in this study be the best predictors of future adherence. As such, the presence of deficits in these areas in particular may serve as a useful warning for clinicians about possible problems with future adherence. Follow-up analyses suggested that primary findings were not meaningfully impacted by differences in the CNS penetration of participants' antiretroviral regimens or changes in participants' substance or alcohol abuse status, but a portion of the apparent adherence advantage conferred by better baseline cognition may have been related to participants' levels of premorbid cognition/intellect. By comparison, higher levels of medication adherence were predictive of improved functioning in a wide range of cognitive abilities, most prominently including information processing speed, but also attention, executive function, and motor function. These latter findings were largely unrelated to participants' premorbid IQ.
Providing support for the existence of a recursive feedback loop between medication adherence and cognition in HIV/AIDS, executive function was involved in each direction of this relationship. This finding suggests that problems with executive function could play a role in perpetuating cognitive decline, and possibly, the progression of disease severity. Therefore, interventions targeting executive functions that may be effective in interrupting this potentially vicious cycle (such as medications, cognitive remediation/compensation, or environmental structuring) are worthy of examination.
Surprisingly, improved antiretroviral adherence was not prospectively predictive of better learning/memory in this study. Follow-up analyses accounting for differences in the CNS penetration of participants' medication regimens did not alter these findings. Considering that negative effects of HIV/AIDS on learning/memory have been well-documented in previous studies,13,17 it is possible that that the antiretroviral medications used by participants in this sample may have offered better protection against decline in functions typically associated with frontal-subcortical neuropathology than the learning/memory functions more commonly associated with medial temporal brain areas. However, to the degree that frontostriatal systems may be differentially sensitive to HIV-related damage,18 these findings might also reflect relatively less potential for decline in learning/memory abilities among our participants during this 6-month study.
However, without the inclusion of an HIV-negative control group, it was impossible to reliably estimate absolute levels of HIV-related decline for participants in this study. Therefore, additional research comparing the neuroprotective benefits of antiretroviral medications by cognitive domain will be needed in order to resolve this issue and to identify medication regimens that provide optimal protection of cognitive functioning in HIV/AIDS.
Multivariate path analysis, as employed in this study, provides a number of noteworthy advantages in the examination of longitudinal relationships of interest. This relatively straightforward, regression-based approach allows for the evaluation and dissociation of overlapping effects (e.g., the potential effects of cognition on adherence, and adherence on cognition), while circumventing the inflated estimates and systematic bias that would otherwise result from the strong relationship of cognition to itself over time. This approach should also offer similar benefits for a variety of other research populations in which neurologic factors and behavioral or psychological factors (e.g., medication or substance use, psychopathology) might be hypothesized to impact each other over time. Additionally, it should be noted that this statistical approach automatically controls for linear changes in measures that are repeated longitudinally. For example, factors such as practice effects and regression to the mean would not be expected to affect estimates of cognition–adherence relationships in the current study.
Nevertheless, a number of limitations may temper the strength and generalizability of the current findings. For example, some variables that might be expected to moderate adherence–cognition relationships, such as age6,19 and regimen complexity,9 were not evaluated due to limitations related to sample size (e.g., reduced statistical power) and demographic constraints (e.g., few older participants). Though it is often impractical to include all potentially relevant variables in any single study or analysis, it should be noted that the exclusion of influential variables may affect the estimates obtained for relationships among variables of interest. Further research examining moderators of the adherence–cognition relationship and comparing differing medication regimens will be crucial to the continued improvement of interventions to prevent and remediate HIV-associated neurocognitive impairment. Additionally, the effects shown here reflect a longitudinal interval of approximately 6 months, and it is possible that some aspects of the adherence–cognition relationship may be better examined using shorter or longer time frames. Related to the known immunologic importance of high antiretroviral medication adherence rates among individuals with HIV/AIDS,3–5 it is also possible that these findings may not generalize well to patient groups with substantially better (or worse) total adherence than the modest 72% mean adherence achieved by participants in this study. Likewise, our sample consisted primarily of males, and therefore it is possible that our results do not adequately represent the relationships of interest among females. Finally, repeated measurements of disease parameters such as CD4 count and HIV viral load were not available, thus limiting our ability to examine the potential relevance of changes in these variables to the adherence–cognition relationship. Further study of these issues using larger samples, a greater proportion of older and female individuals, alternate time frames, and more extensive measurement of HIV disease parameters will be needed to replicate and extend current findings.
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Mark L. Ettenhofer.
DISCLOSURE
Dr. Ettenhofer and Dr. Foley report no disclosures. Dr. Castellon performs and bills for neuropsychological assessment in his clinical practice (5% effort) and receives research support from the NIH (RO1 MH083553 [Co-I] and T32 MH19535 [Co-I]), the Veterans Administration (Merit Review Grant), and the Breast Cancer Research Foundation. Dr. Hinkin performs and bills for neuropsychological assessment in his clinical practice (15% effort) and receives research support from the NIH (RO1 MH083553 [PI] and T32 MH19535 [PI]) and the Veterans Administration (Merit Review Grant).
Supplementary Material
Address correspondence and reprint requests to Dr. Mark L. Ettenhofer, USUHS Department of Medical and Clinical Psychology, 4301 Jones Bridge Road, Bethesda, MD 20814 mark.ettenhofer@usuhs.mil
Supplemental data at www.neurology.org
e-Pub ahead of print on March 10, 2010, at www.neurology.org.
Study funding: Supported by NIH R01 DA13799 to C.H.H. and NIH Ruth L. Kirschstein National Research Service Award T32 MH19535.
Disclosure: Author disclosures are provided at the end of the article.
Received June 6, 2009. Accepted in final form January 27, 2010.
REFERENCES
- 1.Martin EM, Pitrak DL, Novak RM, Pursell KJ, Mullane KM. Reaction times are faster in HIV-seropositive patients on antiretroviral therapy: a preliminary report. J Clin Exp Neuropsychol 1999;21:730–735. [DOI] [PubMed] [Google Scholar]
- 2.Suarez S, Baril L, Stankoff B, et al. Outcome of patients with HIV-1-related cognitive impairment on highly active antiretroviral therapy. AIDS 2001;15:195–200. [DOI] [PubMed] [Google Scholar]
- 3.Clerici M, Seminari E, Maggiolo F, et al. Early and late effects of highly active antiretroviral therapy: a 2 year follow-up of antiviral-treated and antiviral-naive chronically HIV-infected patients. AIDS 2002;16:1767–1773. [DOI] [PubMed] [Google Scholar]
- 4.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr 2000;23:386–395. [DOI] [PubMed] [Google Scholar]
- 5.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30. [DOI] [PubMed] [Google Scholar]
- 6.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS 2004;18 suppl 1:S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinkin CH, Hardy DJ, Mason KI, et al. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc 2002;8:532–538. [DOI] [PubMed] [Google Scholar]
- 8.Waldrop-Valverde D, Ownby RL, Wilkie FL, Mack A, Kumar M, Metsch L. Neurocognitive aspects of medication adherence in HIV-positive injecting drug users. AIDS Behav 2006;10:287–297. [DOI] [PubMed] [Google Scholar]
- 9.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology 2002;59:1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH. Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Int Neuropsychol Soc 2009;15:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert SM, Weber CM, Todak G, et al. An observed performance test of medication management ability in HIV: relation to neuropsychological status and medication adherence outcomes. AIDS Behav 1999;3:121–128. [Google Scholar]
- 12.Cysique LA, Maruff P, Brew BJ. The neuropsychological profile of symptomatic AIDS and ADC patients in the pre-HAART era: a meta-analysis. J Int Neuropsychol Soc 2006;12:368–382. [DOI] [PubMed] [Google Scholar]
- 13.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002;8:410–424. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: history, rationale, and description. Arch Gen Psychiatry 1992;49:624–629. [DOI] [PubMed] [Google Scholar]
- 15.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol 1991;13:933–949. [DOI] [PubMed] [Google Scholar]
- 16.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki PM, Cohen MH, Weber K, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology 2009;72:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaton R, Grant I, Butters N, et al. The HNRC 500-neuropsychology of HIV infection at different disease stages: HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995;1:231–251. [DOI] [PubMed] [Google Scholar]
- 19.Ettenhofer ML, Hinkin CH, Castellon SA, et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry 2009;17:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.