Abstract
One of the first hallmarks of kidney regeneration is the reactivation of genes normally required during organogenesis. Identification of chemicals with the potential to enhance this reactivation could therapeutically promote kidney regeneration. Here, we found that 4-(phenylthio)butanoic acid (PTBA) expanded the expression domains of molecular markers of kidney organogenesis in zebrafish. PTBA exhibits structural and functional similarity to the histone deacetylase (HDAC) inhibitors 4-phenylbutanoic acid and trichostatin A; treatment with these HDAC inhibitors also expanded the renal progenitor cell population. Analyses in vitro and in vivo confirmed that PTBA functions as an inhibitor of HDAC activity. Furthermore, PTBA-mediated renal progenitor cell expansion required retinoic acid signaling. In summary, these results support a mechanistic link among renal progenitor cells, HDAC, and the retinoid pathway. Whether PTBA holds promise as a therapeutic agent to promote renal regeneration requires further study.
The zebrafish embryo is a viable model organism for use in chemical library screens.1,2 Such screens have provided insight into developmental events and have yielded lead compounds for combating human disease.3–5 In zebrafish, the pronephric kidney serves as the functional larval kidney. The pronephros consists of a pair of nephrons connected at the dorsal midline to a compound glomerulus.6 Despite this simplicity, the pronephros contains cell types typical of more complex kidneys.7 Pronephric development depends on the renal progenitor cells that populate the intermediate mesoderm.8 These cells express several genes that define the size of the kidney field, including lhx1a (formerly lim1), pax2a, and pax8.8–10
Regenerating proximal tubule kidney cells express genetic markers normally associated with embryonic renal progenitor cells, including Lhx1, Pax2, Wnt4, Bmp7, and the Notch signaling pathway.11–16 These markers appear within the first 24 hours after injury.12 Expression of these developmental markers is thought to be required for the differentiation of regenerating tubule cells. The mechanism regulating gene reactivation after kidney damage is unknown; however, one study demonstrated that regeneration after renal ischemia is associated with a reduction in histone deacetylase (HDAC) activity.16
A majority of chemicals that function as HDAC inhibitors (HDACis) demonstrate equal efficacy against multiple HDAC classes.17 This functional conservation across diverse structures suggests that the effect of an individual HDACi in vivo could reveal a general mechanistic effect.18 Therapeutically, HDACis have been used against many cancers types, including acute promyelocytic leukemia.19 HDACis have also been shown to reduce the extent of damage in models of ischemic tissue injury in the brain and myocardium.20,21 Importantly, the HDACi trichostatin A (TSA) has been shown to attenuate renal injury in mice.22
One functional consequence of HDAC activity is to regulate retinoic acid (RA) signaling. RA activates transcription by binding to RA receptor (RAR) dimers located on RA response elements in target promoters.23 The absence of RA results in the enlistment of co-repressors by RARs to silence gene activity by engaging HDACs.24 The RA pathway plays an important role in formation of the kidney field. Ectopic activation of RA signaling increases the size of the kidney field, whereas blocking the pathway impairs development.25,26 It has been proposed that HDACis can attenuate RA–RAR complexes, lowering the threshold of RA necessary to activate transcription.27
Evidence suggests that the mechanisms of kidney repair parallel those of organogenesis12,28; therefore, compounds capable of stimulating renal progenitor cell proliferation could improve the regeneration response. Toward this end, we designed a simple small molecule screen using zebrafish embryos. We paired a straightforward indicator of potential kidney effects with a small but diverse chemical library and identified 4-(phenylthio)butanoic acid (PTBA). We demonstrate that PTBA treatment induces an expansion of the renal progenitor cells through a mechanism that involves HDAC inhibition.
Results
PTBA Treatment Expands the Kidney Field
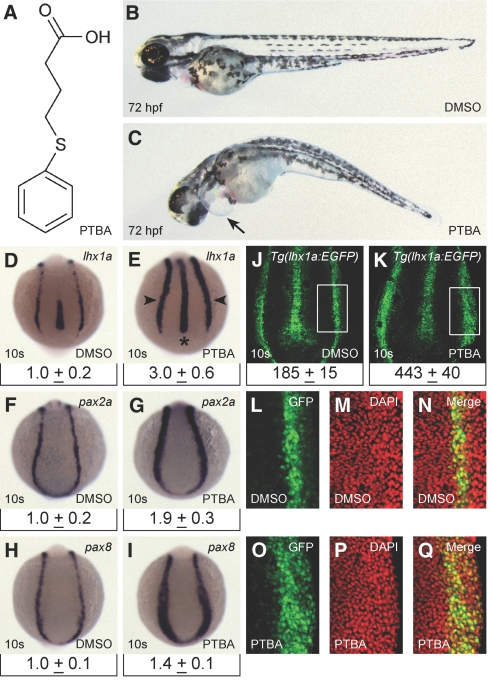
We performed a kidney screen in zebrafish embryos using a library of small molecules with diverse structures (see the Concise Methods section). From this screen, we identified a compound, PTBA (Figure 1A), that caused pericardial edema and axis curvature at 72 hours postfertilization (hpf; Figure 1, B and C). We re-synthesized PTBA and confirmed its structure (see the Supplemental Methods section).
Figure 1.
PTBA treatment expands the pool of renal progenitor cells. (A) Structure of PTBA. (B and C) Zebrafish larvae at 72 hpf treated with 0.5% DMSO (B) or 3 μM PTBA (C). Arrow indicates pericardial edema. (D through I) In situ hybridization for lhx1a (D and E), pax2a (F and G), pax8 (H and I), in 10-somite embryos treated with 0.5% DMSO (D, F, and H) or 3 μM PTBA (E, G, and I). As compared with controls (n = 60 [D], n = 60 [F], and n = 59 [H]), observed expansion in response to PTBA treatment is 95% for lhx1a (n = 60 [E]), 97% for pax2a (n = 60 [G]), and 95% for pax8 (n = 59 [I]). Arrowheads indicate renal progenitor cells, asterisk indicates notochord. Relative qPCR for lhx1a, pax2a, and pax8 in the trunk region of 10-somite embryos (n = 4, 240 embryos) is displayed under corresponding in situ image. Data are mean expression plus 95% confidence interval. Expression is normalized to β-actin and SDHA transcript levels. (J through O) Confocal projections of 10-somite Tg(lhx1a:EGFP)pt303 embryos treated with 0.5% DMSO (n = 18 [J and L through N]) or 3 μM PTBA (n = 21 [K and O through Q]). Boxed areas are regions that were counted for GFP- and DAPI-positive nuclei (J and K) and are shown in L and O (GFP), M and P (DAPI), and N and Q (merge). Cell counts are mean number of positive cells plus 95% confidence interval for each condition (J and K).
To determine whether PTBA affected the kidney field, we performed pax2a in situ hybridization. Embryos treated with 1 to 5 μM PTBA exhibited a concentration-dependent expansion of pax2a expression that coincided with developmental delay (Supplemental Figure 1). To maximize the PTBA effect on expansion of the kidney field while minimizing developmental delay, we chose 3 μM PTBA as our working concentration. Treatment with 3 μM PTBA caused 92% (n = 88) of the embryos to develop edema by 72 hpf without eliciting significant toxicity (as assayed by death; Supplemental Figure 2).
The effects of PTBA on the kidney field prompted us to examine whether the compound influenced renal progenitor cells. To address this, we determined the expression patterns and relative abundance of lhx1a, pax2a, and pax8 at the 10-somite stage. Lhx1a expression was expanded in embryos treated with PTBA, representing a three-fold increase in relative transcript quantity as determined by quantitative PCR (qPCR; Figure 1, D and E). Increased lhx1a expression appeared in the bilateral stripes of intermediate mesoderm that give rise to the pronephros (Figure 1E, arrowheads), as well as in the axial mesoderm (notochord; Figure 1E, asterisk). Expression of pax2a and pax8 was also expanded, with qPCR detecting an approximately two-fold increase for pax2a and a 50% increase for pax8 (Figure 1, F through I).
Although these studies demonstrated that PTBA treatment resulted in increased gene expression, they did not indicate whether there are more renal progenitor cells or simply higher expression levels per cell. To differentiate between these two possibilities, we treated the Tg(lhx1a:EGFP)pt303 reporter line with PTBA and counted the number of renal progenitor cells. As compared with control embryos, PTBA-treated embryos showed a 2.4-fold increase in the number of renal progenitor cells (Figure 1, J through Q).
We next performed a temporal assay to determine the timing of PTBA efficacy. PTBA treatments from 2 hpf through 10 somites (14 hpf) resulted in an increase in pax2a expression as assayed at 24 hpf, but treatments at 15 somites (16.5 hpf) resulted in no kidney field expansion (Supplemental Figure 3). In addition, treatment at 15 somites or later did not affect the functional kidney as assayed by a lack of edema in larvae at 72 hpf (data not shown). The effective temporal treatment window coincides with the period when renal progenitor cells are present.6,8
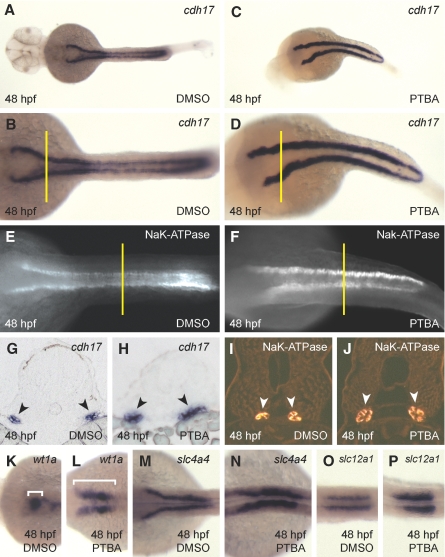
To determine whether PTBA treatment resulted in a transient or persistent expansion of the kidney field, we examined the kidney at 48 hpf using markers of glomerulus and tubule.29–31 As compared with controls, PTBA-treated embryos displayed an expansion of the pan-tubule markers cdh17 and NaK-ATPase at 48 hpf, which indicated a persistent expansion of the kidney (Figure 2, A through F). Cross-sections from the proximal region of the cdh17 expression domain and the distal region of the NaK-ATPase expression domain confirm the expansion (Figure 2, G through J). Finally, in situ hybridization for markers of podocytes (wt1a), proximal tubule (slc4a4), and distal tubule (slc12a1) also showed expansion (Figure 2, K through P). Interestingly, wt1a expression displayed a failure of the expanded podocyte-containing regions to migrate and coalesce at the midline. This failure possibly contributes to a loss of kidney function and the resultant edemic phenotype associated with PTBA treatments.
Figure 2.
PTBA treatment results in a persistent expansion of the kidney field. (A through D) In situ hybridization for cdh17 expression in embryos at 48 hpf treated with 0.5% DMSO (A [higher magnification in B]) or 3 μM PTBA (C [higher magnification in D]). As compared with controls (n = 54 [A and B]), 89% of PTBA-treated embryos exhibit expansion of cdh17 expression at 48 hpf (n = 56 [C and D]). (E and F) Whole-mount antibody staining for NaK-ATPase in embryos at 48 hpf treated with 0.5% DMSO (E) or 3 μM PTBA (F). As compared with controls (n = 10 [E]), 100% of PTBA-treated embryos exhibit expansion of NaK-ATPase expression at 48 hpf (n = 10 [F]). (G and H) Proximal tubule cross-sections (5 μm) taken from cdh17 in situ hybridization of embryos at 48 hpf treated with 0.5% DMSO (G) or 3 μM PTBA (H). Black arrowheads indicate cdh17 expression. Cross-sections are taken from the location indicated in B and D by yellow lines. (I and J) Distal tubule cross-sections (5 μm) taken from NaK-ATPase antibody-stained embryos at 48 hpf treated with 0.5% DMSO (I) or 3 μM PTBA (J). White arrowheads indicate NaK-ATPase protein expression. Cross-sections are taken from the locations indicated in E and F by yellow lines. (K through P) In situ hybridization for wt1a (K and L), slc4a4 (M and N), and slc12a1 (O and P) in embryos at 48 hpf treated with 0.5% DMSO (K, M, and O) or 3 μM PTBA (L, N, and P). As compared with controls (n = 57 [K], n = 58 [M], and n = 56 [O]), observed expansion in response to PTBA treatment is 74% for wt1a (n = 50 [L]), 92% for slc4a4 (n = 60 [N]), and 64% for slc12a1 (n = 59 [P]). Brackets in K and L indicate the expression domain of wt1a.
PTBA Requires Proliferation for Efficacy
The PTBA-mediated increase in kidney field size could result from the fate transformation of nonrenal cells and/or proliferation of renal progenitor cells. To assess the first possibility, we examined the effects of PTBA on markers of two mesodermal tissues juxtaposed to renal progenitor cells: myod1 (somites) and fli1a (vasculature). By in situ hybridization, we observed that myod1 expression in the somites showed a slight decrease after PTBA treatment (Figure 3, A and B); however, subsequent qPCR analysis did not confirm the significance of this observed decrease (Figure 3, A and B). Expression of fli1a in the vasculature remained unchanged by both in situ hybridization and qPCR (Figure 3, C and D). In addition to an increased lhx1a expression in renal progenitor cells, we observed increased lhx1a expression in the notochord (Figure 1E, asterisk). To determine whether this expansion reflected an effect on notochord size or a general increase in lhx1a expression, we assayed the notochord-specific marker ntla. Ntla displayed an expansion that resulted in an approximately 80% increase by qPCR analysis after PTBA treatment (Figure 3, E and F). These results suggest that PTBA treatment cannot be definitively linked to a fate transformation event.
Figure 3.
The efficacy of PTBA requires renal progenitor cell proliferation. (A through F) In situ hybridization for the mesodermal markers myod1 (A and B), fli1a (C and D), and ntla (E and F) in 10-somite embryos treated with 0.5% DMSO (A, C, and E) or 3 μM PTBA (B, D, and F). As compared with controls (n = 60 [A]), myod1 expression is reduced in 95% of PTBA-treated embryos (n = 60 [B]). As compared with controls (n = 59 [C]), fli1a expression is unaffected in 97% of PTBA-treated embryos (n = 60 [D]). As compared with controls (n = 59 [E]), ntla expression is increased in 88% of PTBA-treated embryos (n = 60 [F]). Relative qPCR for myod1, fli1a, and ntla in the trunk region of 10-somite embryos (n = 4, 240 embryos) is displayed under corresponding in situ image. Data are mean expression plus 95% confidence interval. Expression was normalized to β-actin and SDHA transcript levels. (G through J) In situ hybridization for lhx1a expression in 10-somite embryos treated at 5 hpf with 0.5% DMSO (n = 136 [G]), 3 μM PTBA (n = 123 [H]), HUA (n = 131 [I]), or HUA and 3 μM PTBA (n = 104 [J]). As compared with controls (G), lhx1a expression is increased in 97% of PTBA-treated embryos (H) and 13% of the HUA-PTBA–treated embryos (J).
To examine the alternative possibility that PTBA-mediated renal progenitor cell expansion depended on cell proliferation, we tested the efficacy of PTBA in the presence of hydroxyurea and aphidicolin (HUA). HUA treatment is known to block cell division without affecting tissue specification.32 As compared with controls, PTBA-treated embryos exhibited an expansion of lhx1a expression at 10 somites (Figure 3, G and H). HUA treatment alone elicited little effect on lhx1a expression (Figure 3I); however, treatment with a combination of HUA and PTBA diminished the PTBA-mediated expansion of lhx1a expression in renal progenitor cells (Figure 3J). These results suggest that cell proliferation is required for PTBA efficacy.
PTBA Structure-Activity Studies
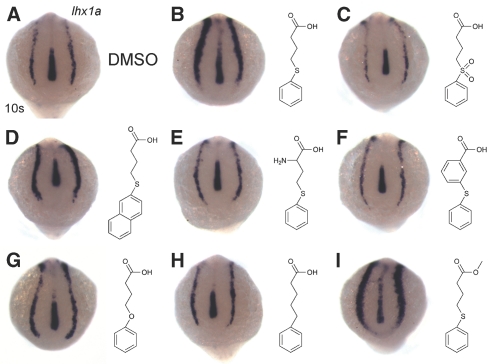
We performed structure-activity analyses using a series of seven analogs (Figure 4). In situ hybridization for lhx1a was performed on 10 somite embryos treated with each analog at 3 μM. The results were compared with control (Figure 4A) and PTBA-treated (Figure 4B) embryos. We observed that replacement of the phenylthio ether with a phenylsulfonyl linkage stripped the compound of its effects on renal progenitor cells (Figure 4C); therefore, the oxidation state of the sulfur atom is a critical activity determinant. However, 4-(naphthalen-2-yl thio)butanoic acid, an analog carrying a naphthalene ring in place of the phenyl moiety of PTBA, still expands lhx1a expression (Figure 4D). Thus, modifications of the ring structure are tolerated and suggest a site for future analog synthesis. Two analogs containing substitutions of the butanoic acid backbone 2-amino-PTBA and 3-(phenylthio)benzoic acid had no effect on lhx1a expression (Figure 4, E and F), suggesting a requirement for a flexible hydrocarbon backbone for biological activity. We also examined 4-phenoxybutanoic acid and 5-phenylpentanoic acid, which contain oxygen and carbon substitutions for the sulfur atom, respectively. 4-Phenyoxybutanoic acid exhibited reduced efficacy compared with the parent compound (Figure 4G), whereas 5-phenylpentanoic acid was inactive (Figure 4H). These results suggest that an atom with a nonbonding electron pair(s) must occupy this position to provide activity. Finally, we determined whether esterification of PTBA elicited any effect on its function. The analog methyl-4-(phenylthio)butanoate demonstrated higher potency than the parent compound (Figure 4I).
Figure 4.
Structure-activity relationship studies reveal essential moieties for PTBA efficacy. (A through I) In situ hybridization for lhx1a expression in 10-somite embryos treated with 0.5% DMSO (n = 53 [A]) or 3 μM of the following compounds: PTBA (100% expansion, n = 52 [B]), PSOBA (no expansion, n = 54 [C]), 4-(naphthalen-2-yl thio)butanoic acid (33% expansion, n = 39 [D]), 2-amino-PTBA (no expansion, n = 64 [E]), 3-(phenylthio)benzoic acid (no expansion, n = 53 [F]), 4-phenoxybutanoic acid (13% expansion, n = 56 [G]), 5-phenylpentanoic acid (no expansion, n = 55 [H]), and methyl-4-(phenylthio)butanoate (100% expansion, n = 41 [I]).
HDACis Mimic the Effects of PTBA
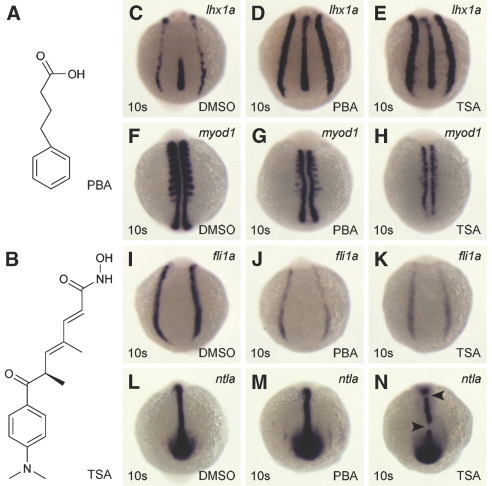
The structure-activity analyses suggested that PTBA functional domains are analogous to 4-phenylbutanoic acid (PBA; Figure 5A), a known HDACi. Furthermore, both compounds resemble the HDACi TSA (Figure 5B). This reflects the general structure-activity relationship of this class of compounds18,33; therefore, we hypothesized that PTBA is an HDACi and, if so, related inhibitors should also expand renal progenitor cells. Concentration-response experiments were performed with PBA and TSA to determine the concentrations necessary to elicit lhx1a expansion (Supplemental Figures 4 and 5). We determined that treatment with 25 μM PBA or 200 nM TSA produced an expansion of renal progenitor cells consistent with that elicited by 3 μM PTBA (Figure 5, C through E, versus Figure 1E); therefore, at least two families of HDACis mimic the ability of PTBA, supporting the idea that PTBA may act through HDAC inhibition.
Figure 5.
Known HDACis expand the pool of renal progenitor cells. (A) Structure of PBA. (B) Structure of TSA. (C through N) In situ hybridization for lhx1a (C through E), myod1 (F through H), fli1a (I through K), ntla (L through N) in 10-somite embryos treated with 0.5% DMSO (C, F, I, and L), 25 μM PBA (D, G, J, and M), or 200 nM TSA (E, H, K, and N). As compared with controls (n = 59 [C]), lhx1a expression is increased in 72% of PBA-treated embryos (n = 56 [D]) and 89% of TSA-treated embryos (n = 58 [E]). As compared with controls (n = 60 [F]), myod1 expression is decreased in 57% of PBA-treated embryos (n = 53 [G]) and 100% of TSA-treated embryos (n = 54 [H]). As compared with controls (n = 54 [I]), fli1a expression is decreased in 78% of PBA-treated embryos (n = 54 [J]) and 95% of TSA-treated embryos (n = 55 [K]). As compared with controls (n = 55 [L]), ntla expression is increased in 87% of PBA-treated embryos (n = 52 [M]) and is disrupted in 86% of TSA-treated embryos (n = 56 [N]). Arrowheads indicate breaks in ntla expression.
We tested how trunk mesoderm juxtaposed to the kidney field is affected by PBA and TSA treatments. TSA is a broad-spectrum HDACi and has been documented to cause disruption of multiple tissues in zebrafish.34,35 In addition, TSA and a second member of the hydroxamic acid family, SAHA, have demonstrated renal toxicity.36 As previously demonstrated, PTBA treatment did not result in drastic structural changes to the somites (myod1), vasculature (fli1a), or notochord (ntla; Figure 3). We treated embryos with 25 μM PBA or 200 nM TSA and compared expression with that of control embryos (Figure 5, F through N). PBA treatment resulted in decreased expression of myod1 and fli1a and increased expression of ntla (Figure 5, G, J, and M). TSA treatment caused severe defects in all three tissues assayed. The somites were almost completely absent, the vasculature was reduced, and the notochord was malformed (Figure 5, H, K, and N).
To determine the toxicity of PBA and TSA, we performed phenotypic concentration-response experiments. Embryos were treated from 2 to 72 hpf over the same concentration range used to test for expansion of the kidney field. When observed at the respective concentrations required to expand the kidney field, PBA treatment resulted in minimal but significant death (7%; n = 90; Supplemental Figure 6), whereas TSA treatment resulted in a high percentage of lethality (61%; n = 90; Supplemental Figure 7).
PTBA Functions as an HDACi
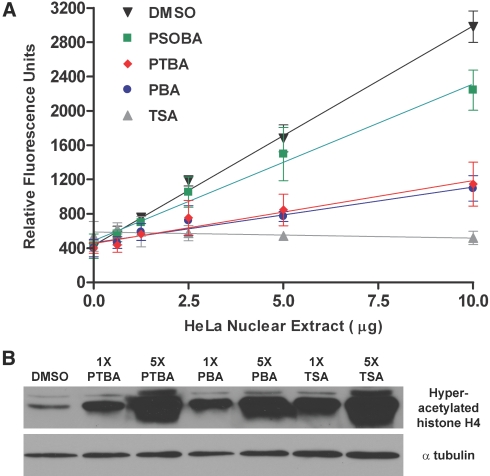
Because PBA and TSA mimic the ability of PTBA to expand renal progenitor cells, we determined whether PTBA shares their ability to inhibit HDACs. We measured the deacetylation of a fluorescent peptide substrate in the presence of HDACs. HDAC activity increased in direct proportion to the amount of HeLa cell nuclear extract added to the assay (Figure 6A, black triangle). Addition of TSA completely blocked HDAC activity at all input levels of nuclear extract added (Figure 6A, gray triangle). Previous work showed that PBA decreased HDAC activity in DSI9 mouse erythroleukemia cells to 19% of the control value at 5 mM.37 To elucidate whether PTBA had similar HDAC inhibition characteristics as PBA, we examined the extent of inhibition for both compounds at 5 mM in HeLa cell nuclear extracts. The two compounds showed similar potency, reducing the HDAC activity elicited by 10 μg HeLa extract to 30% of the control value (Figure 6A, blue circle, red diamond). The PTBA analog 4-(phenylsulfonyl)butanoic acid (PSOBA), showed no apparent ability to expand renal progenitor cells in structure-activity studies (Figure 3C); therefore, we hypothesized that it would function poorly in vitro as an HDACi. At 5 mM, PSOBA reduced the HDAC activity elicited by 10 μg HeLa extract to approximately 70% of the control value (Figure 6A, green square).
Figure 6.
PTBA inhibits HDAC activity both in vitro and in vivo. (A) Fluorescence histone deacetylation assay performed in the presence of 5 mM PTBA, 5 mM PBA, 5 mM PSOBA, 1 μM TSA, or 5% DMSO. At a given concentration of nuclear extract, less fluorescence is indicative of less HDAC activity. Error bars represent the 95% confidence intervals for each data point. (B) Western blot examining the acetylation state of histone H4 isolated from embryos at 30 hpf that had been treated for 6 hours with 0.5% DMSO, 3 μM (1×) or 15 μM (5×) PTBA, 25 μM (1×) or 125 μM (5×) PBA, and 200 nM (1×) or 1 μM (5×) TSA. Western blot for α-tubulin demonstrates equal loading.
We next sought to determine whether PTBA HDACi function was measurable in vivo. We treated 24 hpf embryos with PTBA, PBA, or TSA at their effective concentrations. In addition, to ascertain whether the HDACi activity is concentration-dependent, we also treated at five-fold higher concentrations. After a 6-hour treatment, protein extracts were prepared and immunoblotted with an anti-hyperacetylated histone H4 antibody. A concentration-dependent histone H4 hyperacetylation was observed with all three HDACis (Figure 6B). TSA elicited the strongest hyperacetylation, followed by PTBA then PBA. The in vitro and in vivo results confirm that PTBA functions as an HDACi.
PTBA Treatment Affects RA Signaling
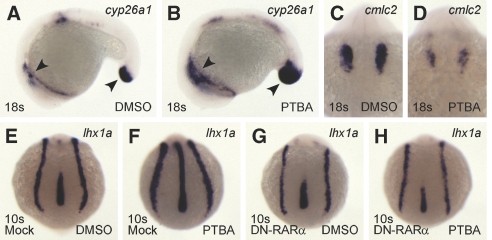
HDACis have been posited to lower the threshold of RA necessary to activate transcription.27 If PTBA treatment hyperactivates the RA pathway, then expression of genes responsive to RA signaling should change. We focused on two genes for this assay, cyp26a1, which is directly activated by RA signaling,38 and the cardiac gene cmlc2, because the heart field is reduced by RA treatments.39 To visualize changes to both genes at the same stage, we collected the treated embryos at the 18-somite stage. Expression of cyp26a1 was increased in PTBA-treated embryos (Figure 7, A and B), whereas cmlc2 expression was reduced (Figure 7, C and D).
Figure 7.
PTBA efficacy is mediated by RA signaling. (A through D) In situ hybridization for cyp26a1 (A and B) and cmlc2 (C and D) in 18-somite embryos treated with 0.5% DMSO (A and C) or 3 μM PTBA (B and D). As compared with controls (n = 58 [A]), cyp26a1 expression is increased in 100% of PTBA-treated embryos (n = 57 [B]). Arrowheads highlight expression domains in cyp26a1 embryos. As compared with controls (n = 58 [C]), cmlc2 expression is decreased in 100% of PTBA-treated embryos (n = 57 [D]). (E through H) In situ hybridization for lhx1a in 10-somite embryos mock-injected with 1% fluorescein dextran (E and F) or injected with 200 pg of DN-RARα mRNA and 1% fluorescein dextran (G and H). At 5 hpf, embryos were treated with 0.5% DMSO (E and G) or 3 μM PTBA (F and H). As compared with controls (n = 80 [E]), lhx1a expression increased in 93% of the mock-injected PTBA-treated embryos (n = 78 [F]) and 19% of DN-RARα-injected PTBA-treated embryos (n = 125 [H]). Normal lhx1a expression is apparent in 92% of the control embryos injected with the DN-RARα construct (n = 130 [G]).
To provide a stronger link between PTBA treatment and RA signaling, a dominant-negative RARα construct (DN-RARα), which is known to block RA signaling,40 was injected before PTBA treatment. As compared with controls, we observed an expansion of lhx1a expression in 90% of the mock-injected PTBA-treated embryos (Figure 7, E and F). Injection of 200 pg of DN-RARα mRNA did not grossly affect lhx1a expression (Figure 7G). Less than 20% of the embryos injected with 200 pg of DN-RARα mRNA and subsequently treated with 3 μM PTBA showed expanded lhx1a expression (Figure 7H); therefore, these data suggest that PTBA-mediated expansion of renal progenitor cells is dependent on the retinoid pathway.
Discussion
The zebrafish is a viable model organism for kidney organogenesis-based small molecule screens. By screening in live embryos, toxic events, such as those elicited by TSA, can be readily identified. From a small molecule screen of 1990 compounds, we identified PTBA, which had not been reported as a positive hit in previously published screens using the National Cancer Institute (NCI) Diversity Set. This compound was found to expand renal progenitor cell populations and was determined to be a new member of the carboxylic acid HDACi family.
PTBA was originally identified for its ability to cause pericardial edema in treated larvae. The edema-associated phenotype suggests that expansion of the renal progenitor cell population disrupts kidney organogenesis and function. In PTBA-treated embryos, wt1a expression domains are expanded, and these domains fail to coalesce and migrate to the midline. This suggests that properly organized glomeruli are absent in treated larvae.29
In addition to PTBA, we demonstrated that PBA and TSA treatments expand the renal progenitor cell population. The ability of several HDACis to expand renal progenitor cell populations fits well with the known functional characteristics of HDACis. Most HDACis share a general structure that facilitates docking in the catalytic pocket of HDACs. What makes PTBA unique is a thioether moiety in the position of the connecting unit, a site that is typically occupied by an amide bond.33 The connecting unit has recently garnered interest as a target for new drug design efforts.
Although all three HDACis expanded the renal progenitor cell population, they exhibited varying effects on other tissues in the embryo. Unlike PTBA, both PBA and TSA caused severe disruption of both somitic and vascular tissue at their effective dosages. This may be indicative of toxicity. Survival studies demonstrated that both PBA and TSA treatment caused significant larval death at their effective concentrations. Interestingly, PTBA and PBA treatment also elicited an expansion of the notochord. This expansion is likely not proliferation based, because PTBA-treated embryos still displayed an expanded notochord in HUA studies. Further investigation is warranted to determine how HDACis of the carboxylic class drive higher gene expression in the notochord.
Mechanistically, PTBA treatment is likely inhibiting HDACs that are part of the RA transcriptional repression complex. This hypothesis is supported by the hyperactivation of a direct target of RA, cyp26a1, in PTBA-treated embryos; therefore, we posit that in the presence of an HDACi, endogenous RA levels can drive the hyperplasic expansion of renal progenitor cells. Interestingly, retinoids have been proposed to be involved in promoting kidney regeneration, because RA has been reported to play a role in inducing kidney tubulogenesis in cell culture.41,42
TSA treatment has been shown to attenuate renal injury in mice.22 One study also demonstrated that renal ischemia-mediated regeneration is associated with a reduction in HDAC5 activity, increased histone acetylation, and reactivation of bone morphogenic protein 716; therefore, inhibitor-mediated attenuation of HDAC activity may increase the expression of reactivated embryonic genes in regenerating renal tubular cells. The ability of HDACis to abate renal damage and increase the number of renal progenitor cells points to a promising therapeutic role for this class of small molecules in kidney regeneration.
Concise Methods
Zebrafish Husbandry
Zebrafish were maintained under standard conditions43 and staged as described previously.44 Embryos were collected from group matings of wild-type AB adults. All animal husbandry adheres to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Small Molecule Screening
The screen was performed in zebrafish embryos using the NCI's Developmental Therapeutics Program (NCI/DTP) Diversity Set. This library contains 1990 compounds selected by pharmacophore modeling to represent more than 140,000 small molecules maintained in the NCI/DTP Open Repository. Compounds from the NCI/DTP Diversity Set were diluted to 10 μM in E3 embryo medium (5 mM NaCl, 0.33 mM CaCl2, 0.33 mM MgSO4, and 0.17 mM KCl) in a final DMSO concentration of 0.5% and arrayed in 96-well plates. Beginning at approximately 2 hpf, embryos were transferred to each well in groups of five using a glass pipette. The plates were incubated at 28.5°C for 70 hours. Individual wells were then scored for a dominant phenotype, representative of at least four of the five embryos. The objective was to identify compounds that caused edema in treated embryos at 72 hpf. Edema can indicate a compromised pronephros, potentially reflecting changes in kidney structure and/or cell number.29 Small molecules generating edema were retested once for verification, before obtaining additional compound from the NCI/DTP Open Repository.
Compound Sources and Treatments
PTBA and methyl-4-(phenylthio)butanoate were synthesized as described in the Supplemental Methods. 4-(Naphthalen-2-yl thio)butanoic acid (NSC2733), 3-(phenylthio)benzoic acid (NSC113994), and 2-amino-PTBA (NSC140113) were obtained from the NCI/DTP Open Repository. PBA, TSA, 4-phenoxybutanoic acid, 5-phenylpentanoic acid, hydroxyurea, and aphidicolin were obtained from Sigma-Aldrich. PSOBA was obtained from Matrix Scientific.
Groups of 20 to 30 chorionated 2-hpf embryos were arrayed in individual wells of 12-well plates. E3 medium was removed with a glass pipette and replaced with 1.5-ml treatment solutions containing 0.5% DMSO in E3 with or without compound at the reported concentrations. Treatments for all studies were initiated at 2 hpf, except for the temporal studies (treated as described in the Results section and Supplemental Information), and the HUA studies. HUA studies were performed as described previously,45 with the following modifications. HUA in 0.5% DMSO was added at early gastrulation (5 hpf) and PTBA was subsequently added at late gastrulation (8 hpf) to allow for penetration of the proliferation inhibitors. All embryos were incubated at 28.5°C until the required developmental stage.
In Situ Hybridization and Immunocytochemistry
In situ hybridization was performed as described previously with some modifications.46 Hybridization temperature was 65°C. Embryos were blocked in 2% blocking reagent (Roche) with 5% sheep serum in MAB (100 mM maleic acid and 150 mM NaCl [pH 7.5]). Whole-mount immunocytochemistry with 1:25 mouse anti-α6F antibody (Developmental Studies Hybridoma Bank) and 1:100 Cy3 secondary antibody (Jackson ImmunoResearch) was performed as described previously.29 Embryos were embedded in JB-4 for sectioning per the manufacturer's instructions (Polysciences), sectioned at 5 μm, and mounted with Cytoseal 60 (Richard-Allan Scientific).
Relative qPCR
Samples for trunk RNA extraction were prepared by cutting embryos just above the first somite with microscissors and discarding the anterior portion. The trunk portions were homogenized with a plastic microcentrifuge pestle in 500 μl of TRI reagent (Ambion), and RNA was isolated using an RNeasy Micro Kit (Qiagen) per the manufacturer's instructions.
Relative qPCR was performed as described previously47 with minor modifications. Details of these modifications, reaction conditions, and information regarding primer sets, primer selection criteria, and qPCR data analysis can be found in the Supplemental Methods section. Mean expression levels (normalized to the control group) and the corrected expression SD were used to generate 95% confidence intervals for each data set.
Transgene Design and Cell Counting
An 8.8-kb genomic fragment of the lhx1a locus, containing green fluorescent protein (GFP) inserted in-frame with the first nine nucleotides of exon 1, was cloned into the pSceI vector and injected into one-cell-stage zebrafish embryos to establish lines. Three independent transgenic lines were isolated, and Tg(lhx1a:EGFP)pt303 was used for subsequent analysis.
After treatment with PTBA, Tg(lhx1a:EGFP)pt303 embryos were fixed in 4% paraformaldehyde in PBS for 8 hours at 4°C. Embryos were washed in PBS containing 0.1% TWEEN 20 (PBT) and incubated in 1 μg/ml DAPI in PBT for 30 minutes at room temperature. Embryos were flat-mounted on glass slides with Cytoseal 60 and imaged with either a Leica M205 FA epifluorescent scope or an Olympus Fluoview 1000 confocal microscope. Confocal projections contained stacks of six 3-μm images.
For cell counting, a predefined box was positioned at the most posterior region of the notochord and encompassed the most lateral GFP-positive cell in a kidney field. The cells that were positive for both GFP and DAPI within this box were counted using Image J. Variances of the control and PTBA-treated groups were compared by F test and found to be unequal; therefore, a two-tailed t test with unequal variance was used to determine significance (α = 0.05).
Histone Hyperacetylation Assays
SDS-PAGE and Western blotting were performed as described previously35 with some modifications. Proteins were separated on 18% SDS-PAGE gels. Membranes were incubated at 4°C overnight with 1:1000 anti-hyperacetylated histone H4 antibody (06-946; Millipore) or 1:1000 anti–α-tubulin antibody (Sigma-Aldrich) in PBT containing 5% nonfat milk.
Fluorescence HDAC Assays
In vitro HDAC activity assays were performed using a fluorescence HDAC assay kit (Active Motif) according to the manufacturer's instructions. For maintaining compound solubility at 5 mM, the final DMSO concentration in all assay wells was increased to 5%. Fluorescence was detected using an M5 Plate Reader (Molecular Dynamics).
mRNA Synthesis and Microinjections
Synthetic mRNA was generated from the XRARα1405/pCD61 construct40 (NotI digested) using a T7 mMessage mMachine kit (Ambion). Zebrafish embryos were injected at the one-cell stage either with 200 pg of synthetic mRNA and 1% fluorescein dextran (Sigma-Aldrich) or with 1% fluorescein dextran alone (mock) and allowed to develop in E3 culture medium at 28.5°C. At the 256-cell stage, only fluorescein dextran–positive embryos were selected for PTBA treatment, which occurred at 5 hpf.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health grants R01DK069403, R01DH053287, and P30DK079307 (to N.A.H.) and R01CA81398 (to T.E.S.).
We thank Michael Tsang for advice and manuscript editing, Brian Cusack and Christine Wasilewski for assistance with zebrafish maintenance and breeding, Andreas Vogt for assistance with in vitro HDAC assay, Greg Gibson for assistance with the Olympus Fluoview 1000 confocal microscope, Bruce Blumberg for the XRARα1405/pCD61 construct, and Emily Noel and Elke Ober for zebrafish protein extraction protocol.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “From Proteus to Prometheus: Learning from Fish to Modulate Regeneration,” on pages 726–728.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC: High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 1: 263–264, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Vogt A, Cholewinski A, Shen X, Nelson SG, Lazo JS, Tsang M, Hukriede NA: Automated image-based phenotypic analysis in zebrafish embryos. Dev Dyn 238: 656–663, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC: Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol 22: 595–599, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Stern HM, Murphey RD, Shepard JL, Amatruda JF, Straub CT, Pfaff KL, Weber G, Tallarico JA, King RW, Zon LI: Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat Chem Biol 1: 366–370, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Yeh JR, Munson KM, Chao YL, Peterson QP, Macrae CA, Peterson RT: AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development 135: 401–410, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Drummond I: Making a zebrafish kidney: A tale of two tubes. Trends Cell Biol 13: 357–365, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Serluca FC, Fishman MC: Pre-pattern in the pronephric kidney field of zebrafish. Development 128: 2233–2241, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M: Nephric lineage specification by Pax2 and Pax8. Genes Dev 16: 2958–2970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP: Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol 223: 77–90, 2000. [DOI] [PubMed] [Google Scholar]
- 11. Kopan R, Cheng HT, Surendran K: Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol 18: 2014–2020, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villanueva S, Cespedes C, Vio CP: Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290: R861–R870, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, Tohda S, Sakano S, Sasaki S: Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int 73: 1240–1250, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S: Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14: 1223–1233, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marumo T, Hishikawa K, Yoshikawa M, Fujita T: Epigenetic regulation of BMP7 in the regenerative response to ischemia. J Am Soc Nephrol 19: 1311–1320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minucci S, Pelicci PG: Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Villar-Garea A, Esteller M: Histone deacetylase inhibitors: understanding a new wave of anticancer agents. Int J Cancer 112: 171–178, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Rasheed WK, Johnstone RW, Prince HM: Histone deacetylase inhibitors in cancer therapy. Expert Opin Investig Drugs 16: 659–678, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ: Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 22: 3549–3560, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, Endres M: Inhibition of histone deacetylation protects wildtype but not gelsolin-deficient mice from ischemic brain injury. Exp Neurol 210: 531–542, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Imai N, Hishikawa K, Marumo T, Hirahashi J, Inowa T, Matsuzaki Y, Okano H, Kitamura T, Salant D, Fujita T: Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells 25: 2469–2475, 2007. [DOI] [PubMed] [Google Scholar]
- 23. Blomhoff R, Blomhoff HK: Overview of retinoid metabolism and function. J Neurobiol 66: 606–630, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Wei LN: Retinoid receptors and their coregulators. Annu Rev Pharmacol Toxicol 43: 47–72, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Cartry J, Nichane M, Ribes V, Colas A, Riou JF, Pieler T, Dolle P, Bellefroid EJ, Umbhauer M: Retinoic acid signalling is required for specification of pronephric cell fate. Dev Biol 299: 35–51, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Wingert RA, Davidson AJ: The zebrafish pronephros: A model to study nephron segmentation. Kidney Int 73: 1120–1127, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Menegola E, Di Renzo F, Broccia ML, Giavini E: Inhibition of histone deacetylase as a new mechanism of teratogenesis. Birth Defects Res C Embryo Today 78: 345–353, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC: Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125: 4655–4667, 1998. [DOI] [PubMed] [Google Scholar]
- 30. Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ: The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet 3: 1922–1938, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sussman CR, Zhao J, Plata C, Lu J, Daly C, Angle N, DiPiero J, Drummond IA, Liang JO, Boron WF, Romero MF, Chang MH: Cloning, localization, and functional expression of the electrogenic Na+ bicarbonate cotransporter (NBCe1) from zebrafish. Am J Physiol Cell Physiol 297: C865–C875, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris WA, Hartenstein V: Neuronal determination without cell division in Xenopus embryos. Neuron 6: 499–515, 1991. [DOI] [PubMed] [Google Scholar]
- 33. Butler KV, Kozikowski AP: Chemical origins of isoform selectivity in histone deacetylase inhibitors. Curr Pharm Des 14: 505–528, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JB: Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J 19: 1166–1168, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Noel ES, Casal-Sueiro A, Busch-Nentwich E, Verkade H, Dong PD, Stemple DL, Ober EA: Organ-specific requirements for Hdac1 in liver and pancreas formation. Dev Biol 322: 237–250, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dong G, Wang L, Wang CY, Yang T, Kumar MV, Dong Z: Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J Pharmacol Exp Ther 325: 978–984, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Lea MA, Tulsyan N: Discordant effects of butyrate analogues on erythroleukemia cell proliferation, differentiation and histone deacetylase. Anticancer Res 15: 879–883, 1995. [PubMed] [Google Scholar]
- 38. Hu P, Tian M, Bao J, Xing G, Gu X, Gao X, Linney E, Zhao Q: Retinoid regulation of the zebrafish cyp26a1 promoter. Dev Dyn 237: 3798–3808, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D: Retinoic acid signaling restricts the cardiac progenitor pool. Science 307: 247–249, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Blumberg B, Bolado J, Jr, Moreno TA, Kintner C, Evans RM, Papalopulu N: An essential role for retinoid signaling in anteroposterior neural patterning. Development 124: 373–379, 1997. [DOI] [PubMed] [Google Scholar]
- 41. Anderson RJ, Ray CJ, Hattler BG: Retinoic acid regulation of renal tubular epithelial and vascular smooth muscle cell function. J Am Soc Nephrol 9: 773–781, 1998. [DOI] [PubMed] [Google Scholar]
- 42. Humes HD, Cieslinski DA: Interaction between growth factors and retinoic acid in the induction of kidney tubulogenesis in tissue culture. Exp Cell Res 201: 8–15, 1992. [DOI] [PubMed] [Google Scholar]
- 43. Westerfield M: The Zebrafish Book, Eugene, University of Oregon Press, 1993. [Google Scholar]
- 44. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF: Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. [DOI] [PubMed] [Google Scholar]
- 45. Zhang L, Kendrick C, Julich D, Holley SA: Cell cycle progression is required for zebrafish somite morphogenesis but not segmentation clock function. Development 135: 2065–2070, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toyama R, O'Connell ML, Wright CV, Kuehn MR, Dawid IB: Nodal induces ectopic goosecoid and lim1 expression and axis duplication in zebrafish. Development 121: 383–391, 1995. [DOI] [PubMed] [Google Scholar]
- 47. Tang R, Dodd A, Lai D, McNabb WC, Love DR: Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin (Shanghai) 39: 384–390, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.