Abstract
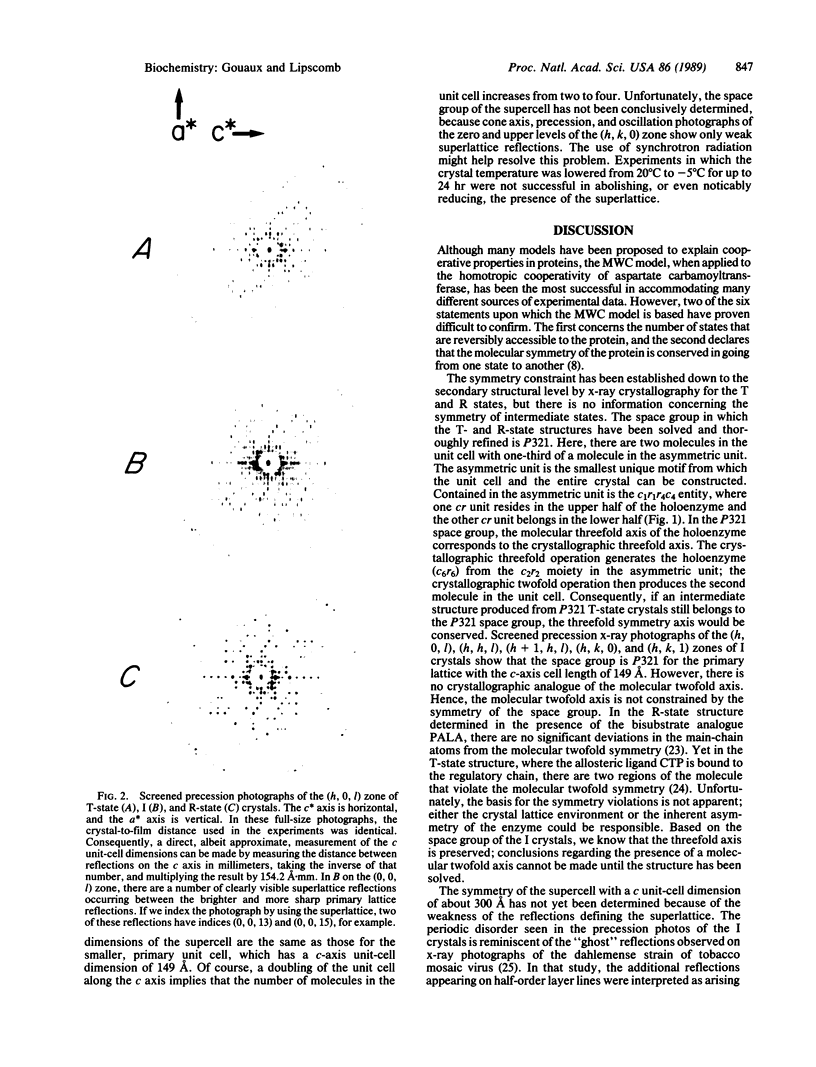
Screened precession x-ray photographs of crystals of native aspartate carbamoyltransferase (EC 2.1.3.2, from Escherichia coli) ligated with L-aspartate and phosphate reveal the presence of a crystal unit-cell dimension that is intermediate between the T (tense) and R (relaxed) states. Characterizing the intermediate (I) crystal is a c-axis unit-cell dimension of 149 A, halfway between the c-axis length of the T (c = 142 A) and R (c = 156 A) states, in the space group P321. Preservation of the P321 space group indicates that the intermediate crystal form retains a threefold axis of symmetry, and therefore the enzyme has at minimum a threefold axis; however, we do not know whether the molecular twofold axis is conserved. The I crystals are formed by soaking T-state crystals with L-aspartate and phosphate. By raising the concentration of L-aspartate we can further transform the I crystals, without fragmentation, to a form that has the same unit-cell dimensions as R-state crystals grown in the presence of N-(phosphonoacetyl)-L-aspartate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bethell M. R., Smith K. E., White J. S., Jones M. E. Carbamyl phosphate: an allosteric substrate for aspartate transcarbamylase of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1442–1449. doi: 10.1073/pnas.60.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn M. N., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Effect of active site ligands on the reactivity of sulfhydryl groups of the regulatory subunits. Biochemistry. 1977 Nov 15;16(23):5084–5091. doi: 10.1021/bi00642a022. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Clarage J., Salunke D. M., Clarage M. Liquid-like movements in crystalline insulin. Nature. 1988 Apr 14;332(6165):659–662. doi: 10.1038/332659a0. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Holmes K. C. Structure of dahlemense strain of tobacco mosaic virus: a periodically deformed helix. J Mol Biol. 1969 Nov 28;46(1):99–133. doi: 10.1016/0022-2836(69)90060-6. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry. 1968 Feb;7(2):531–538. doi: 10.1021/bi00842a007. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Foote J., Schachman H. K. Homotropic effects in aspartate transcarbamoylase. What happens when the enzyme binds a single molecule of the bisubstrate analog N-phosphonacetyl-L-aspartate? J Mol Biol. 1985 Nov 5;186(1):175–184. doi: 10.1016/0022-2836(85)90267-0. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gouaux J. E., Lipscomb W. N. Three-dimensional structure of carbamoyl phosphate and succinate bound to aspartate carbamoyltransferase. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4205–4208. doi: 10.1073/pnas.85.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Changes in the sedimentation coefficient promoted by the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Biochemistry. 1977 Nov 15;16(23):5077–5083. doi: 10.1021/bi00642a021. [DOI] [PubMed] [Google Scholar]
- Kantrowitz E. R., Lipscomb W. N. Escherichia coli aspartate transcarbamylase: the relation between structure and function. Science. 1988 Aug 5;241(4866):669–674. doi: 10.1126/science.3041592. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Pan Z. X., Honzatko R. B., Ke H. M., Lipscomb W. N. Structural asymmetry in the CTP-liganded form of aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1987 Aug 20;196(4):853–875. doi: 10.1016/0022-2836(87)90410-4. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- McClintock D. K., Markus G. Conformational changes in aspartate transcarbamylase. I. Proteolysis of the intact enzyme. J Biol Chem. 1968 Jun 10;243(11):2855–2862. [PubMed] [Google Scholar]
- McClintock D. K., Markus G. Conformational changes in aspartate transcarbamylase. II. Concerted or sequential mechanism? J Biol Chem. 1969 Jan 10;244(1):36–42. [PubMed] [Google Scholar]
- Nowlan S. F., Kantrowitz E. R. Superproduction and rapid purification of Escherichia coli aspartate transcarbamylase and its catalytic subunit under extreme derepression of the pyrimidine pathway. J Biol Chem. 1985 Nov 25;260(27):14712–14716. [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Hammes G. G. Relaxation spectra of aspartate transcarbamylase. Interaction of the native enzyme with an adenosine 5'-triphosphate analog. Biochemistry. 1973 Mar 27;12(7):1400–1408. doi: 10.1021/bi00731a021. [DOI] [PubMed] [Google Scholar]