Abstract
In contrast to pregnancy-associated thrombotic thrombocytopenic purpura, the pathogenesis and presentation of pregnancy-associated atypical hemolytic uremic syndrome (P-aHUS) remain ill-defined. We conducted a retrospective study to assess the presentation and outcomes of patients presenting with P-aHUS and the prevalence of alternative C3 convertase dysregulation. P-aHUS occurred in 21 of the 100 adult female patients with atypical HUS, with 79% presenting postpartum. We detected complement abnormalities in 18 of the 21 patients. The outcomes were poor: 62% reached ESRD by 1 month and 76% by last follow-up. The risk for P-aHUS was highest during a second pregnancy. Thirty-five women, 26 (74%) of whom had complement abnormalities, had at least one pregnancy before the onset of a non-pregnancy–related aHUS. Outcomes did not differ between patients with pregnancy-related and non-pregnancy–related aHUS. Mutations in the SCR19-20 domains of factor H were less frequent in P-aHUS patients compared with non-pregnancy–related aHUS. Pregnancies in female patients with complement abnormalities (n = 44) were complicated by fetal loss and preeclampsia in 4.8% and 7.7%, respectively. Better understanding of complement dysregulation in pregnancy complications is essential, especially to guide development of pharmacologic agents to modulate this system.
Pregnancy-associated thrombotic microangiopathy (P-TMA) is a rare disorder with an estimated incidence of approximately 1 in 25,000 pregnancies1; however, it is associated with a significant perinatal or maternal morbidity and mortality. P-TMA is defined by the occurrence of fibrin and/or platelet thrombi in the microvasculature, resulting in hemolytic mechanical hemolysis and thrombocytopenia during the antepartum or postpartum period. In the last decade, two major findings have significantly improved our understanding of thrombotic microangiopathy (TMA). On one hand, an acquired or constitutional deficiency in ADAMTS13, a von Willebrand factor (vWF)-processing enzyme, has emerged as a cause of a peculiar type of TMA with profound thrombocytopenia and minor renal involvement—thrombotic thrombocytopenic purpura (TTP).2,3 On the other hand, mutations in one or more genes coding for proteins involved in regulation or activation of the alternative pathway of complement have been established as a risk factor for another type of TMA with predominant renal involvement—atypical hemolytic uremic syndrome (aHUS) (i.e., non Shiga-toxin-related HUS). The complement system, a part of the innate immune system, is a complex multiproteic cascade involved in protection against invading microorganisms, removal of debris from plasma and tissues, and enhancement of cell-mediated immune responses. The complement cascade is activated through three distinct pathways (i.e., classical, lectin, and alternative pathways) that converge in the generation of C3 convertases. Because the alternative pathway is initiated spontaneously, the alternative C3 convertase (C3bBb) is tightly regulated by plasma and membrane-bound factors, mainly factor H (FH), factor I (FI), membrane cofactor protein (MCP), and decay-accelerating factor (DAF).4 It is assumed that a lack of control of the alternative C3 convertase leads to complement-induced lesions of the host cells, mainly endothelial cells. Acquired (anti-FH antibodies) or constitutional (inactivating mutations in FH, FI, or MCP coding genes or activating mutations in factor B and C3 coding genes) dysregulation of the alternative C3 convertase has been established as a risk factor for the occurrence of aHUS.5 It is assumed that excessive activation of the alternative C3 convertase leads to complement-induced lesions of the host cells, mainly endothelial cells.
Pregnancy may trigger the onset or subsequent relapses of ADAMTS-13 deficiency-related TTP6 as well as complement dysregulation associated-aHUS. More recently, complement dysregulation was also associated with another pregnancy disorder, HELLP (Hemolysis Elevated Liver enzymes and Low Platelet count) syndrome, which shares several features with P-TMA.7
To the best of our knowledge, no study to date has specifically assessed the implication of alternative complement pathway dysregulation in pregnancy-associated aHUS (P-aHUS). Thus, we conducted a study to assess the incidence of complement dysregulation in patients presenting with P-aHUS in a French cohort of adult patients with TMA.
Results
P-aHUS occurred in 21 of the 100 adult female patients with aHUS (21%).
Clinical and Biologic Characteristics of the 21 Patients with P-aHUS
Table 1 shows the characteristics of the 21 patients at diagnosis. The mean age of patients at disease onset was 28 years. Three patients presented with a relapsing aHUS, with onset of the first episode occurring 2 months to 6 years before P-aHUS. Patient 3 had a familial history of aHUS and presented at month 4 of her second pregnancy with GN with exclusive glomerular C3 deposits associated with a nephrotic syndrome. The disease occurred during the first pregnancy in eight patients (8 of 21, 38%). Twelve of 21 patients had had one (n = 9) or more than one (n = 3) pregnancy before the P-aHUS episode. These patients had a total of 17 pregnancies before the occurrence of P-aHUS, which were complicated by preeclampsia in one case. Three patients (patients 4, 6, and 8) presented with preeclampsia during the pregnancy that was complicated by P-aHUS.
Table 1.
Characteristics of 21 patients with pregnancy-associated atypical hemolytic uremic syndrome
| Patient | Number of Previous Pregnancies | Medical History | Age at P-a HUS Onset | Time of Occurrence of aHUS | Hb | Plts (g/L) | LDH (IU/L) | Hapto | Schiz | SCr (mmol/L) | TRT | P-aHUS Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 26 | M3 PP | 4.9 | 44 | 5000 | <0.06 | + | HD | PE FFP Cs | GFR at 18 ml/min/1.73 m2 (M2); 33 ml/min/1.73 m2 (Y11) | |
| 2 | 2 | 25 | D3 PP | 4.7 | 78 | NA | <0.06 | + | 474 | PE | CR | |
| 3 | 1 | NS at M4 of P due to GNC3. ESRD due to TMA/GNC3 in father | 29 | W3 PP | NA | <70 | >750 | NA | NA | HD | NA | ESRD; Lost 2 RT due to TMA |
| 4 | 2 | 41 | W28 G | 7.5 | 178 | 630 | <0.1 | + | 84 | None | CKD | |
| 5 | 1 | 31 | W5 PP | 5.5 | 310 | 670 | <0.06 | + | HD | PE Cs | ESRD | |
| 6 | 1 | 41 | W3 PP | 5.5 | 120 | 2469 | <0.06 | + | HD | PE | ESRD | |
| 7 | 0 | aHUS at the age of 18 years | 23 | W29 G | 7 | 101 | 573 | <0.06 | + | 500 | PE | ESRD |
| 8 | NA | 23 | D10 PP | 6.5 | 100 | 2373 | <0.06 | + | HD | Nonea | ESRD | |
| 9 | 1 | aHUS at the age of 30 years | 39 | W36 G | 8.9 | 221 | 658 | 0.08 | + | 353 | PE Cs | CKD |
| 10 | 0 | 27 | NA | NA | <100 | >700 | NA | NA | HD | NA | ESRD | |
| 11 | 0 | 23 | M4 PPb | NA | <100 | NA | <0.08 | NA | HD | PE IVIg | ESRD | |
| Lost 2 RT due to TMA | ||||||||||||
| 12 | 0 | 23 | NA | NA | <100 | NA | NA | NA | HD | PE | ESRD | |
| 13 | 1 | 38 | W2 PP | 6.9 | 78 | 2630 | <0.15 | + | HD | PE | ESRD | |
| P at the age of 33 y | ||||||||||||
| 14 | 0 | 22 | W1PP | 9.2 | 12 | >700 | <0.07 | + | HD | PE Cs | ESRD | |
| cardiomyopathy during P-aHUS | ||||||||||||
| 15 | 0 | 27 | PP | NA | <100 | NA | NA | + | HD | NA | ESRD | |
| 16 | 1 | 35 | D15 PP | 6.7 | 54 | NA | <0.01 | + | HD | PE | CKD | |
| 17 | 1 | 30 | D3 PP | NA | NA | NA | NA | NA | HD | PE | Cardiomyopathy during P-aHUS | |
| CKD; ESRD at Y3 | ||||||||||||
| 18 | 1 | 23 | M4 PPb | 6.8 | 118 | NA | <0.01 | + | HD | PE Cs | CKD; ESRD at M19 | |
| 19 | 1 | 30 | W2 PP | 6 | 330 | 3200 | <0.01 | HD | PE | ESRD | ||
| 20 | 1 | 24 | W3 PP | NA | NA | NA | NA | NA | HD | NA | ESRD | |
| aHUS recurrence after RT | ||||||||||||
| 21 | 3 | aHUS 2M before pregnancy | 32 | 4w G | 7.2 | 108 | 626 | NA | + | HD | PE | CKD; Recurrence at Y1; ESRD at Y3 |
Pt, patient; Hb, hemoglobin; Plts, platelets; LDH, lactate dehydrogenase; Hapto, haptoglobin; Schizo, schizocytes; SCr, serum creatinin; KB, kidney biopsy; TRT, treatment; M, months; D, days; W, weeks; HD, hemodialysis; G, glomerular; A, arteriolar; CR, complete remission; RT, renal transplants; CKD, chronic kidney disease; NS, nephritic syndrome; GNC3, GN with exclusive glomerular C3 deposits; PrE, preeclampsia; PE, plasma exchange; FFP, fresh frozen plasma; NA, not available.
aRenal MRI showed cortical necrosis and PE was not started.
bUse of a contraceptive pill.
P-aHUS occurred mainly (15 of 19, 79%) in the postpartum period (Figure 1). Four patients developed aHUS at 4, 28, 29, and 36 weeks of gestation. The time of occurrence of P-aHUS was not available for two patients.
Figure 1.
Risk of aHUS in patients with documented complement dysregulation is highest during the second pregnancy.
Renal involvement was severe: 81% patients (17 of 21) required hemodialysis at the acute phase of the disease and 62% (13 of 21) patients reached ESRD less than 1 month after the episode of P-aHUS. In contrast, thrombocytopenia was moderate, with a platelet count >100,000/mm3 in 40% (8 of 20) patients. Renal biopsy showed typical features of TMA (arteriolar and capillary thrombi, “double contour” aspects, mesangiolysis) in all eight performed renal biopsies. None of the patients presented neurologic symptoms. Treatment consisted mainly of plasma exchange (15 of 18, 83%). Long-term outcome was poor: 76% (16 of 21) of patients had reached ESRD or had undergone renal transplantation at last follow-up. Sixteen pregnancies led to a live birth, and one pregnancy was complicated by an early fetal loss (patient 21). Data are not available for three patients.
Complement Component Assessment and Molecular Characterization of Genetic Abnormality in 21 Patients with P-aHUS
Complement component assessment was performed at a median time of 19 months (range: 2 to 181) from the episode of P-aHUS. C3 was low at the time of the first complement assessment in 12 of 21 patients (57%) (Table 2). A complement abnormality (i.e., mutations in genes involved in control of the alternative complement pathway or encoding components of the alternative C3 convertase) was detected in 18 of 21 patients (86%). Ten patients (45%) had a CFH mutation, two (9%) had a CFI mutation, two had a C3 mutation (9%), one (4%) had a MCP mutation, and three (14%) had more than one mutation (CFH and C3, CFH and MCP, CFI/CFI). None of these mutations was detected in the control group. No anti-FH antibodies were detected in any of the patients. Three patients (3 of 21; 14%) had no detected genetic defect. A total of 12 different mutations scattered throughout the CFH coding region were identified; two of them were located in the SCR19-20 domains (2 of 12, 16%). FH levels were decreased in 6 of 12 patients with a heterozygous CFH mutation, a finding compatible with the definition of type I mutation (lack of protein expression from the mutant allele). Three type II mutations were detected in the SCR1-3 domains of FH. Two of them (Q81P in SCR1 and A161S in SCR3) are part of the C3b binding site, and these mutations most probably affect the direct binding to C3b and C3 convertase regulation. Mutation R53C affects an exposed residue on the opposite surface of SCR1, which, although not in contact with C3, could affect the local protein conformation. One type II mutation was found in SCR6 (R341H) and affects the residue that is most likely involved in the secondary heparin binding site in the SCR6-7 to 8 regions of FH. Two mutations leading to defective binding of FH to C3b, heparin, and endothelial cells were found in SCR20 of FH and have been previously reported in aHUS.5
Table 2.
Results of complement components assays in 21 patients with P-aHUS
| Patient | C3, mg/L | C4, mg/L | CFB, mg/L | CFH, mg/L | CFI, mg/L | MCP, MFI | Genetic Abnormality | Functional Significant of the Mutation |
|---|---|---|---|---|---|---|---|---|
| 1 | 220 | 120 | 120 | 250 | NA | NA | CFH (p.H893R; SCR16) | Type I mutation (quantitative FH deficiency) |
| 2 | 567 | 233 | 142 | 306 | 76 | 1311 | CFH (p.G397R; SCR7) | Type I mutation (quantitative FH deficiency) |
| 3 | 1260 | 293 | 292 | 464 | 98 | NA | CFH(p.R1210C; SCR 20) | Type II mutation, previously reported in aHUS,11 alter the C3b/polyanions-binding site in the C-terminal region41 |
| MCP (p.C35Y) | Type I mutation (quantitative MCP deficiency) | |||||||
| 4 | 893 | 393 | 124 | 638 | 50 | 788 | CFI (p.G119R; G101R) | Undetermined |
| CFI (p.G424D;G406D) | Undetermined | |||||||
| 5 | 733 | 248 | 97 | 530 | 76 | 996 | CFH (p.A161S; SCR 03) | Type II mutation, previously reported in aHUS,42 may alter the direct binding site to C3b |
| 6 | 463 | 390 | 80 | 332 | 78 | NA | CFH (p.K584X; SCR10) | Type I mutation (quantitative FH deficiency) |
| 7 | 345 | 344 | 56 | 326 | 52 | 1301 | CFH (p.G218E; SCR 4) | Type I mutation (quantitative FH deficiency) |
| 8 | 637 | 330 | 69 | 724 | 75 | NA | CFH (p.R341H; SCR6) | Type II mutation, may alter the secondary heparin binding site in the SCR6-8 region of FH |
| C3 (p.R161W) | Type II mutation, with is located within or in very close proximity to the FH binding site20 | |||||||
| 9 | 563 | 210 | 82 | 760 | 62 | NA | no | |
| 10 | 509 | 160 | 85 | 689 | 65 | 1267 | CFH (p.R53C; SCR 1) | Type II mutation, could affect the local conformation of FH |
| 11 | 652 | 207 | 73 | 357 | 59 | 987 | CFH (p.V1197A; SCR20) | Type II mutation, previously reported in aHUS,5 alter the C3b/polyanions-binding site in the C-terminal region41 |
| 12 | 833 | 340 | 130 | 525 | 51 | 806 | no | |
| 13 | 814 | 170 | 119 | 546 | 77 | NA | C3(p.I1095S) | Type II mutation, which is located within or in very close proximity to the FH binding site20 |
| 14 | 435 | 294 | 60 | 571 | 66 | NA | C3 (p.R161W) | Type II mutation, which is located within or in very close proximity to the FH binding site20 |
| 15 | 931 | 201 | 150 | 668 | 71 | NA | no | |
| 16 | 239 | 138 | 62 | 301 | 55 | 1131 | CFH(p.C431Y; SCR7) | Type I mutation (quantitative FH deficiency) |
| 17 | 497 | 326 | 59 | 418 | 57 | 921 | CFH (p.Q81P; SCR1) | Type II mutation, may alter the direct binding site to C3b |
| 18 | 698 | 182 | 116 | 490 | 59 | 409 | MCP (IVS2–2) | Type I mutation (quantitative MCP deficiency)35 |
| 19 | 580 | 260 | 75 | 270 | 67 | NA | CFH (p.C864S; SCR14) | Type I mutation (quantitative CFH deficiency) |
| 20 | 836 | 225 | 101 | 704 | 41 | 611 | CFI (p.G119R; G101R) | Undetermined |
| 21a | 528 | 180 | 59 | 459 | 37 | NA | CFI (p.G474X) | Type I mutation (quantitative FI deficiency) |
| Normal values | 660 to 1250 | 93 to 380 | 90 to 320 | 338 to 682 | 42 to 78 | 600 to 1400 |
FI levels were decreased in two of three patients with CFI mutation, a finding suggestive of the type I mutation. Mutations in the C3 gene are located within or in a very close proximity to the FH binding site. Alteration of the direct binding of FH to C3 is the most probable functional consequence for these two mutations. The functional consequences of the mutations in CFH, CFI, MCP, and C3 genes are summarized in Table 2.
Four of the 18 tested patients were homozygous for the at-risk CD46GGAAC haplotype (4 of 18, 22%), and 9 of 20 tested patients were homozygous for the at-risk CFHGTGT (9 of 20, 45%) haplotype. Among the P-aHUS patients, 57% of patients presented at least one additional risk factor for aHUS (11 of 19). None of the 18 tested patients presented a homozygous CFHR1 deletion.
Pregnancy Outcome in Patients with aHUS Unrelated to Pregnancy
Among the 100 patients with aHUS, we identified 35 women who had had at least one pregnancy in the absence of P-aHUS. The study population consisted of 11, 10, 8, 2, and 3 women who had had 1, 2, 3, 4, and 6 pregnancies, respectively. The mean age of patients at the onset of aHUS was 35 years. The outcome of aHUS did not differ between these two groups of patients (Table 3). In this group of patients, a complement abnormality was detected in 26 of 35 patients (74%) consisting in CFH mutations (n = 14), CFI mutations (n = 6), MCP mutations (n = 3), C3 mutations (n = 1), and FB mutations (n = 2). In patients with at least one pregnancy and aHUS unrelated to pregnancy, 43% of CFH mutations (6 of 14) were located in SCR19-20. The percentage of mutations located in SCR19-20 was significantly lower in patients with P-aHUS compared with those with aHUS unrelated to pregnancy (P = 0.01). In the group of nulliparous women and aHUS (median age of aHUS: 29 years), 66% of the CFH mutations were located in SCR19-20. The SCR distribution of FH mutations in female patients is shown in Figure 2.
Table 3.
Characteristics of patients with P-aHUS and patients with aHUS not related to pregnancy
| Patients with P-aHUS (n = 21) | Patients with aHUS Not Related to Pregnancy (n = 35) | P | |
|---|---|---|---|
| Age at aHUS onset (years) | 26 ± 5 | 33 ± 12 | P < 0.05 |
| Number of pregnancies | 2 ± 0.8 | 2.3 ± 1.5 | NS |
| Number of patients reaching ESRD <6 months after the first aHUS episode | 11 (52%) | 20 (57%) | NS |
| Number of patients with aHUS relapses | 3 (14%) | 4 (11%) | NS |
| Number of patients reaching ESRD at last follow-up | 16 (76%) | 26 (74%) | NS |
| Number of patients with complement abnormality | 18 (86%) | 26 (74%) | NS |
| CFH | 10 (48%) | 14 (40%) | NS |
| CFI | 3 (14%) | 6 (17%) | NS |
| MCP | 1 (5%) | 3 (8.5%) | NS |
| C3 | 2 (9.5%) | 1 (3%) | NS |
| FB | 0 (0%) | 2 (5.5%) | NS |
| More than one mutation | 2 (9.5%) | 1 | NS |
NS, not statistically significant.
Figure 2.
CFH mutations in female patients with aHUS are located mainly outside the SCR19-20 domains. Three patients have two mutations: CFH/C3, CFH/MCP, and CFH/CFH.
There were a total of 118 pregnancies in the group of patients with aHUS. In patients with mutations in the CFH, C3, CFI, and MCP genes, aHUS was triggered by pregnancy in 20%, 28%, 11%, and 17% of patients, respectively (Table 4). Mutations in SCR19-20 of CFH and CFI genes were associated with a lower frequency of P-aHUS compared with mutations in C3, MCP, and SCR1-18 of CFH.
Table 4.
Frequency of P-aHUS according to the type of complement dysregulation
| Patients | Number of Pregnancies | P-aHUS (%) |
|---|---|---|
| CFH mutations (n = 23)a | 49 | 10 (20%) |
| Mutations in SCR19-20 (n = 6) | 10 | 1 (10%) |
| Mutations in other SCR (n = 17) | 38 | 9 (24%) |
| CFI mutations (n = 8) | 26 | 3 (11%) |
| MCP mutations (n = 4) | 6 | 1 (17%) |
| C3 mutations (n = 3) | 7 | 2 (28%) |
| CFB mutations (n = 2) | 7 | 0 (0%) |
| More than one mutation (n = 4)b | 5 | 3 (60%) |
| No mutation (n = 10) | 15 | 3 (20%) |
aThree patients with two mutations in CFH (SCR 9 and 19)—in C3/CFH and in MCP/CFH—were excluded from the analysis.
bPatients with two mutations in CFH (SCR 9 and 19)—in C3/CFH (patient 8), in MCP/CFH (P3), and in CFI/CFI (patient 4)
Pregnancy Outcome in All Patients with an Abnormal Control of the Complement Alternative Pathway
Forty-four patients with complement dysregulation (CFH = 24, CFI = 9, MCP = 4, C3 = 3, CFB = 2, >1 mutation = 3) had 103 pregnancies (Table 5). Pregnancy was uneventful in 74.7% of patients and complicated with fetal loss in 4.8% of patients, preeclampsia in 7.7% of patients, and aHUS in 17.4% of patients. The risk of P-aHUS in these patients was highest during the second pregnancy (Figure 3). Ten patients with aHUS and no documented complement dysregulation had 15 pregnancies complicated by aHUS in three patients (20%) and were uneventful in 80% of patients. Three patients with P-aHUS and no detected genetic abnormality had four pregnancies complicated by aHUS in three patients and were uneventful in one patient.
Table 5.
Pregnancy outcome in 44 women with aHUS and genetic defects (CFH = 23, CFI = 9, MCP = 4, C3 =3, CFB = 2, more than one mutation = 3) and in 10 patients with aHUS and no detectable genetic defect
| Number of Pregnancies | Fetal Loss | Preeclampsia | P-aHUS | Uneventful Pregnancy | |
|---|---|---|---|---|---|
| Patients with genetic abnormality (n = 44) | 103 | 5 (4.8%) | 8 (7.7%) | 18 (17.4%) | 77 (74.7%) |
| Patients with no genetic abnormality (n = 10) | 15 | 0 (0%) | 0 (0%) | 3 (20%) | 12 (80%) |
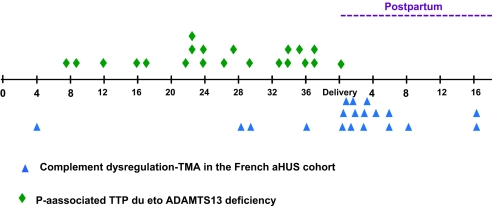
Figure 3.
Complement dysregulation-associated aHUS in the French cohort (18 patients) occurs mainly in the postpartum, whereas ADAMTS13 deficiency-associated TTP (23 previously published cases)21–30 occurs mainly during pregnancy.
Discussion
In contrast to pregnancy-associated TTP, the pathogenesis and presentation of P-aHUS remain ill-defined. For instance, data regarding complement dysregulation in P-aHUS are extremely scarce and are based, to the best of our knowledge, on less than 15 patients reported mostly in cohort studies without available detailed clinical data.8–11 Nonetheless, our study clearly underlines that P-aHUS is a frequently encountered subtype of aHUS, accounting for approximately 20% of all patients with aHUS occurring in the French aHUS registry. In a previous report by Caprioli et al., P-aHUS represented 10% (nine patients) of all aHUS patients.8 Our data clearly underline that pregnancy is definitely an important triggering factor for aHUS. Moreover, the outcome is severe, with more than two-thirds of patients reaching ESRD, mostly in the month after the onset.
P-aHUS shares with non-pregnancy-related aHUS a high incidence of mutations in complement genes (86% and 76%, respectively), with CFH mutations being the most frequently encountered (48% of patients). The most striking finding in our series is the occurrence of >75% of cases of P-aHUS in the postpartum period. This finding may seem paradoxical, because pregnancy per se triggers complement activation. During pregnancy, an immunological conflict takes place in the placenta—an interface between a semiallograft, the fetus, and the mother. The placenta is potentially subject to complement-mediated immune attack at the feto-maternal interface with the potential risk of fetal loss. Indeed, complement components (C3b, C4b) are detected in the placenta of normal and pathologic pregnancies.12–14 In normal pregnancy, uncontrolled complement activation is prevented mainly by the three regulatory proteins DAF, MCP, and CD59, which are positioned on the surface of trophoblasts.12 In addition, serum concentrations of C3, C4, and CH50 gradually increase during pregnancy by 10% to 50%.12 DAF is the predominant regulatory protein expressed on trophoblasts and is anchored through its lipophilic, diacylglycerol, membrane-bound fragment in the outer lipid bilayer of the trophoblast. Similarly, FH is sequestered on the trophoblast surface through its interaction with bone sialoprotein and ostepontin attached to their specific receptors.15 FH is also probably synthesized and secreted by trophoblasts.16 DAF, MCP, and FH downregulate the alternative C3 convertase through binding to C3b, cofactor activity to FI, and/or C3 convertase dissociation. Such control is crucial for the development of the fetus, as outlined by several animal models.17,18 Thus, in patients with defective control of the alternative C3 convertase due to CFH, CFI, C3, and MCP mutations, the integrity of the regulatory factors DAF and CR1 provides an effective control of the complement activation at the feto-maternal interface during the pregnancy. Furthermore, paternally inherited fetal complement regulatory factors may help to compensate for maternal-deficient alternative C3 convertase. In contrast, after delivery, inflammation due to delivery per se, the release in the maternal circulation of fetal cells, infections, and hemorrhage lead to systemic activation of the alternative pathway, which, in the absence of effective regulatory mechanisms, ultimately induces postpartum aHUS. The importance of alternative pathway activation in the genesis of P-aHUS may explain the high incidence of mutations occurring in the regulation of the alternative pathway C3 convertase in patients with P-aHUS.
In aHUS, most CFH mutations cluster in the C-terminus domain, which is believed to mediate FH interaction with endothelial cells. Mutations in SCR19-20 probably lead to altered FH fixation to endothelia and hence hinder FH capacity to protect the endothelia from complement-induced lesions.19 Interestingly, CFH mutations in patients with P-aHUS do not aggregate in the SCR19-20 but are detected in various FH SCR domains (Figure 2). Patients with P-aHUS had only 17% (2 of 12) of their CFH mutations occurring in the SCR19-20 domains, compared with 43% (6 of 14) in patients with at least one pregnancy and 66% (8 of 12) in nulliparous patients with aHUS.
In six patients with P-aHUS related to CFH mutations, mutated genes do not encode any circulating protein and lead to a quantitative FH deficiency (e.g., 50% of detected serum CFH concentration). The remaining four mutations associated with normal FH levels (type II mutations) are located in SCR1, 3, and 6 of FH. Type II mutations occurring outside of the C terminal domains of FH, which might impair the binding of FH to surface-bound C3b, have been previously reported in children with aHUS.19 Moreover, in two patients with P-aHUS, we identified two mutations in C3 genes that are located within or in very close proximity to the FH binding site. This finding suggests that the functional consequence of these two mutations is probably the alteration of the direct binding of FH to C3b.20
The postpartum period probably requires an effective control of the alternative pathway in the fluid phase. This may account for the high frequency of mutations located in the C3b binding site or associated with a quantitative deficiency in FH in P-aHUS patients. The different distribution of CFH mutations between P-aHUS cases and other types of aHUS cases remains unexplained but highly suggests that the pathologic significance of SCR domains in FH outside of the C-terminal region have been underrecognized
In contrast, plasma vWF increases steadily during gestation and reaches a maximum (200% to 500% of normal) at term with the appearance of the highly thrombogenic ultralarge vWF multimers.6 In contrast, a review of 23 previously published cases of pregnancy-related TMA associated with ADAMTS13 deficiency21–30 clearly shows this type of TMA occurs predominantly in the late second and third trimesters. This is in accordance with a recent review of 166 pregnancy-related TTP cases in which the median time of TTP occurrence was 23 weeks.31 Plasma ADAMTS13 activity gradually declines throughout pregnancy from the end of the first trimester up to the end of early puerperium6 and may reach an insufficient level to counterbalance the inhibitory effect of anti-ADAMTS13 antibodies in the late second and third trimesters. Thus, the late second and third trimesters are the gestation period with the highest risk for TTP. However, in ADAMTS13 deficiency and C3 convertase dysregulation-associated P-aHUS, confounding factors such as infections may lead to the occurrence of pregnancy related-TMA earlier in pregnancy.
Treatment of aHUS mainly includes plasma infusions and plasma exchange. Because of the extreme severity of P-aHUS with more than two-thirds of patients reaching ESRD less than 1 month after P-aHUS, aggressive treatment with high-dose plasma exchange needs to be started rapidly. Complement activation modulators (eculizumab and human FH) represent promising therapeutic options for severe forms of aHUS (e.g., P-aHUS)32; however, the efficiency of such treatments remains to be clearly established in aHUS.
In addition to the data regarding P-aHUS, our study suggests that fetal losses and preeclampsia tend to occur more frequently in patients with genetic complement abnormalities compared with the general population, with an incidence of 4.6% (versus 2% to 3% in the general population) and 7.4% (versus 4% to 5% in the general population), respectively. As previously discussed, an effective control of complement activation is required for the development of a normal pregnancy. Thus, one cannot exclude that complement dysregulation could be involved in other pregnancy complications such as preeclampsia and fetal losses.
The counseling of young female patients with documented C3 convertase dysregulation who wish to undergo a pregnancy remains an ill-addressed issue. The identification of these mutations does not allow an accurate prediction of the risk of P-aHUS. The incomplete penetrance of the disease in individuals carrying mutations is relatively high, and additional variants in FH and MCP act as additional susceptibility factors for the development of aHUS.5 In our series of patients with P-aHUS, the risk-associated haplotype CFHGTGT or MCPGGAAC was present in homozygosity in 57% of patients. Our data indicate that the concurrence of both mutations and risk polymorphisms may be required for the development of the disease, but pregnancy is not sufficient per se to trigger aHUS.
On the basis of our results, patients with complement dysregulation should be informed of the relatively high risk (20%) of P-aHUS, and any pregnancy should be closely monitored, but especially in these patients. The risk for pregnancy complications is probably overestimated in our series, which included patients with severe clinical manifestations associated with a C3 convertase dysregulation. Preventive treatment with aspirin and/or plasma infusions during pregnancies in women with previous manifestations related to C3 convertase dysregulation remains a matter of debate.
In all, alternative C3 convertase dysregulation is probably associated with a wide range of pregnancy complications including HELLP syndrome and, most importantly, P-aHUS. A precise assessment of the implication of complement dysregulation in pregnancy complications is crucial, especially regarding the use of anti-complement-targeted agents in the treatment of these disorders.
Concise Methods
Patients and Methods
Using a computerized database, we identified 100 adult women (age: 18 to 85 years, median 31 years) with aHUS referred between 2000 and 2008 to the laboratory of immunology at Hôpital Européen Georges Pompidou (Paris, France), a reference center for the evaluation of complement disorders in human diseases. A diagnosis of aHUS was defined by the coexistence of hemolytic mechanical anemia (hemoglobin <10 g/dl, lactate dehydrogenase level >600 IU/L, and presence of schistocytes on blood smear), thrombocytopenia (platelet count <150 000/ mm3), and acute renal failure. P-aHUS was defined as an aHUS occurring during the antepartum or the postpartum period. Preeclampsia was defined by the occurrence after 20 weeks' gestation of hypertension (systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg) and proteinuria >300 mg/d.
The MEDLINE database was used to search for published P-TMA cases (case reports or series) with the following keywords: thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, TTP, HUS, thrombotic microangiopathy, pregnancy, ADAMTS13, complement, Factor H, Factor I, MCP, membrane cofactor protein, C3, Factor B. The search was restricted to 1996 to 2008 because the identification of ADAMTS13 and complement dysregulation in TMA was after 1996.
All patients' medical records were reviewed and relevant clinical and biologic data were collected. All patients gave informed consent for genetic analysis according to the Declaration of Helsinki. Complement analysis was done as part of the usual workup regularly performed in all TMA patients seen in nephrology departments.
EDTA plasma samples were obtained for all patients. Plasma concentrations of FH and FI were measured by ELISA; C4, C3, and FB were measured by nephelometry; and MCP expression was analyzed on granulocytes using anti-CD46 phycoerythrin-conjugated antibodies (Serotec, United Kingdom). All coding sequences for FH (CFH), FI (CFI), membrane cofactor protein (MCP), C3, and Factor B genes were sequenced as described previously.33–37
DNA samples available from 100 local blood donors with the same genetic background as the patients (70% of women and 30% of men) were also sequenced for the aforementioned genes to differentiate polymorphisms (detectable in healthy volunteers) from mutations (undetectable in healthy volunteers).
The haplotype CD46GGAAC, which is associated with an increased risk of aHUS,38 is defined by the following single nucleotide polymorphisms: −652 A>G (rs2796267), −366 A>G (rs2796268), IVS9-78 G>A (rs1962149), IVS12 + 638 G>A (rs859705), and c.4070 T>C (rs7144). The at-risk haplotype was not available for three patients. The risk CFH –H3 haplotype39 was tagged by genotyping rs800292 (c.184G>A; p.Val62Ile), rs1061170 (c.1204T>C; p.Tyr402His), rs3753396 (c.2016A>G; p.Gln672Gln), and rs1065489 (c.2808G>T; p.Glu936Asp). The at-risk haplotype was not available for one patient. CFHR1 deletion was screened using multiplex ligation-dependent probe amplification as previously reported.40
Disclosures
None.
Acknowledgments
This work was supported by grants from Assistance Publique-Hôpitaux de Paris (Programme Hospitalier de Recherche Clinique [AOM05130/P051065] 2005 and [AOM 08198] 2008) and by an INSERM grant (ANR 2009 GENOPAT) to V.F.B. L.T.R. is a recipient of an EMBO Long Term Fellowship ALTF 444-2007. We gratefully acknowledge the colleagues participating in this study: C. Allard, Department of Nephrology, CHU de Metz-Thionville, Metz; O. Toupance, Department of Nephrology, CHU de Reims, Reims; Cédric Rafat, Department of Nephrology, Hôpital Tenon, Paris; Thierry Lobbedez, CHU de Caen; Antoine Jacquet, Department of Nephrology, CHU du Kremlin Bicêtre, Le Kremlin Bicêtre; Jean-Philippe Rérolle, Department of Nephrology, CHU de Limoges, Limoges; Benoit Barrou, Department of Nephrology, CHU Pitie Salpetrière, Paris; Philippe Lesavre, Department of Nephrology, CHU Necker, Paris; and Christophe Legendre, Department of Transplantation, CHU Necker, Paris.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Pulling the Trigger in Atypical Hemolytic Uremic Syndrome: The Role of Pregnancy,” on pages 731–732.
REFERENCES
- 1. Dashe JS, Ramin SM, Cunningham FG: The long-term consequences of thrombotic microangiopathy (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome) in pregnancy. Obstet Gynecol 91: 662–668, 1998. [DOI] [PubMed] [Google Scholar]
- 2. Furlan M, Robles R, Galbusera M: von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med 339: 1578–1584, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Levy GG, Nichols WC, Lian EC: Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 413: 488–494, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Walport MJ: Complement. First of two parts. N Engl J Med 344: 1058–1066, 2001. [DOI] [PubMed] [Google Scholar]
- 5. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 200911 [DOI] [PubMed] [Google Scholar]
- 6. Sanchez-Luceros A, Farias CE, Amaral MM: von Willebrand factor-cleaving protease (ADAMTS13) activity in normal non-pregnant women, pregnant and post-delivery women. Thromb Haemost 92: 1320–1326, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Fakhouri F, Jablonski M, Lepercq J: Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood 112: 4542–4545, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Caprioli J, Noris M, Brioschi S: Genetics of HUS: The impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108: 1267–1279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iannuzzi M, Siconolfi P, D'Angelillo A: A post-partum hemolytic-uremic-like-syndrome in a patient with pre-eclampsia: Description of a clinical case. Transfus Apher Sci 34: 11–14, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Kavanagh D, Richards A, Noris M: Characterization of mutations in complement factor I (CFI) associated with hemolytic uremic syndrome. Mol Immunol 45: 95–105, 2008. [DOI] [PubMed] [Google Scholar]
- 11. Martinez-Barricarte R, Pianetti G, Gautard R: The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 19: 639–646, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girardi G: Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol Invest 37: 645–659, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Girardi G, Salmon JB: The role of complement in pregnancy and fetal loss. Autoimmunity 36: 19–26, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Shamonki JM, Salmon JE, Hyjek E: Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol 196: e161–165, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fedarko NS, Fohr B, Robey PG: Factor H binding to bone sialoprotein and osteopontin enables tumor cell evasion of complement-mediated attack. J Biol Chem 275: 16666–16672, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Goldberg M, Luknar-Gabor N, Keidar R: Synthesis of complement proteins in the human chorion is differentially regulated by cytokines. Mol Immunol 44: 1737–1742, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Wu X, Spitzer D, Mao D: Membrane protein Crry maintains homeostasis of the complement system. J Immunol 181: 2732–2740, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu C, Mao D, Holers VM: A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287: 498–501, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Saunders RE, Abarrategui-Garrido C, Frémeaux-Bacchi V: The interactive factor H-atypical hemolytic uremic syndrome mutation database and website: Update and integration of membrane cofactor protein and factor I mutations with structural models. Hum Mutat 28: 222–234, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Wu J, Wu YQ, Ricklin D: Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol 10: 728–733, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cosmai EM, Puzis L, Tsai HM: Thrombocytopenic purpura and cardiomyopathy in pregnancy reversed by combined plasma exchange and infusion. Eur J Haematol 68: 239–242, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Ducloy-Bouthors AS, Caron C, Subtil D: Thrombotic thrombocytopenic purpura: Medical and biological monitoring of six pregnancies. Eur J Obstet Gynecol Reprod Biol 111: 146–152, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Fujimura Y, Matsumoto M, Kokame K: Pregnancy-induced thrombocytopenia and TTP, and the risk of fetal death, in Upshaw-Schulman syndrome: A series of 15 pregnancies in 9 genotyped patients. Br J Haematol 144: 742–754, 2009. [DOI] [PubMed] [Google Scholar]
- 24. Rehberg JF, Briery CM, Hudson WT: Thrombotic thrombocytopenic purpura masquerading as hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome in late pregnancy. Obstet Gynecol 108: 817–820, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Scully M, Starke R, Lee R: Successful management of pregnancy in women with a history of thrombotic thrombocytopaenic purpura. Blood Coagul Fibrinolysis 17: 459–463, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Strasser SM, Kwee A, Fijnheer R: Transient severe fetal heart rate abnormalities in a pregnancy complicated by thrombotic thrombocytopenic purpura. Obstet Gynecol 111: 517–521, 2008. [DOI] [PubMed] [Google Scholar]
- 27. Vesely SK, George JN, Lammle B: ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 102: 60–68, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Zheng XL, Kaufman RM, Goodnough LT: Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood 103: 4043–4049, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Furlan M, Robles R, Solenthaler M: Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 89: 3097–3103, 1997. [PubMed] [Google Scholar]
- 30. Gerth J, Schleussner E, Kentouche K, Busch M, Seifert M, Wolf G: Pregnancy associated thrombotic thrombocytopenic purpura. Thromb Haemost 101: 248–251, 2009. [PubMed] [Google Scholar]
- 31. Martin JN, Jr., Bailey AP, Rehberg JF: Thrombotic thrombocytopenic purpura in 166 pregnancies: 1955–2006. Am J Obstet Gynecol 199: 98–104, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Nurnberger J, Witzke O, Opazo Saez A: Eculizumab for atypical hemolytic-uremic syndrome. N Engl J Med 360: 542–544, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Fremeaux-Bacchi V, Dragon-Durey MA, Blouin J: Complement factor I: A susceptibility gene for atypical haemolytic uraemic syndrome. J Med Genet 41: e84, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C: Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: Report and genetic analysis of 16 cases. J Am Soc Nephrol 15: 787–795, 2004. [DOI] [PubMed] [Google Scholar]
- 35. Fremeaux-Bacchi V, Moulton EA, Kavanagh D: Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J Am Soc Nephrol 17: 2017–2025, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Fremeaux-Bacchi V, Miller EC, Liszewski MK: Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood 112: 4948–4952, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roumenina LT, Jablonski M, Hue C: Hyperfunctional C3 convertase leads to complement deposition on endothelial cells and contributes to atypical hemolytic uremic syndrome. Blood 114: 2837–2845, 2009. [DOI] [PubMed] [Google Scholar]
- 38. Fremeaux-Bacchi V, Kemp EJ, Goodship JA: The development of atypical haemolytic-uraemic syndrome is influenced by susceptibility factors in factor H and membrane cofactor protein: Evidence from two independent cohorts. J Med Genet 42: 852–856, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pickering MC, de Jorge EG, Martinez-Barricarte R: Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med 204: 1249–1256, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dragon-Durey MA, Blanc C, Marliot F: The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet 46: 447–450, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Ferreira VP, Herbert AP, Cortés C: The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol 182: 7009–7018, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA: Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 18: 2392–2400, 2007. [DOI] [PubMed] [Google Scholar]