Abstract
Cancer is a widely recognized complication of transplantation, and the effects of various immunosuppressive drugs on cancer risk remains controversial. This randomized trial allocated 489 recipients of first cadaveric renal transplants to one of three groups: Azathioprine and prednisolone, cyclosporine monotherapy, or cyclosporine monotherapy followed by a switch to azathioprine and prednisolone after 3 months. Here, we report cancer outcomes by non–skin cancer (including melanoma) and skin cancer (excluding melanoma) for 481 patients during a median follow-up of 20.6 years. A total of 226 patients developed at least one cancer: 95 with non–skin cancer and 171 with skin cancer. In the intention-to-treat analysis, mean times to first non–skin cancer (16.0, 15.3, and 15.7 years for groups 1 through 3, respectively) and first skin cancer (13.6, 14.3, and 15.2 years, respectively) were not different among the three groups or between any subgroup. In multivariate analyses, non–skin cancer associated with increasing age and previous smoking history, whereas skin cancer associated with increasing age, nonbrown eye color, fairer skin, and a functioning transplant. Treatment allocation did not associate with development of either form of cancer in multivariate analyses. In conclusion, these immunosuppressive regimens, widely used in recent decades, carry similar risks for carcinogenicity after kidney transplantation.
Kidney transplantation is the preferred treatment for end-stage kidney disease because of improved patient survival and quality of life and lower treatment costs compared with dialysis. Cancer is a widely recognized complication of transplantation1,2 and is likely to become more common among transplant recipients as donation criteria are extended to allow older donors, the age of waiting list patients increases, and transplant recipients live longer.
The relative carcinogenicity of the specific immunosuppressive agents or combinations of agents is not well understood. One reason for this is that randomized trials of various immunosuppressive regimens have not reported cancer outcomes or followed kidney transplant recipients long enough for cancers to develop. Second, although observational data from registries have made an important contribution to our knowledge,1–3 their validity in answering drug intervention questions is limited because of the unequal distribution of confounders (both known and unknown). These factors combine to limit our knowledge and impair clinicians' attempts to mitigate patients' cancer risk.
The Australian Multicenter Trial of Cyclosporine Withdrawal randomly assigned patients to three arms: Standard therapy of azathioprine and prednisolone (AP), long-term cyclosporine (CY), and short-term cyclosporine followed by withdrawal and maintenance azathioprine and prednisolone (WDL). We previously reported graft and patient survival outcomes at 15 and 20 years of median follow-up.4,5 In this study, we compare incident cancer rates and spectrum of cancers across the three arms using the extremely prolonged follow-up provided by registry linkage, the unbiased distribution of confounders arising from the trial randomization, and extensive baseline data on cancer risk.
Results
A total of 489 patients were randomly allocated to one of the three treatment groups between August 1983 and November 1986. Follow-up was available for 481 (98%) patients; the median follow-up of surviving patients was 20.6 years (interquartile range 20.0 to 21.5 years) after randomization. The baseline characteristics of the three treatment groups, including measured risk factors for non–skin and skin malignancies, were similar (Table 1). Following provision according to the study protocol, more recipients in the AP group received antithymocyte globulin (ATG) than in either of the CY group or the WDL group. A total of 226 (46%) patients developed at least 1 cancer. Ninety-five (19%) patients had at least one non–skin cancer (NSC) and 171 (35%) had at least one skin cancer excluding melanoma (SCEM; 60% squamous cell carcinomas and 40% basal cell carcinomas) during the study follow-up (Table 2). Adenocarcinomas made up the largest group of NSCs (37%) followed by SCEMs (20%)
Table 1.
Characteristics of trial participants
| Characteristic | AP | CY | WDL | Total |
|---|---|---|---|---|
| No. randomly assigned | 158 | 166 | 165 | 489 |
| Male gender (n [%]) | 88 (56) | 98 (59) | 93 (56) | |
| Age at transplantation (years; n [%]) | ||||
| <35 | 40 (25) | 50 (30) | 46 (28) | 136 |
| 35 to 44 | 39 (25) | 34 (20) | 37 (22) | 110 |
| 45 to 54 | 38 (24) | 42 (25) | 57 (35) | 137 |
| ≥55 | 41 (26) | 40 (24) | 24 (15) | 105 |
| Years on dialysis (median [range]) | 1.24 (0.20 to 21.70) | 1.16 (0.10 to 11.50) | 1.21 (0.10 to 8.60) | 1.22 (0.00 to 21.70) |
| History of ischemic heart disease (n [%]) | 22 (14) | 29 (17) | 22 (13) | 73 |
| BMI at transplantation (kg/m2; [n]) | ||||
| <25 | 98 (63) | 109 (67) | 112 (70) | 319 |
| ≥25 | 57 (37) | 53 (33) | 47 (30) | 157 |
| Smoking (ever/never; n) | 81/77 | 96/67 | 87/76 | 262/220 |
| Latitude of primary residence (n [%]) | ||||
| >35°Sa | 27 (18) | 26 (16) | 30 (19) | 83 |
| 30 to 35°S | 70 (46) | 77 (48) | 72 (46) | 219 |
| <30°S | 55 (36) | 57 (36) | 56 (35) | 168 |
| Eye color (brown/not brown; n) | 60/98 | 66/99 | 49/114 | 175/311 |
| Skin type (n [%]) | ||||
| fair and freckles | 16 (10) | 10 (6) | 8 (5) | 34 |
| fair, no freckles | 27 (17) | 27 (16) | 24 (15) | 78 |
| olive | 66 (42) | 69 (42) | 78 (48) | 213 |
| darker skin | 49 (31) | 59 (36) | 54 (33) | 162 |
| Previous skin cancer (yes/no; n) | 3/155 | 3/163 | 1/163 | 7/481 |
| Cold ischemia time (hours; median [range]) | 22 (7 to 60) | 22 (4 to 30) | 23 (8 to 39) | 22 (4 to 60) |
| HLA mismatches (n [%]) | ||||
| 0 | 2 (1) | 2 (1) | 1 (1) | 5 |
| 1 to 2 | 52 (33) | 55 (33) | 58 (36) | 165 |
| ≥3 | 102 (65) | 108 (65) | 100 (63) | 310 |
| 2 DR mismatches | 18 (12) | 19 (12) | 20 (13) | 57 |
| PRA (current; n) | ||||
| ≤20% | 132 (89) | 138 (87) | 130 (85) | 400 |
| >20% | 16 (11) | 21 (13) | 23 (15) | 60 |
| Received ATG (n [%]) | 94 (59) | 9 (5) | 8 (5) | 111 |
aIncludes those with predominant residential history of NZ, northern and southern Europe and Pacific Islands.
BMI, body mass index; PRA, panel-reactive antibodies.
Table 2.
Cancer types across all treatment groups out to 20 years after randomization
| Cancer Type | AP | CY | WDL | Total |
|---|---|---|---|---|
| NSC | ||||
| total recipients | 29 | 35 | 31 | 95 |
| adenocarcinoma | 8 | 15 | 12 | 35 |
| colon | 4 | 4 | 1 | 9 |
| prostate | 0 | 3 | 2 | 5 |
| kidney | 0 | 2 | 1 | 3 |
| breast | 0 | 1 | 2 | 3 |
| esophagus/stomach | 1 | 1 | 1 | 3 |
| other sites | 3 | 3 | 2 | 8 |
| unknown primary | 0 | 1 | 3 | 4 |
| squamous cell carcinoma | 4 | 11 | 4 | 19 |
| cervix | 1 | 3 | 0 | 4 |
| lung | 0 | 3 | 1 | 4 |
| head and neck | 2 | 3 | 3 | 8 |
| other | 1 | 2 | 0 | 3 |
| melanoma | 7 | 1 | 1 | 9 |
| lymphoma (all) | 2 | 3 | 4 | 9 |
| leukemia | 1 | 0 | 1 | 2 |
| transitional cell carcinoma | 3 | 0 | 2 | 5 |
| Kaposi | 0 | 1 | 1 | 2 |
| multiple myeloma | 1 | 0 | 0 | 1 |
| other/unknown | 3 | 4 | 6 | 13 |
| Skin cancer | ||||
| total recipients | 51 | 65 | 55 | 171 |
| squamous cell carcinoma | 32 | 36 | 34 | 102 |
| basal cell carcinoma | 19 | 28 | 21 | 68 |
| other | 0 | 1 | 0 | 1 |
Risk for Cancer by Randomized Group
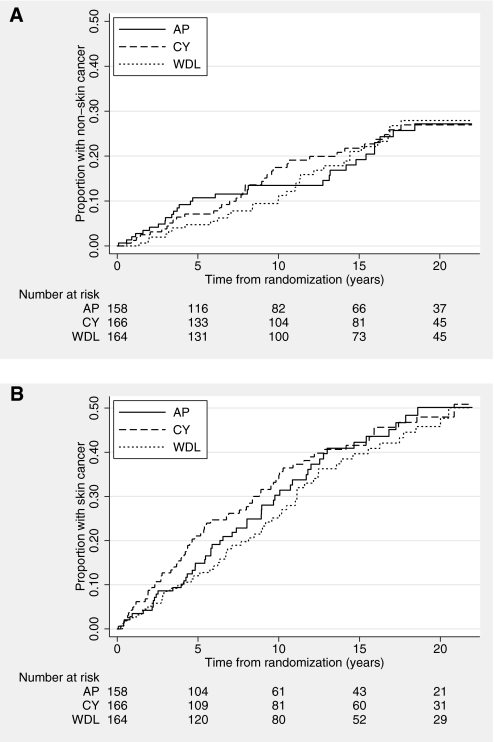
The mean time to first NSC was not different among treatment groups: AP 16.0 years (95% confidence interval [CI] 15.0 to 17.0 years), CY 15.3 years (95% CI 14.5 to 16.1 years), and WDL 15.7 years (95% CI 15.0 to 16.4 years). The overall cumulative incidence of NSC at 20 years after transplantation was 27% and not different among the three treatment groups (27, 27, and 28%, respectively; P = 0.96; Figure 1A). The proportions of adenocarcinomas and squamous cell carcinomas within each treatment group were not different (P = 0.44 and P = 0.1, respectively).
Figure 1.
Cumulative incidence of cancer out to 20 years posttransplant. (A) Cumulative incidence of NSC or melanoma, by treatment allocation, over time since randomization. (B) Cumulative incidence of SCEM, by treatment allocation, over time since randomization.
The mean time to the first SCEM was not different among groups: AP 13.6 years (95% CI 12.4 to 14.8 years), CY 14.3 years (95% CI 13.0 to 15.6 years), and WDL 15.2 years (95% CI 14.0 to 16.4 years). The overall cumulative incidence of skin cancer was 48% at 20 years and was not different among the three treatment groups (50, 48, and 46%; P = 0.69; Figure 1B). Seven trial participants had a history of skin cancer before transplantation, but none went on to develop an additional SCEM after transplantation.
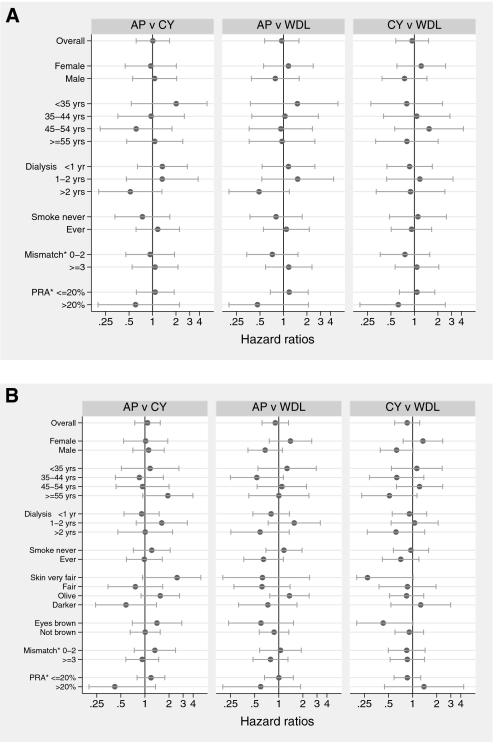
Analyses of NSC incidence by baseline subgroups of gender, age, dialysis duration, smoking history, HLA matching, and panel-reactive antibody levels suggested no differences between any treatment groups (Figure 2A). Similarly, for SCEM, analysis by baseline subgroups of gender, age, dialysis duration, smoking history, HLA mismatching, panel-reactive antibody levels, skin type, and eye color did not suggest any differences between any treatment groups (Figure 2B).
Figure 2.
Treatment effects upon cancer risk by potential effect modifiers. (A) Effects of randomized study of immunosuppressive treatments on NSC, stratified by potential effect modifiers. *Mismatch is the number of HLA mismatches between donor and recipient (grouped as shown); PRA is the panel-reactive antibody values for recipients (grouped as shown). (B) Effects of randomized study of immunosuppressive treatments on SCEM, stratified by potential effect modifiers. *Mismatch is the number of HLA mismatches between donor and recipient (grouped as shown); PRA is the panel-reactive antibody values for recipients (grouped as shown).
Risk for Cancer by Multivariate Modeling
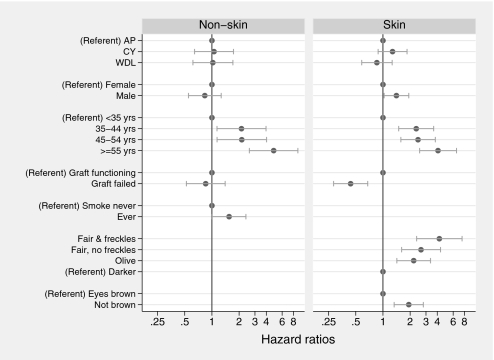
In the univariate analysis of risk factors, only increasing age and history of smoking were significantly associated with the development of NSC after transplantation. Gender, exposure to ATG, body mass index, history of ischemic heart disease, and graft failure showed no association. After allowing for their independent effects in a multivariate model, age and smoking history continued to be significantly associated with NSC development, but there was no demonstrable effect of immunosuppressive treatment allocation on NSC risk (Figure 3). Using these variables to define groups at low and high risk for NSC after transplantation, the predicted incidence varies between these groups by approximately six-fold over time (Table 3).
Figure 3.
Risk factors for NSA and SCEM by multivariate modeling.
Table 3.
Predicted risk for NSC and skin cancer over time after transplantation
| Risk Stratum | Group Characteristics | No. (95% CI) with Cancers Predicted per 100 People Treated |
||
|---|---|---|---|---|
| 5 years | 10 years | 20 years | ||
| NSC | ||||
| low | <35 years old, never smoked | 2.5 (0.9 to 4.1) | 4.9 (2.1 to 7.7) | 12.0 (5.8 to 18.0) |
| high | ≥55 years old, previous or current smoker | 17.0 (9.8 to 24.0) | 32.0 (20.0 to 41.0) | 61.0 (43.0 to 74.0) |
| SCEM | ||||
| low | Female, <35 years old, brown eyes, dark skin | 1.9 (0.7 to 3.1) | 4.2 (1.7 to 6.6) | 9.3 (4.1 to 14.0) |
| high | Male, ≥55 years old, nonbrown eyes, fair skin | 56.0 (29.0 to 73.0) | 84.0 (55.0 to 95.0) | 99.0 (82.0 to 100.0) |
In SCEM, the univariate modeling showed that continued transplant function, increased age at transplantation, nonbrown eye color, and fairer skin type all were significantly associated with SCEM development after transplantation. Receipt of ATG, history of smoking, and allocated immunosuppressive treatment were not. After allowing for their independent effects in the multivariate model, male gender, continued transplant function, increased age at transplantation, nonbrown eyes, and fairer skin type showed significant association with SCEM (Figure 3). Using these variables to define groups at low and high risk for SCEM after transplantation, the predicted incidence varies from 10- to 28-fold over time (Table 3).
Discussion
These results suggest that patients who were treated with these three immunosuppressive regimens, commonly used in renal transplantation for the past 30 years, show no difference in the development of both NSC and skin cancer out to 20 years after transplantation. We demonstrated no difference in drug effects for any patient subgroups, but the risk for cancer was strongly associated with patient characteristics known at the time of transplantation. As in the general population, age and smoking history are the major determinants of posttransplantation NSC risk. The results also highlight the inexorable rise in skin cancer during time after transplantation and the reduction in risk after graft loss, presumably mediated through a reduced need for subsequent immunosuppression.
Despite the existing knowledge regarding cancer risk, no immunosuppressive treatment strategies have been proved to reduce this risk. Much debate has focused on the effects of the various immunosuppressive agents and dose regimens, with concerns raised about azathioprine,4,6,7 cyclosporine,8,9 mycophenolate mofetil,8 tacrolimus,10 and triple therapy.11 Our data suggest that azathioprine- and cyclosporine-based regimens are associated with similar overall long-term cancer risks, suggesting that the risk may be mediated by the total burden of immunosuppression more than the agent. It remains possible that small differences in drug effects do exist but that we did not have the statistical power to detect them even after 20 years of follow-up. The clinical implications of such small differences, if they do exist undetected by our analysis, is questionable, however, because most modern transplant regimens use combinations of antiproliferative agents and calcineurin antagonists. Our analysis also underlines that other factors contribute much more to cancer risk after transplantation than the specific immunosuppressive therapy and that risk estimation is possible.
One recent publication reported a possible reduction in cancer risk at 24 months of follow-up with sirolimus,12 but this has not been confirmed by other trials13 or a meta-analysis.14 Apart from the association of anti-CD3 mAbs with posttransplantation lymphoproliferative disease,15 the clinical evidence for one treatment regimen over another in reducing posttransplantation malignancy is varied and not compelling. This analysis suggests that longer term follow-up of recent comparative immunosuppressive trials (e.g., Efficacy Limiting Toxicity Elimination [ELITE]-Symphony Trial13) could provide important data on cancer outcomes and that better markers of the degree of immunosuppression may also aid in reducing cancer risk.
A strength of these data is that they arose from a randomized trial, using a crossed synthesis design made possible using the Australian and New Zealand Transplant Registry, demonstrating that it is feasible and informative to obtain long-term results from trials with almost complete outcome data. The randomized allocation of immunosuppressive treatment ensures that the measure of no difference should be the least biased estimate of effect possible. Nonetheless, there are some limitations to the results of this trial. The trial was designed to test a primary outcome of graft survival, although cancer incidence was a prespecified outcome and important cancer risk factors were collected at baseline. The increase in cancer risk after transplantation has been widely documented, but the reported rates do vary and this is likely a function of regional variations in cancer epidemiology as well as differing study methods. Patients in this study come from a largely white population arising from the broader Australian general population, who have among the highest rates of skin cancer in the world. Thus, the absolute estimates of risk may differ for transplant recipients in different settings, but it is likely that the relative differences will hold true. In addition, some of the immunosuppressive regimens used in the trial, most notably cyclosporine monotherapy, the absence of mandated cyclosporine drug monitoring, and the absence of new antiproliferative agents (e.g., mycophenolate), differ from current immunosuppressive practice, so caution should be exercised when extrapolating the results to contemporary immunosuppressive protocols. It is likely, however, that today's immunosuppressive regimens, with their lower acute rejection rates, represent more potent immunosuppression.
Although the large transplant registries have provided an invaluable real-world insight into posttransplantation malignancy,1,2,16 their value in clarifying the effects of individual drugs and drug regimens is limited by selection bias as a result of the absence of random allocation to treatments and limited recording of drug exposures. The major burden of cancer after transplantation is seen more than 5 years after engraftment, beyond the scope and affordability of routine clinical trial follow-up. Cancer event rates seen in our study were approximately 1.25% per annum for NSC and 2.5% per annum for SCEM, making it difficult and expensive to power a study using cancers as an outcome. The rates of cancer seen in other studies have been higher17 but still low in absolute terms. In such a context, deriving long-term follow-up of large randomized trials from patient registries through similar cross-synthesis designs offers the best hope of identifying treatments to reduce the cancer burden in transplant recipients.
Concise Methods
Details of the trial, including the extent of deviations from protocol therapy, have been previously reported4,5,18 but will be summarized in brief here.
Participants
Eligible participants were adult patients who were receiving primary, deceased-donor renal transplants. Exclusion criteria were insulin-dependent diabetes, abnormal liver function tests, previous malignancy, malabsorption, active infection, or a known contraindication to azathioprine. Patients were randomly assigned at the time of transplantation between 1983 and 1986 in seven centers throughout Australia. The randomization sequence was centrally generated by computer and stratified by center, with patient assignment by sequentially labeled, opaque, sealed envelopes delivered to each center. The study was open-label; patients, treating physicians, data entry staff, and data analysts were not blinded to treatment allocation
Randomized Interventions
AP Group.
Participants who were allocated to AP received treatment with azathioprine and prednisolone with the option of induction ATG therapy. Oral azathioprine (3 mg/kg) was given preoperatively followed by 2 mg/kg per d. Intravenous methylprednisolone was given preoperatively (1000 mg) and on day 1 (500 mg) followed by oral prednisolone daily tapering to maintenance doses of 10 to 15 mg/d. Two of the seven centers involved in the trial opted for routine use of prophylactic ATG (5 mg/kg) for all of their recipients, dosed every other day for a total of seven doses.
CY Group.
Participants who were allocated to CY received long-term cyclosporine monotherapy. Intravenous cyclosporine (5 mg/kg) was given preoperatively and on day 1 (4 mg/kg) followed by oral cyclosporine 12.5 mg/kg tapering to 7.5 mg/kg by 3 months after transplantation. Intravenous prednisolone was given at operation (1000 mg) and on day 1 (500 mg). Patients with clinical signs of nephrotoxicity had their cyclosporine dosage reduced to 7.5 mg/kg, with therapy changed to azathioprine and prednisolone when there was no improvement within 1 week. Patients who were receiving cyclosporine could receive additional maintenance prednisolone if they experienced three or more episodes of rejection.
WDL Group.
Participants who were allocated to WDL received identical treatment as CY for the first 3 months after transplantation. When at 3 months there was no evidence of rejection (either clinically or on renal biopsy) in the previous 2 weeks, treatment was changed to azathioprine (2 mg/kg per d) and prednisolone (20 mg/d). Therapy transition was completed after a 4-day overlap of the two strategies for the majority of recipients.
Outcomes
The primary and secondary outcomes of the study—death-censored graft survival, patient survival, and the combined end point of death or graft loss4,5,18—have previously been reported. Cancer outcomes were prespecified in the original trial and were briefly reported at 15 and 20 years4,5 but were not previously analyzed and reported in detail. Cancer outcomes were measured by linking the trial database with the Australia and New Zealand Dialysis and Transplant Registry database, using data up to and including the census of December 2005.
Statistical Analysis
An intention-to-treat analysis of cancer outcomes by randomized treatment allocation was assessed using cumulative incidence curves and Cox proportional hazard models. Pair-wise differences between treatment groups were conducted both overall and by patient subgroups. In all analyses, recipients were censored from the analysis at their last known date of follow-up (alive, lost to follow-up, or death) when they did not experience the cancer event of interest.
A secondary analysis was conducted to examine the risk factors for NSC using a Cox proportional hazards model and similarly for SCEM. Age, gender, allocated treatment group, and graft status (functioning versus failed) were included in the multivariate models, regardless of their statistical significance. Graft status was a time-varying variable. Other variables that were considered possible risk factors for cancer were included in the multivariable modeling at their univariate P ≤ 0.3 and remained in the final model at the multivariable P ≤ 0.05. Potential effect modification was investigated by testing for interaction between age and gender and also between graft status and all other variables remaining in the model. For both analyses, no interaction term had a P < 0.05; hence, no interaction terms were included in either final model. The assumption of proportion hazards was checked for each variable by including a time-varying covariate of the variable of interest, multiplied by log(time). Schoenfeld residuals were also plotted to check potential deviation from the proportional hazards assumption. Statistical analyses was conducted in SAS 9.1.
Disclosures
M.G. and V.P. have received speaking fees from pharmaceutical companies that manufacture immunosuppressive agents used in transplantation; J.E. has received travel assistance from and chairs advisory boards of pharmaceutical companies that manufacture immunosuppressive agents; and the Australia and New Zealand Dialysis and Transplant Registry has received contributions in the past from pharmaceutical companies that manufacture immunosuppressive agents.
Supplementary Material
Acknowledgments
This study was supported by Sandoz out to 10 years of follow-up, but this analysis received no dedicated support. Authors have received support from the Australian National Heart Foundation Postdoctoral Fellowship (V.P.) and Australian National Health and Medical Research Council Fellowship (A.C.). The Australia and New Zealand Dialysis and Transplant Registry is funded by the Australian Department of Health and Ageing, the NZ Ministry of Health, and Kidney Health Australia.
Parts of the data reported here were supplied by the Australia and New Zealand Dialysis and Transplant Registry.
We thank those who instigated the original study, especially Bruce Hall, David Tiller, Ian Hardie, John Mahony, Tim Matthew, Geoffry Thatcher, Peter Miach, Nip Thompson, and Ross Sheil, along with all of the clinicians at all of the centers participating in the trial, and Royal Prince Alfred Hospital (Sydney), Princess Alexandra Hospital (Brisbane), Queen Elizabeth Hospital (Adelaide), Royal North Shore Hospital (Sydney), Monash Medical Centre (Melbourne), Austin Hospital (Melbourne), and Sir Charles Gairdner Hospital (Perth).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
The interpretation and reporting of the data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Australia and New Zealand Dialysis and Transplant Registry.
REFERENCES
- 1. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frödin L, et al. : Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer 60: 183–189, 1995. [DOI] [PubMed] [Google Scholar]
- 3. Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR: Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: A cohort study of 15,183 recipients. Am J Transplant 7: 2140–2151, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher M, Jardine M, Perkovic V, Cass A, McDonald S, Petrie J, Eris J: Cyclosporine withdrawal improves long-term graft survival in renal transplantation. Transplantation 87: 1877–1883, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Gallagher MP, Hall B, Craig J, Berry G, Tiller DJ, Eris J: A randomized controlled trial of cyclosporine withdrawal in renal-transplant recipients: 15-Year results. Transplantation 78: 1653–1660, 2004. [DOI] [PubMed] [Google Scholar]
- 6. Scharf J, Nahir M, Eidelman S, Jacobs R, Levin D: Carcinoma of the bladder with azathioprine therapy. JAMA 237: 152, 1977. [PubMed] [Google Scholar]
- 7. Krutchik AN, Buzdar AU, Tashima CK: Azathioprine and breast carcinoma. JAMA 239: 107–108, 1978. [DOI] [PubMed] [Google Scholar]
- 8. Guba M, Graeb C, Jauch KW, Geissler EK: Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 77: 1777–1782, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP: Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: Randomised comparison of two cyclosporin regimens. Lancet 351: 623–628, 1998. [DOI] [PubMed] [Google Scholar]
- 10. Imao T, Ichimaru N, Takahara S, Kokado Y, Okumi M, Imamura R, Namba Y, Isaka Y, Nonomura N, Okuyama A: Risk factors for malignancy in Japanese renal transplant recipients. Cancer 109: 2109–2115, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Penn I, First MR: Development and incidence of cancer following cyclosporine therapy. Transplant Proc 18: 210–215, 1986. [PubMed] [Google Scholar]
- 12. Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF: Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-Month efficacy and safety results from the CONVERT trial. Transplantation 87: 233–242, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, Acevedo RR, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, Halloran P: Calcineurin inhibitor minimization in the SYMPHONY study: Observational results 3 years after transplantation. Am J Transplant 9: 1876–1885, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Webster AC, Lee VW, Chapman JR, Craig JC: Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev CD004290, 2006. [DOI] [PubMed] [Google Scholar]
- 15. Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW: Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation 76: 1289–1293, 2003. [DOI] [PubMed] [Google Scholar]
- 16. Robson R, Cecka JM, Opelz G, Budde M, Sacks S: Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 5: 2954–2960, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C: Cancer after kidney transplantation in the United States. Am J Transplant 4: 905–913, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Hall BM, Tiller DJ, Hardie I, Mahony J, Mathew T, Thatcher G, Miach P, Thomson N, Sheil AG: Comparison of three immunosuppressive regimens in cadaver renal transplantation: Long-term cyclosporine, short-term cyclosporine followed by azathioprine and prednisolone, and azathioprine and prednisolone without cyclosporine. N Engl J Med 318: 1499–1507, 1988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.