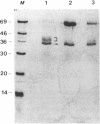
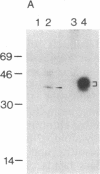
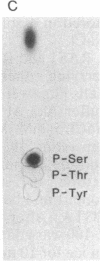
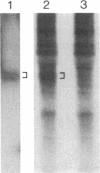
Abstract
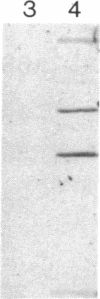
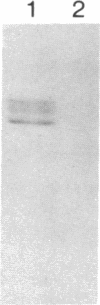
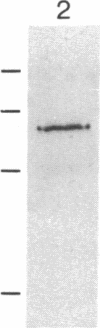
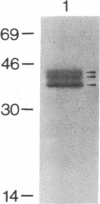
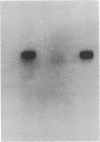
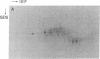
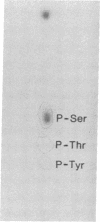
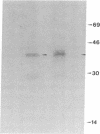
RAS1 and RAS2 proteins of Saccharomyces cerevisiae are guanine nucleotide-binding proteins involved in the regulation of adenylate cyclase. In this paper, we report that these proteins are phosphorylated. The phosphorylation of RAS1 protein is demonstrated by treating with alkaline phosphatase as well as by labeling with [32P]orthophosphate. The phosphorylation occurs exclusively on serine residues and phosphorylated RAS1 protein is predominantly membrane localized. The phosphorylation of RAS2 protein is demonstrated by similar 32P-labeling experiments. The phosphorylation occurs exclusively on serine residues and phosphopeptide analyses suggest that only two major phosphorylated tryptic peptides are generated from the RAS2 protein. These results provide evidence for the phosphorylation of RAS proteins in vivo. Furthermore, our demonstration that the phosphorylation occurs exclusively on serine residues and that the RAS2 protein contains only two major phosphorylated tryptic peptides argues that the phosphorylation may be physiologically significant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Furth M. E., Rosen O. M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J Biol Chem. 1987 Feb 25;262(6):2688–2695. [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- De Vendittis E., Zahn R., Fasano O. Regeneration of the GTP-bound from the GDP-bound form of human and yeast ras proteins by nucleotide exchange. Stimulatory effect of organic and inorganic polyphosphates. Eur J Biochem. 1986 Dec 1;161(2):473–478. doi: 10.1111/j.1432-1033.1986.tb10468.x. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama A., Matsumoto K., Tamanoi F. A novel yeast mutant defective in the processing of ras proteins: assessment of the effect of the mutation on processing steps. EMBO J. 1987 Jan;6(1):223–228. doi: 10.1002/j.1460-2075.1987.tb04742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama A., Tamanoi F. Processing and fatty acid acylation of RAS1 and RAS2 proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1266–1270. doi: 10.1073/pnas.83.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Miyajima A., Arai K., Matsumoto K. Possible involvement of RAS-encoded proteins in glucose-induced inositolphospholipid turnover in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8172–8176. doi: 10.1073/pnas.83.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Cameron S., Toda T., Ferguson K. M., Wigler M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987 Nov;1(9):931–937. doi: 10.1101/gad.1.9.931. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Papageorge A., Lowy D., Scolnick E. M. Comparative biochemical properties of p21 ras molecules coded for by viral and cellular ras genes. J Virol. 1982 Nov;44(2):509–519. doi: 10.1128/jvi.44.2.509-519.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Resnick R. J., Racker E. Phosphorylation of the RAS2 gene product by protein kinase A inhibits the activation of yeast adenylyl cyclase. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2474–2478. doi: 10.1073/pnas.85.8.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikumar P., Ulsh L. S., Clanton D. J., Huang K. P., Shih T. Y. Novel phosphorylation of c-ras p21 by protein kinases. Oncogene Res. 1988;3(3):213–222. [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., DeFeo-Jones D., Tatchell K., Ellinger M. S., Scolnick E. M. Expression and characterization of ras mRNAs from Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2298–2305. doi: 10.1128/mcb.4.11.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Turner B. M. Use of alkaline phosphatase-conjugated antibodies for detection of protein antigens on nitrocellulose filters. Methods Enzymol. 1986;121:848–855. doi: 10.1016/0076-6879(86)21081-2. [DOI] [PubMed] [Google Scholar]