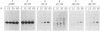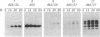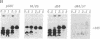Abstract
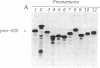
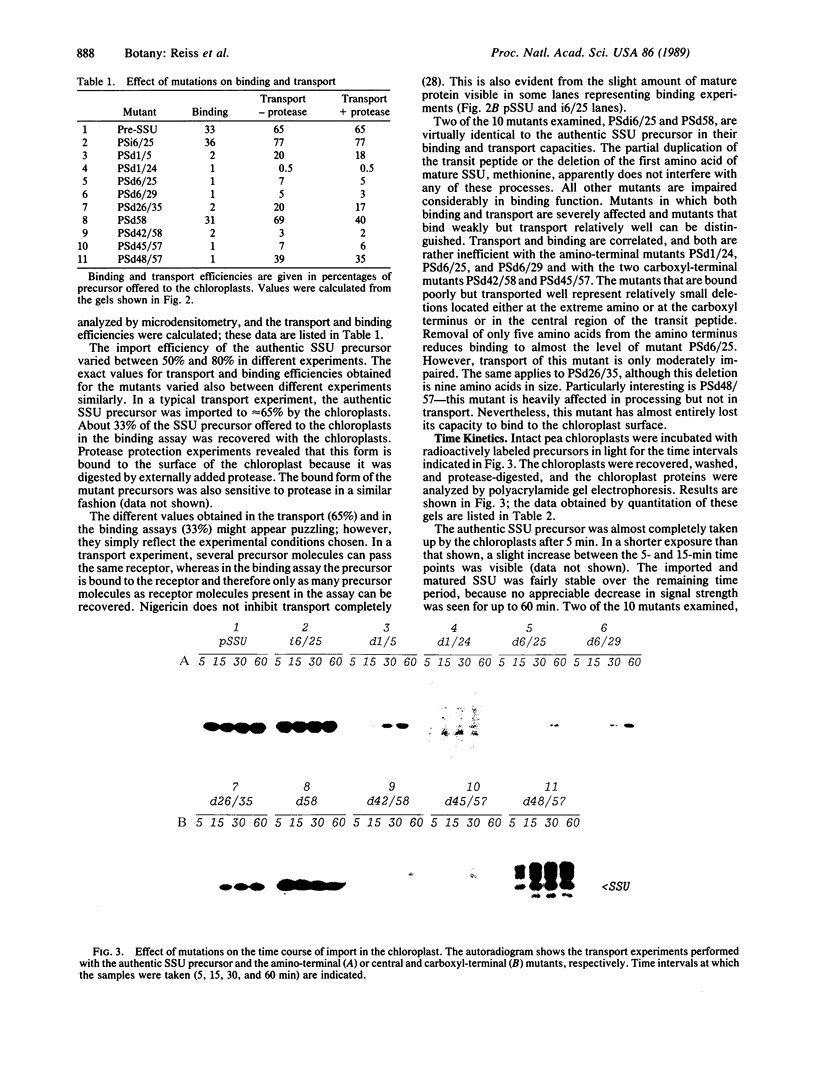
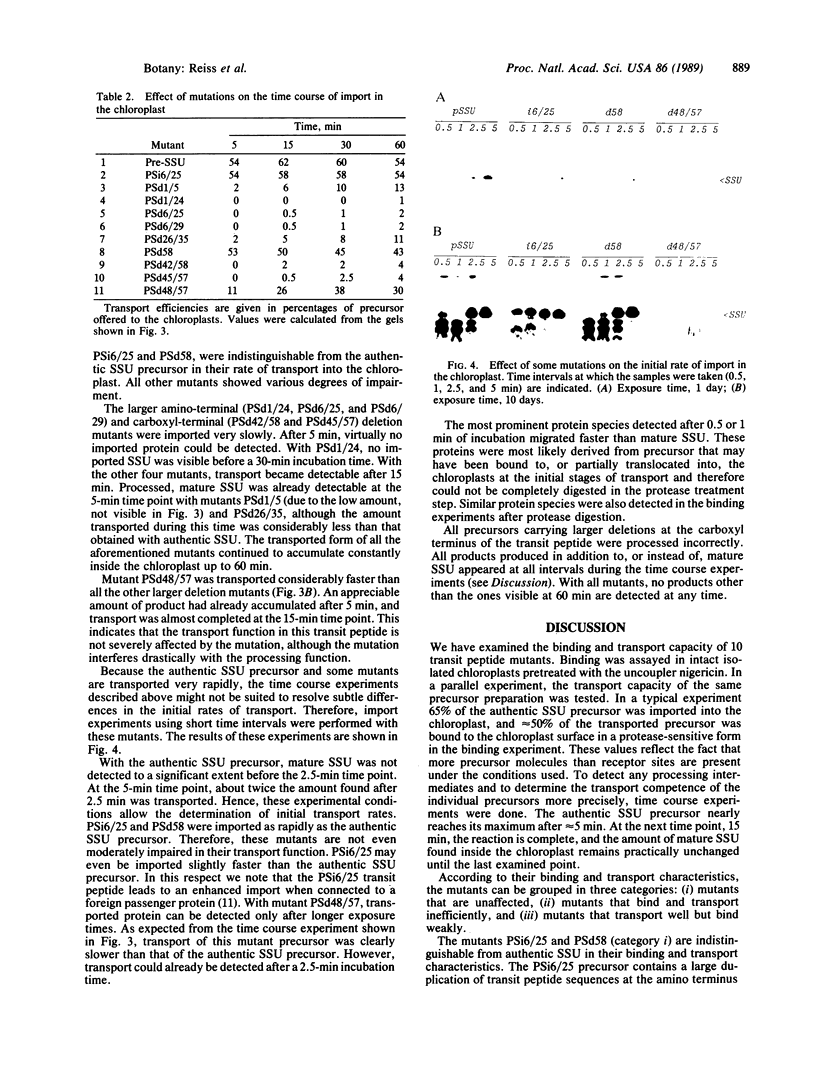
We studied transport and binding to intact chloroplasts of 10 mutants in three regions of the transit peptide of a precursor to the small subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase [3-phospho-D-glycerate carboxy-lyase (transphosphorylating), E.C.4.1.1.39]. Transport was assayed in a reconstituted system using isolated pea chloroplasts and radioactively labeled precursor. Binding to the chloroplast envelope was assayed in a similar manner using chloroplasts pretreated with nigericin. Most mutants showed a dramatically decreased capacity of binding, although some of them transported relatively well. The accumulation of the mutant proteins inside the chloroplast as a function of time was examined. Although the authentic small subunit precursor was imported rapidly, uptake of most mutant precursors was considerably slower and continued until the last time point examined. In terms of assigning functions to individual regions, we found that at least the middle region and parts of the amino and the carboxyl termini of the transit peptide are more important for receptor binding than for translocation. A two-step processing mechanism has been postulated for the maturation of the small subunit precursor. This model predicts the occurrence of processing intermediates. When precursors carrying carboxyl-terminal deletions were presented to the chloroplast, no defined intermediates could be detected. Instead, a number of proteins, probably resulting from aberrant processing, accumulated simultaneously inside the chloroplasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Transport of proteins into mitochondria and chloroplasts. J Cell Biol. 1979 Jun;81(3):461–483. doi: 10.1083/jcb.81.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Andrews J., Mersey B., Newcomb E. H., Keegstra K. Separation and characterization of inner and outer envelope membranes of pea chloroplasts. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3595–3599. doi: 10.1073/pnas.78.6.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Cornwell K. L., Keegstra K. Evidence that a Chloroplast Surface Protein Is Associated with a Specific Binding Site for the Precursor to the Small Subunit of Ribulose-1,5-Bisphosphate Carboxylase. Plant Physiol. 1987 Nov;85(3):780–785. doi: 10.1104/pp.85.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Cioppa G., Bauer S. C., Klein B. K., Shah D. M., Fraley R. T., Kishore G. M. Translocation of the precursor of 5-enolpyruvylshikimate-3-phosphate synthase into chloroplasts of higher plants in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6873–6877. doi: 10.1073/pnas.83.18.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Tobin E. M. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986 Jan;5(1):9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lubben T. H., Keegstra K. Efficient in vitro import of a cytosolic heat shock protein into pea chloroplasts. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5502–5506. doi: 10.1073/pnas.83.15.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind M. L., Wessler S. R., Schmidt G. W. Functional determinants in transit sequences: import and partial maturation by vascular plant chloroplasts of the ribulose-1,5-bisphosphate carboxylase small subunit of Chlamydomonas. J Cell Biol. 1985 Jan;100(1):226–234. doi: 10.1083/jcb.100.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain D., Kanwar Y. S., Blobel G. Identification of a receptor for protein import into chloroplasts and its localization to envelope contact zones. Nature. 1988 Jan 21;331(6153):232–237. doi: 10.1038/331232a0. [DOI] [PubMed] [Google Scholar]
- Reiss B, Wasmann C C, Bohnert H J. Regions in the transit peptide of SSU essential for transport into chloroplasts. Mol Gen Genet. 1987 Aug;209(1):116–121. doi: 10.1007/BF00329845. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. The precursor of small subunit of ribulose bisphosphate carboxylase is processed to the mature size in two steps. Eur J Biochem. 1984 Jul 16;142(2):343–346. doi: 10.1111/j.1432-1033.1984.tb08292.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Seftor E. A., Schell J., Bohnert H. J. The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J. 1985 Jan;4(1):25–32. doi: 10.1002/j.1460-2075.1985.tb02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Maher P. A., Yaffe M. P. On the translocation of proteins across membranes. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1015–1019. doi: 10.1073/pnas.84.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Smeekens S., van Binsbergen J., Weisbeek P. The plant ferredoxin precursor: nucleotide sequence of a full length cDNA clone. Nucleic Acids Res. 1985 May 10;13(9):3179–3194. doi: 10.1093/nar/13.9.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck G., Timko M. P., Kausch A. P., Cashmore A. R., Van Montagu M., Herrera-Estrella L. Targeting of a foreign protein to chloroplasts by fusion to the transit peptide from the small subunit of ribulose 1,5-bisphosphate carboxylase. 1985 Jan 31-Feb 6Nature. 313(6001):358–363. doi: 10.1038/313358a0. [DOI] [PubMed] [Google Scholar]
- Wasmann C. C., Reiss B., Bohnert H. J. Complete processing of a small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from pea requires the amino acid sequence Ile-Thr-Ser. J Biol Chem. 1988 Jan 15;263(2):617–619. [PubMed] [Google Scholar]