Abstract
Seaweed has been used in traditional cosmetics and as a herbal medicine in treatments for cough, boils, goiters, stomach ailments, and urinary diseases, and for reducing the incidence of tumors, ulcers, and headaches. Despite the fact that seaweeds are frequently used in the practice of human health, little is known about the role of seaweed in the context of inflammation. This study aimed to investigate the influence of Jeju endemic seaweed on a mouse macrophage cell line (RAW 264.7) under the stimulation of lipopolysaccharide (LPS). Ethyl acetate extracts obtained from 14 different kinds of Jeju seaweeds were screened for inhibitory effects on pro-inflammatory mediators. Our results revealed that extracts from five seaweeds, Laurencia okamurae, Grateloupia elliptica, Sargassum thunbergii, Gloiopeltis furcata, and Hizikia fusiformis, were potent inhibitors of the production of pro-inflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Based on these results, the anti-inflammatory effects and low cell toxicity of these seaweed extracts suggest potential therapeutic applications in the regulation of the inflammatory response.
Keywords: Nitric oxide, Interleukin-6 (IL-6), Prostaglandin E2 (PGE2), Tumor necrosis factor-α (TNF-α), Seaweeds, Pro-inflammatory mediators
1. Introduction
The inflammatory response serves to protect the host against tissue wounds and microbial infections. An appropriate and proper inflammatory response depends on the careful regulation of a number of mediators such as cytokines, which are secreted by inflammatory cells such as macrophages and neutrophils (Park et al., 2006; Zhou et al., 2007). Macrophages are known to play a pivotal role in the host’s defense against harmful materials and are involved in a variety of diseases including autoimmune diseases, pathogenic infections, and inflammatory disorders (Dokka et al., 2001; Kang et al., 2008). An inflammatory stimulus such as lipopolysaccharide (LPS) can activate macrophages to produce a variety of pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), and other inflammatory mediators including prostaglandins and nitric oxide (NO), which are catalyzed by cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), respectively.
Overproduction of NO by iNOS has been implicated in the pathology of several inflammatory disorders including septic shock, tissue damage following inflammation, and rheumatoid arthritis. Therefore, NO production induced by LPS through iNOS can reflect the degree of inflammation, and a change in NO level through inhibition of iNOS enzyme activity or iNOS induction provides a means of assessing the effect of agents on the inflammatory process (Zhou et al., 2007; Choi et al., 2008; Kanwar et al., 2009; O′Connor and O′Brien, 2009; Murakami, 2009). Prostaglandins also play a major role as mediators of the inflammatory response. COX is an enzyme that converts arachidonic acid to prostaglandin. It is responsible for the production of large amounts of pro-inflammatory prostaglandins at the inflammatory site, and its uncontrolled activity is thought to play an important role in the pathogenesis of many chronic inflammatory diseases (Zhou et al., 2007; Rao and Knaus, 2008; Scher and Pillinger, 2009; Iyer et al., 2009). In addition, cytokines like TNF-α and IL-6 have been reported to be pro-inflammatory in vitro and in vivo, and the production of TNF-α is known to be crucial for the induction of NO synthesis in interferon-γ (IFN-γ)and/or LPS-stimulated macrophages. TNF-α elicits a number of physiological effects that include septic shock, inflammation, cachexia, and cell death. Similarly, IL-6 is also considered to be a pivotal pro-inflammatory cytokine; for example, it is regarded as an endogenous mediator of LPS-induced fever (Kim J.Y. et al., 2008; de Benedetti, 2009; Fonseca et al., 2009; Radovits et al., 2009; Esposito and Cuzzocrea, 2009). Therefore, pharmacological interference with the production of NO, PGE2, and cytokines such as TNF-α and IL-6 is postulated to be useful for reducing many inflammatory disease states that are mediated by excessive and/or prolonged activation of macrophages.
Marine organisms have proven to be rich sources of structurally novel and biologically active natural compounds. These compounds have served as important chemical prototypes for the discovery of new drugs for use in the treatment of various human diseases (Usami, 2009; Zhang and Kim, 2009; Blunt et al., 2009). Jeju Island, the largest island in Korea, is located in the southwest of the Korean Strait, and is well known for its distinctive environment. In particular, the sea levels around this island are known to fluctuate rapidly as a result of global warming. Therefore, in response to this unusual environment, the seaweeds that are present on Jeju Island may possess substantial endogenous protective mechanisms (Kim K.N. et al., 2009; Kim M.M. et al., 2009).
Some studies on seaweed-derived anti-inflammatory compounds have investigated potential inhibitory effects in in vitro systems, using LPS-stimulated macrophages. Therefore, bacterial LPS has become one of the best characterized stimuli used to induce the up-regulation of pro-inflammatory proteins. However, there is still a lack of methods for evaluating the anti-inflammatory efficacy and mechanisms of action of anti-inflammatory compounds (Dang et al., 2008; Kim S.K. et al., 2008; Kim K.N. et al., 2009; Kim M.M. et al., 2009). Hence, in this study we examined the inhibitory effect of Jeju seaweeds on NO, PGE2, TNF-α, and IL-6 production.
2. Materials and methods
2.1. Plant materials
Most seaweeds were collected between March and July, 2006 from Jeju Island, Korea. The voucher specimens are deposited at the herbarium of the Jeju Biodiversity Research Institute (JBRI), Jeju, Korea. The materials for extraction were cleaned, dried at room temperature for two weeks, and ground into a fine powder. The dried algae (50 g) were extracted with 80% ethanol (EtOH; 2 L) at room temperature for 24 h and then evaporated under vacuum. The evaporated EtOH extract (10 g) was suspended in water (1 L) and partitioned with ethyl acetate (EtOAc; 1 L), and this partition was repeated three times.
2.2. Analysis of total phlorotannin
To determine the total phlorotannin content in the seaweed extracts, the adjusted method with Folin-Ciocalteu reagent (Merck) was used. We added 550 μl of distilled water/Folin-Ciocalteu solution (10:1, v/v) to 50 μl of diluted extract (1 mg/ml of ethanol). After 3 min, 200 μl of 2 mol/L sodium carbonate (Na2CO3) and 300 μl of distilled water were added. After 1 h standing at laboratory temperature, absorbance was measured at 725 nm. The total phlorotannin content was calculated as a phloroglucinol equivalent from the calibration curve of phloroglucinol standard solutions (concentration range, 0–1.0 mg/ml). All measurements were conducted in triplicate.
2.3. Cell culture
Murine RAW 264.7 macrophages were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (w/v) fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin, all from GIBCO (Grand Island, NY, USA), in an incubator at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
2.4. Cell viability
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. RAW 264.7 cells were cultured in 96-well plates for 18 h, followed by treatment with various concentrations (3.125, 6.25, 12.5, 25, 50, or 100 µg/ml) of the EtOAc fractions of Jeju seaweeds. After a 24-h incubation, MTT was added to the medium for 4 h. Finally, the supernatant was removed and the formazan crystals were dissolved in dimethyl sulphoxide (DMSO). Absorbance was measured at 540 nm. The percentage of cells showing cytotoxicity was determined relative to the control group.
2.5. Determination of NO concentration
RAW 264.7 cells (5×105 cells/well) were incubated in 24-well dishes for 18 h with 1 µg/ml of LPS and various concentrations (3.125, 12.5, 25, 50, or 100 µg/ml) of the EtOAc fractions of Jeju seaweeds. The presence of nitrite, a stable oxidized product of NO, was determined in cell-culture media by a modified Griess method. The culture supernatant (100 µl) was mixed with the same volume of Griess reagent (1% (w/v) sulfanilamide and 0.1% (w/v) N-[1-naphthyl]-ethylenediamine dihydrochloride in 5% (w/v) phosphoric acid) for 10 min, and the absorbance was measured at 540 nm.
2.6. Measurement of PGE2
The RAW 264.7 cells were cultured in 24-well plates for 18 h, followed by treatment with LPS in the presence of various concentrations (3.125, 12.5, 25, 50, or 100 µg/ml) of samples. After a 24-h incubation, PGE2 in the culture supernatants was measured by an enzyme-linked immunosorbent assay (ELISA) kit.
2.7. Cytokine assays
The amounts of TNF-α and IL-6 in the cell-culture supernatant and in serum were measured using an ELISA kit (R & D, Minneapolis, MI). RAW 264.7 cells were plated in a 24-well cell-culture plate at a density of 2.5×105 cells/well and incubated with various concentrations (3.125, 12.5, 25, 50, or 100 µg/ml) of seaweed extracts in 1 µg/ml LPS for 24 h. The culture supernatant was collected and assayed according to the manufacturer’s instructions to determine the amounts of TNF-α and IL-6 that had been released from the cells.
2.8. Statistical analysis
All results were expressed as mean±standard error (SE). Each experiment was repeated at least three times. Statistical significances were compared between each treated group and analyzed by the Student’s t-test. Data with P<0.05 were considered statistically significant.
3. Results and discussion
Since the overproduction of NO is harmful and results in various inflammatory and autoimmune diseases, pharmacological interference with the NO production cascade offers promising strategies for therapeutic intervention in inflammatory disorders. Therefore, we first investigated the effect of Jeju seaweed extracts on NO synthesis in activated macrophages. Murine macrophage RAW 264.7 cells can induce iNOS transcription and protein synthesis, and subsequent NO production in response to LPS stimulation alone. This cell system is an excellent model for evaluating topical agents and for screening potential inhibitors of the pathways that induce iNOS and NO production. In our search for natural products that possess anti-inflammatory activity, we prepared 80% ethanol crude extracts from 14 native seaweeds from Jeju Island, Korea. The evaporated EtOH extract was partitioned with ethyl acetate. All ethyl acetate seaweed fractions were dissolved in 80% ethanol and diluted with sterile water to normalise the concentration of the test sample. The Griess reaction, a spectrophotometric determination for nitrite, was carried out to quantify the nitrite levels in the conditioned medium of RAW 264.7 cells treated with LPS. The final concentration of ethanol in the culture media was 0.1%, and this concentration of ethanol did not show any effect on the assay systems.
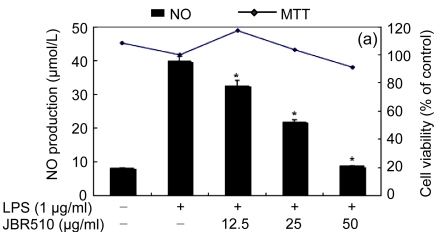
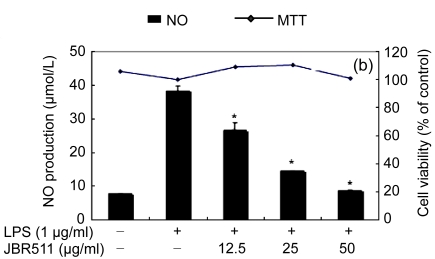
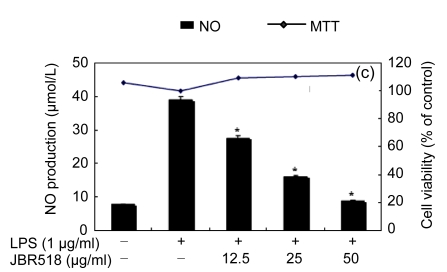
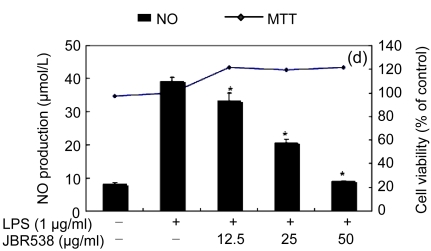
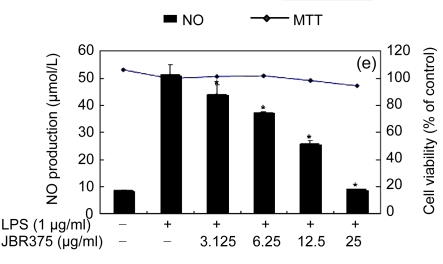
Table 1 shows the inhibitory effect of seaweed extracts on NO production by LPS-activated macrophages. Of the 14 extracts, 13 extracts showed greater than 50% inhibition of NO production at a concentration of 100 μg/ml in the culture medium. Among these 12 extracts, 5 (Laurencia okamurae, Grateloupia elliptica, Sargassum thunbergii, Gloiopeltis furcata, and Hizikia fusiformis) showed the most potent inhibition in a concentration-dependent manner (Fig. 1). Their half maximal inhibitory concentration (IC50) values were 12.6, 28.5, 20.2, 21.2, and 26.8 μg/ml, respectively. The numbers of viable activated macrophages were not significantly altered by the 14 extracts as determined by MTT assays (Table 1 and Fig. 1), thereby indicating that the inhibition of NO synthesis by the seaweed extracts was not simply the result of cytotoxic effects (Yang et al., 2009).
Table 1.
Inhibitory effect of Jeju seaweeds on the production of pro-inflammatory mediators
| Specimen number | Scientific name | Inhibition rate (%) |
Phlorotannin content (µg/ml) | ||||
| NO | PGE2 | IL-6 | TNF-α | Cytotoxicity | |||
| JBR243 | Sargassum nipponicum | 72.1±2.7** | 86.2±0.8** | 74.9±7.2** | 90.0±2.6** | 132.6±5.2 | 58.0 |
| JBR256 | Myelophycus simplex | 81.9±0.3** | 65.0±2.2** | 74.5±4.5** | 51.8±1.2** | 103.5±1.6 | 33.0 |
| JBR270 | Chondrus ocellatus | 78.2±0.3** | 90.7±1.0** | 83.1±2.4** | 71.0±1.9** | 106.1±12.4 | 37.2 |
| JBR271 | Ishige okamurae | 30.8±0.9* | NE | 47.1±0.7* | 3.1±0.8* | 118.6±3.8 | 332.5 |
| JBR274 | Bonnemaisonia hamifera | 81.8±0.1** | NE | 18.0±0.6** | 73.8±0.0** | 99.5±1.0 | 72.2 |
| JBR345 | Scytosiphon lomentaria | 70.4±0.2** | 89.9±1.5** | 90.4±0.2** | 55.2±0.3** | 93.6±6.9 | 65.0 |
| JBR354 | Callophyllis crispata | 83.6±0.0** | 84.7±3.1** | 90.4±0.5** | 75.7±0.9** | 79.7±5.9 | 40.0 |
| JBR368 | Padina arborescens | 59.7±0.4** | 80.5±0.9** | 71.3±3.7** | 80.9±0.7** | 87.8±8.7 | 124.0 |
| JBR375a | Laurencia okamurae | 82.4±0.1** | 82.1±4.2** | 76.9±0.7** | 52.5±0.0** | 91.4±2.4 | 37.3 |
| JBR376 | Chondria crassicaulis | 75.7±0.0** | 88.4±1.6** | 84.8±0.6** | 67.1±0.3** | 87.5±5.3 | 54.7 |
| JBR510b | Grateloupia elliptica | 78.2±0.4** | 81.0±4.8** | 85.7±0.8** | 63.8±0.8** | 89.4±6.9 | 47.7 |
| JBR511b | Sargassum thunbergii | 77.3±0.2** | 73.8±4.0** | 46.9±4.4** | 63.1±1.2** | 98.0±3.7 | 44.8 |
| JBR518b | Gloiopeltis furcata | 77.6±0.5** | 85.6±0.6** | 82.1±2.2** | 70.9±1.3** | 108.3±4.8 | 53.3 |
| JBR538b | Hizikia fusiformis | 77.2±0.1** | 81.0±4.8** | 69.9±6.7** | 61.5±3.9** | 105.8±3.5 | 43.3 |
The presence of nitrite, as a stable oxidized product of NO, was determined in cell-culture media by a modified Griess method. The amounts of PGE2, TNF-α, and IL-6 in the cell-culture supernatant and serum were measured using an ELISA kit. RAW 264.7 cells were incubated with various concentrations of seaweed extracts (25a, 50b, or 100 µg/ml). Cell viability was determined by MTT assay. The data represent the mean±SD of triplicate experiments
P<0.05 vs. LPS alone
P<0.01 vs. LPS alone
NE: no effects
Fig. 1.
Effect of Jeju seaweed extracts on nitric oxide production in LPS-stimulated RAW 264.7 cells
The cells were stimulated with 1 µg/ml of LPS only or with a combination of LPS and various concentrations (3.125, 12.5, 25, or 50 µg/ml) of seaweed varieties JBR510 (a), JBR511 (b), JBR518 (c), JBR538 (d), and JBR375 (e) for 24 h. Nitric oxide production was determined by the Griess reagent method. Cell viability was determined using the 24-h culture of cells stimulated with LPS (1 µg/ml) in the presence of each sample. The data represent the mean±SD of triplicate experiments. * P<0.05, ** P<0.01 vs. LPS alone
COX is a key enzyme catalyzing the rate-limiting step in the biosynthesis of prostaglandins from arachidonic acid. Growing evidence indicates that COX-2 plays a key role in several biological processes such as inflammation and tumorigenesis. This is because the targeted inhibition of COX-2 is a promising approach to inhibiting inflammation and carcinogenesis. Therefore, we examined the effects of Jeju seaweed extracts on PGE2 production in LPS-stimulated RAW 264.7 macrophages.
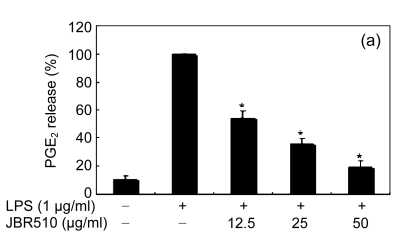
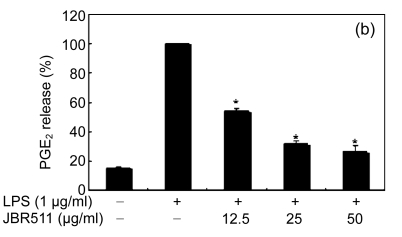
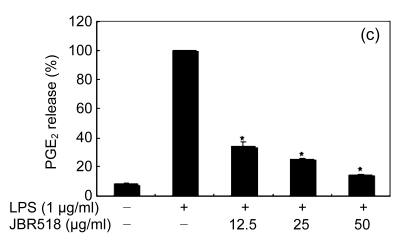
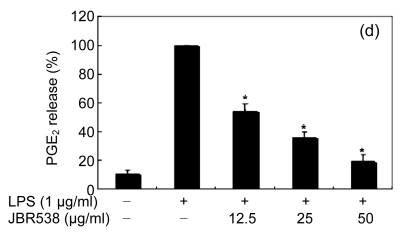
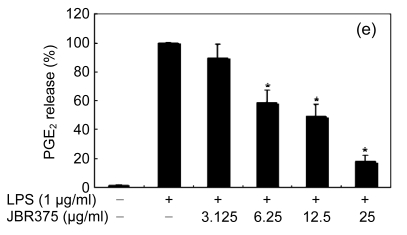
When the macrophages were stimulated with LPS (1 μg/ml) for 24 h, the levels of PGE2 increased in the culture medium. Except for Ishige okamurae and Bonnemaisonia hamifera, 100 μg/ml of all seaweed samples suppressed the LPS-induced PGE2 production (Table 1). Among the seaweed species, extracts from L. okamurae, G. elliptica, S. thunbergii, G. furcata, and H. fusiformis produced the most active preparations, giving IC50 values of 11.7, 15.0, 14.9, 9.7, and 15.0 μg/ml, respectively. They also inhibited LPS-induced PGE2 production in a dose-dependent manner (Fig. 2).
Fig. 2.
Effect of Jeju seaweed extracts on PGE2 production in LPS-stimulated RAW 264.7 cells
The cells were stimulated with 1 µg/ml of LPS only or with a combination of LPS and various concentrations (3.125, 12.5, 25, or 50 µg/ml) of JBR510 (a), JBR511 (b), JBR518 (c), JBR538 (d), and JBR375 (e) for 24 h. PGE2 produced and released into the culture medium was assayed by the ELISA method. The data represent the mean±SD of triplicate experiments. * P<0.05, ** P<0.01 vs. LPS alone
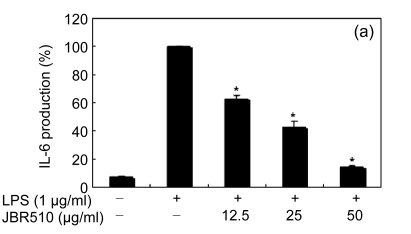
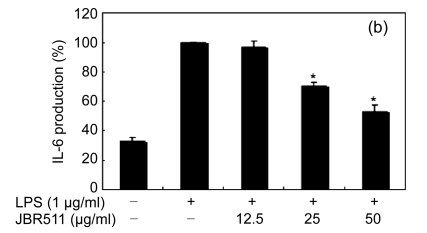
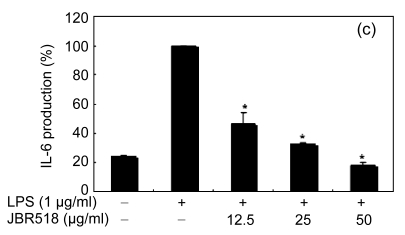
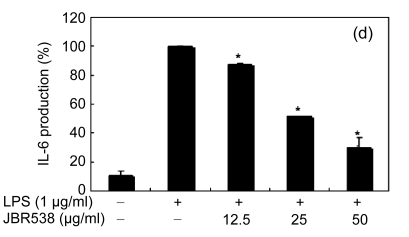
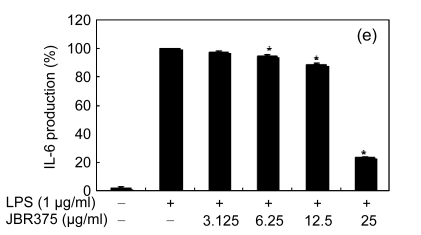
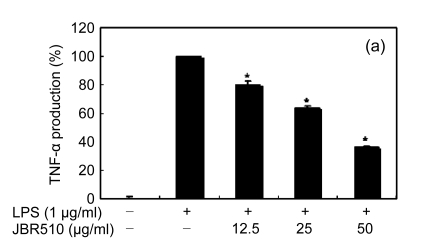
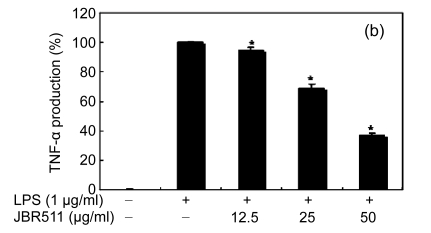
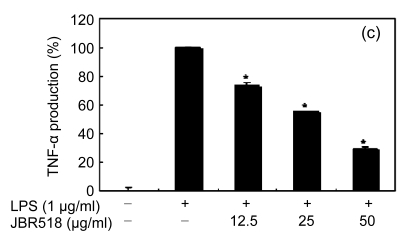
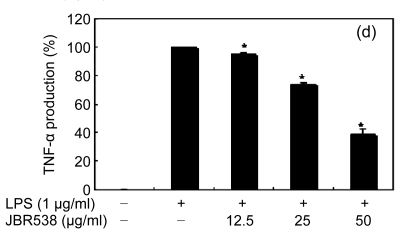
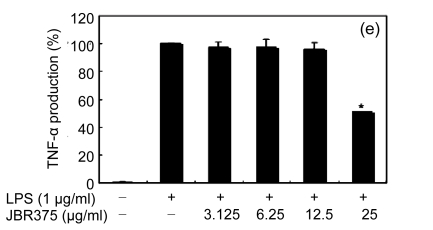
Inflammation is activated in response to appropriate extracellular stimulation, most often by stress or pro-inflammatory cytokines including TNF-α and IL-6, and by bacterial components including LPS through the Toll-like receptors. As Jeju seaweed extracts potently inhibited the pro-inflammatory mediators, we further investigated their effects on LPS-induced IL-6 and TNF-α release by enzyme immunoassay. After 24-h incubation with both LPS (1 μg/ml) and solvent fractions of seaweed (12.5, 25, or 50 μg/ml), there was remarkable inhibition of TNF-α and IL-6. Extracts from L. okamurae, G. elliptica, S. thunbergii, G. furcata, H. fusiformis and other seaweeds inhibited the production of IL-6 and TNF-α in a dose-dependent manner (Figs. 3 and 4). Furthermore, the L. okamurae fractions (25 μg/ml) significantly decreased the levels of IL-6 and TNF-α production by up to 76.9% and 52.5%, respectively.
Fig. 3.
Effect of Jeju seaweed extracts on IL-6 production in LPS-stimulated RAW 264.7 cells
The cells were stimulated with 1 µg/ml of LPS only or with a combination of LPS and various concentrations (3.125, 12.5, 25, or 50 µg/ml) of JBR510 (a), JBR511 (b), JBR518 (c), JBR538 (d), and JBR375 (e) for 24 h. IL-6 produced and released into the culture medium was assayed by the ELISA method. The data represent the mean±SD of triplicate experiments. * P<0.05, ** P<0.01 vs. LPS alone
Fig. 4.
Effect of Jeju seaweed extracts on TNF-α production in LPS-stimulated RAW 264.7 cells
The cells were stimulated with 1 µg/ml of LPS only or with a combination of LPS and various concentrations (3.125, 12.5, 25, or 50 µg/ml) of JBR510 (a), JBR511 (b), JBR518 (c), JBR538 (d), and JBR375 (e) for 24 h. TNF-α produced and released into the culture medium was assayed by the ELISA method. The data represent the mean±SD of triplicate experiments. * P<0.05, ** P<0.01 vs. LPS alone
Phlorotannins are aromatic secondary plant metabolites, widespread in the seaweeds that are associated with the nutritional, anti-inflammatory and antioxidant properties of various foods. Therefore, the total phlorotannin content of each of the 14 seaweeds was assessed. Four extracts, from Ishige okamurae, Padina arborescens, Bonnemaisonia hamifera, and Scytosiphon lomentaria, showed high phlorotannin content (>60 μg/mg) (Table 1).
Considering these results, we suggest that the Jeju seaweed extracts in this study, especially those from L. okamurae, G. elliptica, S. thunbergii, G. furcata, and H. fusiformis, may be considered as possible candidates for anti-inflammatory agents. Further investigations will focus on the in vivo assessment of the biological activity of seaweed extracts and on the chemical identification of the major active components responsible for anti-inflammatory activity in the efficacious seaweed extracts. The efficacies of I. okamurae (Kim K.N. et al., 2009; Kim M.M. et al., 2009), S. thunbergii (Samee et al., 2009) and G. furcata (Bae and Choi, 2007) extracts have already been reported. To the best of our knowledge, this is the first report demonstrating the in vitro anti-inflammatory activities of S. nipponicum, M. simplex, C. ocellatus, B. hamifera, S. lomentaria, C. crispata, P. arborescens, L. okamurae, C. crassicaulis, G. elliptica, and H. fusiformis extracts, and providing a scientific basis for their application in human health.
Footnotes
Project partially supported by the Jeju Sea-green Program for the Regional Innovation System and the Regional Technology Innovation Program (No. RTI04-02-07) of the Ministry of Knowledge and Economy, Korea
References
- 1.Bae SJ, Choi YH. Methanol extract of the seaweed Gloiopeltis furcata induces G2/M arrest and inhibits cyclooxygenase-2 activity in human hepatocarcinoma HepG2 cells. Phytotherapy Research. 2007;21(1):52–57. doi: 10.1002/ptr.2020. [DOI] [PubMed] [Google Scholar]
- 2.Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Natural Product Reports. 2009;26(2):170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 3.Choi HJ, Eun JS, Park YR, Kim DK, Li R, Moon WS, Park JM, Kim HS, Cho NP, Cho SD, et al. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and nuclear factor-kappaB signaling pathways. European Journal of Pharmacology. 2008;601(1-3):171–188. doi: 10.1016/j.ejphar.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Dang HT, Lee HJ, Yoo ES, Shinde PB, Lee YM, Hong J, Kim DK, Jung JH. Anti-inflammatory constituents of the red alga Gracilaria verrucosa and their synthetic analogues. Journal of Natural Products. 2008;71(2):232–240. doi: 10.1021/np070452q. [DOI] [PubMed] [Google Scholar]
- 5.de Benedetti F. Targeting interleukin-6 in pediatric rheumatic diseases. Current Opinion in Rheumatology. 2009;21(5):533–537. doi: 10.1097/BOR.0b013e32832f1445. [DOI] [PubMed] [Google Scholar]
- 6.Dokka S, Shi X, Leonard S, Wang L, Castranova V, Rojanasakul Y. Interleukin-10-mediated inhibition of free radical generation in macrophages. The American Journal of Physiology-Lung Cellular and Molecular Physiology. 2001;280(6):L1196–L1202. doi: 10.1152/ajplung.2001.280.6.L1196. [DOI] [PubMed] [Google Scholar]
- 7.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Current Medicinal Chemistry. 2009;16(24):3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca JE, Santos MJ, Canhão H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmunity Reviews. 2009;8(7):538–542. doi: 10.1016/j.autrev.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Iyer JP, Srivastava PK, Dev R, Dastidar SG, Ray A. Prostaglandin E(2) synthase inhibition as a therapeutic target. Expert Opinion on Therapeutic Targets. 2009;13(7):849–865. doi: 10.1517/14728220903018932. [DOI] [PubMed] [Google Scholar]
- 10.Kang OH, Chae HS, Choi JG, Oh YC, Lee YS, Kim JH, Seung MJ, Jang HJ, Bae KH, Lee JH, et al. Ent-pimara-8(14), 15-dien-19-oic acid isolated from the roots of Aralia cordata inhibits induction of inflammatory mediators by blocking NF-kappaB activation and mitogen-activated protein kinase pathways. European Journal of Pharmacology. 2008;601(1-3):179–185. doi: 10.1016/j.ejphar.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Kanwar JR, Kanwar RK, Burrow H, Baratchi S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Current Medicinal Chemistry. 2009;16(19):2373–2394. doi: 10.2174/092986709788682155. [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, Lee KT. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. European Journal of Pharmacology. 2008;584(1):175–184. doi: 10.1016/j.ejphar.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Kim KN, Ham YM, Moon JY, Kim MJ, Kim DS, Lee WJ, Lee NH, Hyun CG. In vitro cytotoxic activity of Sargassum thunbergii and Dictyopteris divaricata (Jeju seaweeds) on the HL-60 tumour cell line. International Journal of Pharmacology. 2009;5(5):298–306. doi: 10.3923/ijp.2009.298.306. [DOI] [Google Scholar]
- 14.Kim MM, Rajapakse N, Kim SK. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-kappaB transcription factor in RAW 264.7 cells. Phytotherapy Research. 2009;23(5):628–634. doi: 10.1002/ptr.2674. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK, Lee DY, Jung WK, Kim JH, Choi I, Park SG, Seo SK, Lee SW, Lee CM, Yea SS, et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: role of suppressor of cytokine signaling. Biomedicine and Pharmacotherapy. 2008;62(5):289–296. doi: 10.1016/j.biopha.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Murakami A. Chemoprevention with Phytochemicals Targeting Inducible Nitric Oxide Synthase. In: Yoshikawa T, editor. Food Factors for Health Promotion; Forum of Nutrition; Basel, Karger. 2009. pp. 193–203. [DOI] [PubMed] [Google Scholar]
- 17.O′Connor DM, O′Brien T. Nitric oxide synthase gene therapy: progress and prospects. Expert Opinion on Biological Therapy. 2009;9(7):867–878. doi: 10.1517/14712590903002047. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Oh SM, Lim SS, Lee YS, Shin HK, Oh YS, Choe NH, Park JH, Kim JK. Induction of heme oxygenase-1 mediates the anti-inflammatory effects of the ethanol extract of Rubus coreanus in murine macrophages. Biochemical and Biophysical Research Communications. 2006;351(1):146–152. doi: 10.1016/j.bbrc.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Radovits BJ, Kievit W, Laan RF. Tumour necrosis factor-alpha antagonists in the management of rheumatoid arthritis in the elderly: a review of their efficacy and safety. Drugs and Aging. 2009;26(8):647–664. doi: 10.2165/11316460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Rao P, Knaus EE. Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond. Journal of Pharmacy and Pharmaceutical Sciences. 2008;11(2):81s–110s. doi: 10.18433/j3t886. [DOI] [PubMed] [Google Scholar]
- 21.Samee H, Li ZX, Lin H, Khalid J, Guo YC. Anti-allergic effects of ethanol extracts from brown seaweeds. Journal of Zhejiang University-SCIENCE B. 2009;10(2):147–153. doi: 10.1631/jzus.B0820185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. Journal of Investigative Medicine. 2009;57(6):703–708. doi: 10.2310/JIM.0b013e31819aaa76. [DOI] [PubMed] [Google Scholar]
- 23.Usami Y. Recent synthetic studies leading to structural revisions of marine natural products. Marine Drugs. 2009;7(3):314–330. doi: 10.3390/md7030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang EJ, Yim EY, Song G, Kim GO, Hyun CG. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdisciplinary Toxicology. 2009;2(4):245–249. doi: 10.2478/v10102-009-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Kim SK. Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: the current situation and future prospects. Marine Drugs. 2009;7(2):71–84. doi: 10.3390/md7020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou HY, Shin EM, Guo LY, Zou LB, Xu GH, Lee SH, Ze KR, Kim EK, Kang SS, Kim YS. Anti-inflammatory activity of 21(alpha,beta)-methylmelianodiols, novel compounds from Poncirus trifoliata Rafinesque. European Journal of Pharmacology. 2007;572(2-3):239–248. doi: 10.1016/j.ejphar.2007.07.005. [DOI] [PubMed] [Google Scholar]