Abstract
Bee venom phospholipase A2 (BvPLA2) is a lipolytic enzyme that catalyzes the hydrolysis of the sn-2 acyl bond of glycerophospholipids to liberate free fatty acids and lysophospholipids. In this work, a new BvPLA2 (AccPLA2) gene from the Chinese honeybee (Apis cerana cerana) venom glands was inserted into bacmid to construct a recombinant transfer vector. Tn-5B-4 (Tn) cells were transfected with the recombinant bacmid DNA for expression. Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis revealed a double band with molecular weights of 16 and 18 kDa. Products of hexahistidine AccPLA2 fusion protein accumulated up to 5.32% of the total cellular proteins. The AccPLA2 fusion protein was cross reactive with the anti-AmPLA2 (BvPLA2 of the European honeybee, Apis mellifera) polyclonal serum. The reaction resulted in a double glycosylation band, which agrees with the band generated by the native AmPLA2 in Western blot analysis. The PLA2 activity of the total extracted cellular protein in the hydrolyzing egg yolk is about 3.16 μmol/(min·mg). In summary, the recombinant AccPLA2 protein, a native BvPLA2-like structure with corresponding biological activities, can be glycosylated in Tn cells. These findings provided fundamental knowledge for potential genetic engineering to produce AccPLA2 in the pharmaceutical industry.
Keywords: Apis cerana cerana, Bee venom phospholipase A2 (BvPLA2), Insect cell, Expression
1. Introduction
Phospholipases A2 (PLA2s; EC 3.1.1.4) are a large super-family of lipolytic enzymes that catalyze the hydrolysis of the sn-2 acyl bond of glycerophospholipids to liberate free fatty acids and lysophospholipids (Mingarro et al., 1995). PLA2s have important functions in the cellular processes that modulate the release of arachidonic acid and the precursor of eicosanoids of potent inflammatory mediators (Rodriguez de Turco et al., 2002). Additionally, PLA2s play a critical role in host defense, atherosclerosis, signal transduction processes, membrane remodeling (Murakami and Kudo, 2002; Dennis, 1994; 1997), and delaying oxidant-induced cell death (Zhao et al., 2001). PLA2s are also associated with numerous human disorders such as rheumatoid arthritis, autoimmune uveitis, respiratory distress syndrome, myocardial infarction, and septic and endotoxic shock (Mukherjee et al., 1994).
PLA2s enzymes arise from a variety of sources including the mammalian pancreas, reptile venom and insect venom, and synovial fluids (Scott et al., 1990b). These enzymes have been systematically classified into 15 closely structure-related groups on the basis of the level of homology among their nucleotide and amino acid sequences. Based on their properties, PLA2s can be historically classified into secretory (sPLA2), cytosolic Ca2+-dependent (cPLA2), and cytosolic Ca2+-independent (iPLA2). Group I gobra/krait venom and mammalian pancreas, Group II crotalid and viper venom, and Group III bee/lizard/scorpion venom are all secretory, low-molecular mass (13–18 kDa) and Ca2+-dependent PLA2s (Balsinde et al., 2002). Bee venom PLA2 (BvPLA2) is a typical Group III sPLA2 member and makes up approximately 12% of the dry weight of venom in the European honeybee, Apis mellifera (Habermann, 1972; Dennis, 1997). BvPLA2 is the most lethal honeybee venom peptide. It acts as an allergen cooperating with other components to defend the colony against predators and intruders (Schmidt, 1995; King and Spangfort, 2000). The activity of BvPLA2 can be enhanced by melittin, the most abundant constituent of honey bee venom (Mingarro et al., 1995). BvPLA2 has a wide variety of pharmacological properties including anti-human immunodeficiency virus (HIV) activity, neurotoxicity, myo-toxicity, and neurite outgrowth induction (Fenard et al., 1999; 2001; Nakashima et al., 2004).
A nucleotide sequence of BvPLA2 (AmPLA2) from the European honeybee has been determined. The deduced amino acid sequence of AmPLA2 consists of a signal peptide of 18 amino acid residues (preregion of PLA2), a proregion of 15 residues, and a mature peptide of 134 amino acid residues. The mature peptide contains 10 cystine residues which can form 5 disulfide bonds (Kuchler et al., 1989; Shipolini et al., 1974a; 1974b). The crystal structure and catalyzing activity of PLA2 were also well documented (Annand et al., 1996; Scott et al., 1990a; 1990b). A synthetic gene encoding the mature peptide of AmPLA2 was expressed in Escherichia coli, but the biological activities of the expressed protein were low because it can form an inclusion body with incorrect folding in the prokaryotic cell (Dudler et al., 1992). The similar phenomenon was found for another recombinant bee venom allergen, hyaluronidase (BvHya) from the European honeybee. The biological activity of the BvHya expressed in E. coli was only 20%–30% of that of the BvHya expressed in the baculovirus-infected insect cells (Soldatova et al., 1998).
The Asiatic honeybee, Apis cerana, is another commonly-domesticated species distributed in southern and southeastern Asia. It has been appreciated that the European honeybee derived from its closed relative Asiatic honeybee in western or central Asia and subsequently spread into Europe and Africa (Oldroyd and Wongsiri, 2006; Whitfield et al., 2006). The Chinese honeybee, Apis cerana cerana, one subspecies of Asiatic honeybee, has more than 3000 years of history. The total number of the Chinese honeybee colonies maintained by Chinese farmers is 1.2 million, while European honeybee has as much as 7.8 million colonies in recent years. This species was the only breeding honeybee in China before the European honeybee was introduced at the beginning of the last century. The Chinese honeybee has unique biological characters and behaviors that are significantly different from the European honeybee, and has played important roles in the formation of the unique botanical layer in China by pollination (Zhang et al., 2008; Xu et al., 2009). We previously amplified the BvPLA2 (AccPLA2) gene that encodes a mature peptide of the Chinese honeybee (Shen et al., 2002). The expression of AccPLA2 appears from 5 to 42 d of the worker bee of the Chinese honeybee. PLA2 modification between the Chinese honeybee and the European honeybee was compared (Li et al., 2005). The aim of the present study was to investigate the expression, glycosylation, immunologic properties, and the enzymatic activity of the recombinant AccPLA2 in insect cells.
2. Materials and methods
2.1. Materials
The plasmid, pGEM®-AcPLA2, was constructed as previously described (Shen et al., 2002). Trichoplusia ni cell line (Tn-5B-4 (Tn) cell) was maintained in our laboratory. New Zealand white rabbits were purchased from the animal center of Chinese Traditional Medical Institute of Zhejiang. The restriction enzymes EcoRI and HindIII, DL2000 and DL15 000 markers were purchased from TaKaRa Company (Dalian, China). The commercially-purified native AmPLA2 was purchased from Sigma Company (St. Louis, MO, USA). The T4 DNA ligase, X-gal, isopropyl beta-D-thiogalactopyranoside (IPTG), Lipofectin, TNM-FH insect cell medium and bovine calf serum, the transfer vector pFastBacHTa, and E. coli strains DH10B were purchased from Invitrogen Corporation (Carlsbad, USA). The Taq DNA polymerase, protein molecular marker, nitrocellulose filter (NC filter), and the goat anti-rabbit IgG (Fc) conjugate were purchased from Sino-Promega Company (Shanghai, China). Plasmid DNA extraction kit was purchased from Omega Bio-Tek Corporation (Norcross, USA). The calcium chloride, sodium deoxycholate, and bovine serum albumin (BSA) were purchased from Sangon Company (Shanghai, China). All the biochemical reagents were of highest commercially available purity.
2.2. Construction of baculovirus transfer vector and recombinant bacmid
The pGEM®-AccPLA2 was amplified in E. coli TG1 cells and extracted with a plasmid DNA extraction kit. The EcoRI/HindIII-digested cDNA fragment of AccPLA2 from pGEM®-AccPLA2 was directionally inserted into the transfer vector pFastBacHTa. The recombinant transfer vectors were identified by using digestion and polymerase chain reaction (PCR) with the primers AccF1 5′-AGAATTCATGATAATATATCCAGGAACG-3′ (EcoRI site was added and underlined) and AccR1 5′-GAAGCTTAATACTTGCGAAGATCG-3′ (HindIII site was added and underlined) of AccPLA2, following the described procedures (Sambrook and Russell, 2002). Then it was sequenced with a DNA sequencer (performed by Sangon Company of Shanghai). After transformed into competent E. coli DH10B cells, the AccPLA2 fragment in recombinant transfer vector was transposed to the recombinant baculovirus shuttle vector, bacmid, by Tn7 transposition. We extracted DNA from the positive clones that had been verified by PCR using primers AccF1 and AccR1 of AccPLA2, M13F1 (5′-GTAAAACGACGGCCAGT) and M13R1 (5′-AACAGCTATGACCATG) of PUC/M13, following the described procedures (Luckow et al., 1993; Shang et al., 2007).
2.3. Culture of insect cells and Lipofectin-mediated transfection
Tn cells were used for the routine transfection and propagation of recombinant bacmid. TNM-FH medium supplemented with 10% (v/v) fetal bovine calf serum (complete medium) was used for propagation of the insect cells. The recombinant bacmid DNA was introduced into Tn cells mediated using a Lipofectin kit according to the supplier’s instruction. Then the cells were incubated with serum-free medium. After the cells were exposed to DNA-lipid-medium for 24 h, the medium was replaced by the complete medium. The 2-d-old conditioned medium was collected. After transfection, Tn cells were incubated for 24 h and the medium was replaced with serum-free medium. The 2-d-old conditioned medium was collected. After centrifugation at 1500×g at 4 °C for 3 min, the pellet of infected cells was stored at −80 °C, and the supernatant containing the virus particles was used to propagate in Tn cells. After propagation for 4 generations, the genomic viral DNA isolated from the infected cells was confirmed by PCR with primers AccF1 and AccR1.
2.4. Preparation of anti-AmPLA2 polyclonal serum
New Zealand white rabbits were immunized with the mixture of 300 mg commercially-purified native AmPLA2 and 1 ml of phosphate buffered saline (PBS)/complete Freund’s adjuvant emulsion. The rabbits were bled three weeks after primary immunization with 200 mg of the enzyme emulsified in incomplete Freund’s adjuvant (1 ml), followed by injection (200 mg of enzyme in 1 ml of saline) for three weeks. The effect value of the serum extracted from the blood sample after one week of the second enhanced immunization was detected with native AmPLA2 by the gel diffused protocol as described (Sambrook and Russell, 2002). The serum was stored at −80 °C.
2.5. Recombinant protein analysis
The intracellular proteins of the infected cells, a negative control (normal insect cells), and positive control (native AmPLA2) were analyzed using 10% (v/v) sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described (Sambrook and Russell, 2002). Gels were stained with Coomassie brilliant blue R250. The concentration of expressed fusion protein was measured by thin layer scanning of the SDS-PAGE gel. For Western blots, the separated unstained proteins were transferred onto unmodified NC membrane in a Bio-Rad transblot apparatus. The fusion protein was analyzed using rabbit antiserum against native AmPLA2 prepared as above. The proteins on the blot were detected using goat anti-rabbit IgG (Fc) conjugate as described (Sambrook and Russell, 2002).
2.6. Assay of PLA2 activity
Titrimetric assay of PLA2 was based on a modified method used by Owen et al. (1990). The fresh egg yolk substrate blended in 200 ml of distilled and deionized water was prepared on daily basis. Five milliliters of egg yolk substrate was mixed with 1 ml of 3×10−2 mol/L calcium chloride, 1 ml of 1.3×10−2 mol/L sodium deoxycholate, 1 ml of 5% (v/v) BSA, and 2 ml of water. This mixture was heated to 37 °C and NaOH was added to bring the pH value of mixture to 8.0 in a thermostatted microreaction vessel. A pH meter system was used to perform pH-stat titration with the pH value held at 8.0 by addition of 0.005 mol/L of NaOH. The concentration of the solubilized proteins of the extracts of the infected cells was measured and then the extracts were added to the substrate mixture. The rate of release of fatty acids was determined by the amount of added NaOH to maintain the pH at 8.0. One unit of PLA2 activity is defined as the amount of enzyme required to hydrolyze 1 μmol of substrate per minute at pH 8.0 and 37 °C. The purified native AmPLA2 and native bee venom powder diluted in distilled and deionized water, respectively, were used as positive control. The extracts of the normal Tn cells were used as negative control.
3. Results
3.1. Construction of baculovirus transfer vector
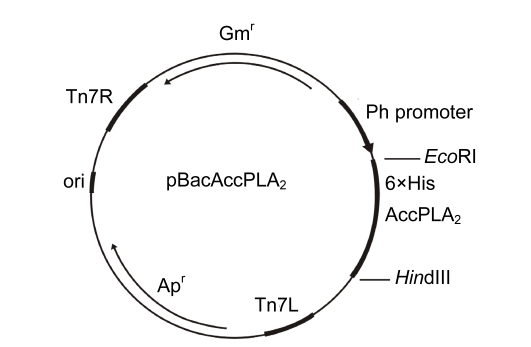
The EcoRI/HindIII-digested cDNA fragment of AccPLA2 from pGEM®-AccPLA2 was inserted into the transfer vector pFastBacHTa (Fig. 1). The products of restricted digestion and PCR of the recombinant plasmid had an approximate 400-bp band, which is identical to the AccPLA2 fragment (GenBank accession No. AF321087). Nucleotide sequencing confirmed that the inserted fragment is a correct open-reading frame (ORF) for protein expression (Fig. 2). In accordance with the schematic representation of the recombinant transfer vector pFastBac-AccPLA2, the inserted gene was under the control of the Autographa californica nuclear polyhedrosis virus (AcNPV) polyhedrin promoter. The expressed gene was designed to generate a fusion protein composed of hexahistidine resides (6×His) at amino-terminus, which allows a single-step purification using immobilized Ni2+ ion affinity chromatography.
Fig. 1.
Schematic representation of the donor vector
Fig. 2.
cDNA sequence of AccPLA2
The deduced amino acid sequence (matured peptide) is given below the nucleotide sequence. Forward primer and reversed primer for PCR are indicated with arrows, respectively
3.2. Construction of recombinant of bacmid and transfection of insect cells
After the recombinant transfer vector pFastBac-AccPLA2 was transformed into E. coli DH10B competent cells that contain baculovirus shuttle vector bacmid, the recombinant colonies were further cultured. Then the PCR identification was conducted with the bacmid DNA isolated from the positive colonies as a template using primers AccPLA2 and PUC/M13, respectively. Two fragments, about 400 bp (Fig. 3a) and 2700 bp (Fig. 3b), were generated. These fragments are identical to AccPLA2 fragment within the multiple cloning sites (MCSs) and the bacmid fragment of 2300 bp within the left and right ends of bacterial transposon Tn7 plus AccPLA2 fragment, which confirms that the recombinant bacmid, rBacmid-AccPLA2, had been correctly constructed.
Fig. 3.
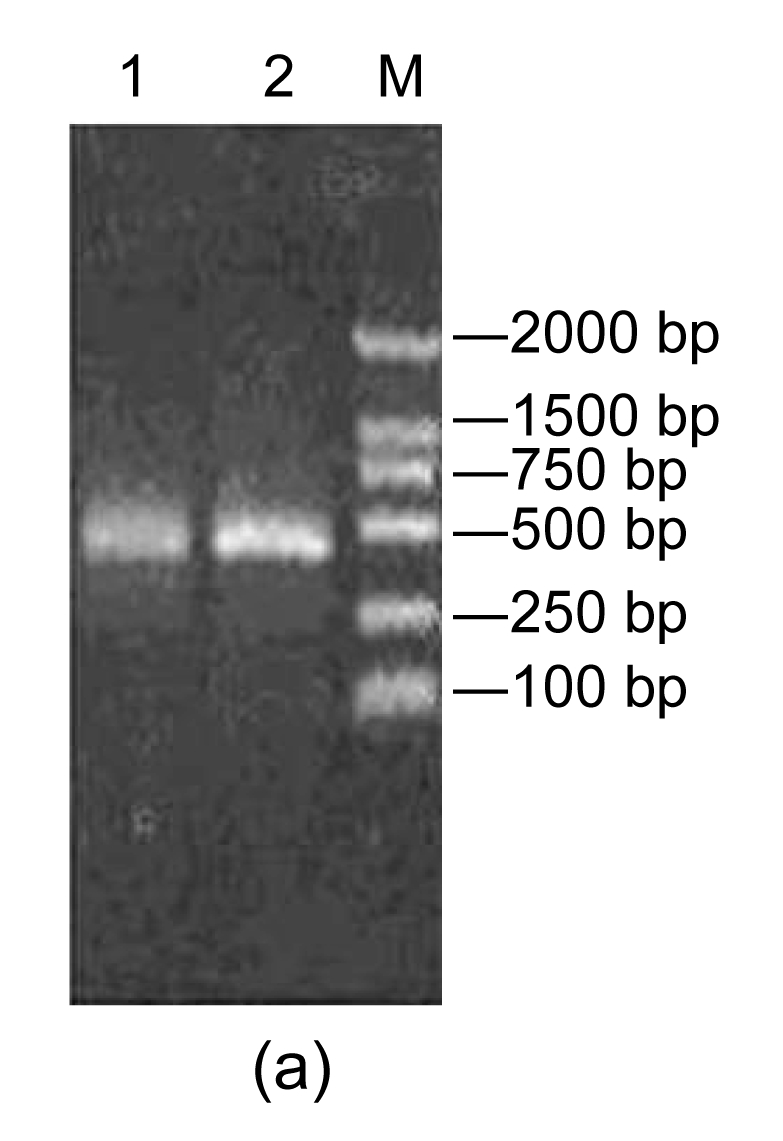
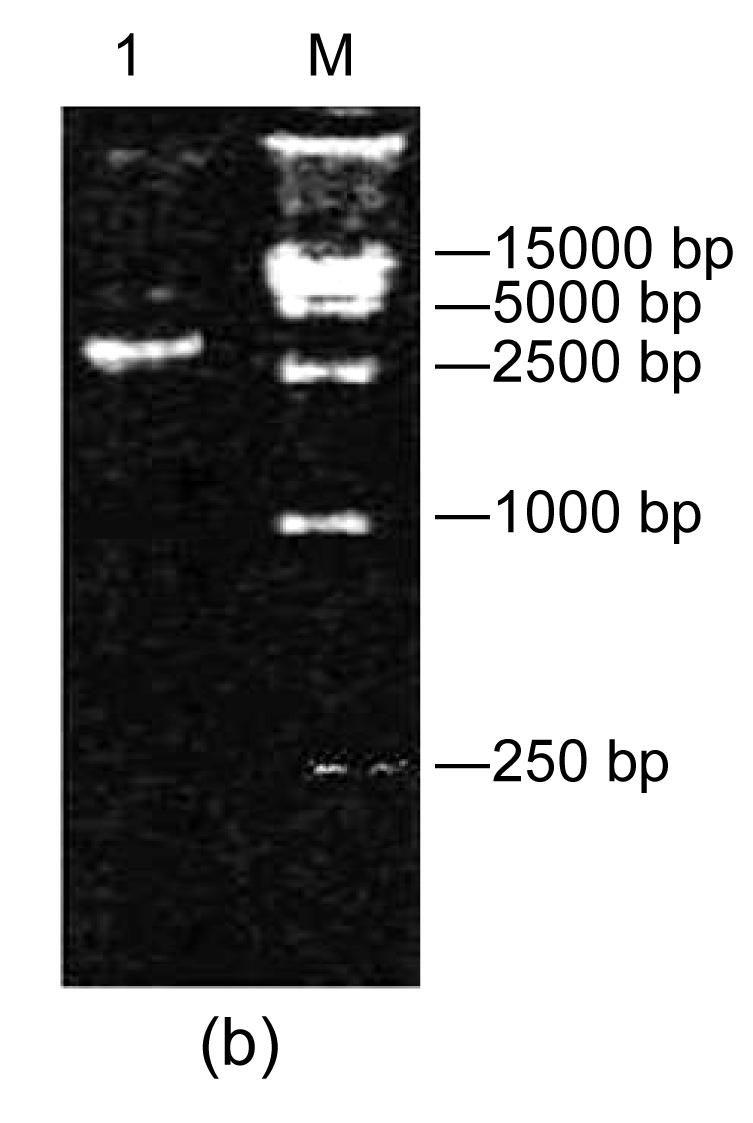
PCR assays with AccPLA2 and PUC/M13 primers
(a) AccPLA2 primer. Lane 1: rBacmid-AccPLA2 PCR; Lane 2: pGEM-AccPLA2 PCR product; Lane M: DNA molecular weight marker (DL2000); (b) PUC/M13 primer: Lane 1: rBacmid-AccPLA2 PCR product; Lane M: DNA molecular weight marker (DL15 000)
With mediation of the Lipofectin, the rBacmid-AccPLA2 DNA was introduced into Tn cells in medium with free bovine calf serum. AccPLA2 appeared in 3 d after infection of rBacmid-AccPLA2 DNA. After centrifugation for harvesting cells, the amplified rBacmid in Tn cells, rTnV-Bac-AccPLA2, in supernatants was used to propagate.
3.3. PCR identification of rTnV-Bac-AccPLA2
After propagation for four generations, PCR identification was performed using the rTnV-Bac-AccPLA2 genomic DNA isolated from the infected cells as a template. The agarose electrophoretic analysis profile showed that the PCR products were of approximate 400 bp, identical with AccPLA2 gene, confirming that rTnV-Bac-AccPLA2 had been propagated in Tn cells.
3.4. SDS-PAGE and Western blot analysis
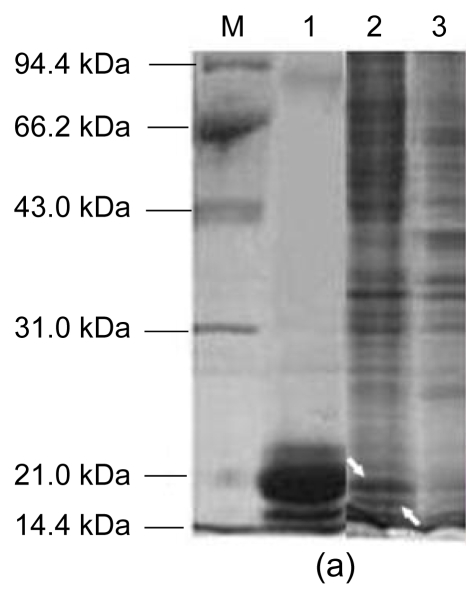
The SDS-PAGE analysis showed that the expressed product has a double band of 16 to 18 kDa (Fig. 4a). The 18-kDa band agrees with the expected molecular weight for the expressed fusion protein of AccPLA2 (134 amino acids, 10.45 kDa) and 25 amino acids of N-terminal 6×His tag protein (2.8 kDa), a spacer region and rTEV protease cleavage site (3 kDa).
Fig. 4.
SDS-PAGE analysis and Western blot of AccPLA2 expression products
(a) SDS-PAGE analysis: Lane M: protein molecular weight marker proteins; Lane 1: native AmPLA2; Lane 2: extracts from Tn cells infected with rBacmid-AccPLA2 (the expressed bands were indicated with arrows); Lane 3: extracts from normal Tn cells; (b) Western blot: Lane 4: native AmPLA2; Lane 5: extracts from Tn cells infected by rBacmid-AccPLA2; Lane 6: extracts from normal Tn cells
The gel diffusion detection showed that there was precipitation between the anti-AmPLA2 polyclonal serum (1:8, 1:16, 1:32, 1:64, and 1:128) and antigen (1:1000). It has been confirmed that the prepared rabbit antibody could cross-react with native AmPLA2. Following resolution on SDS-PAGE, the proteins of the infected cells were transferred electrophoretically into unmodified NC membrane probed with the anti-AmPLA2 polyclonal serum (1:3000). The expressed fusion protein was recognized by the AmPLA2-antibody, which confirms the expression of AccPLA2 proteins, and shows that the Tn cells infected with the recombinant virus exhibited a double-immunoreaction band with molecular weights of 16 to 18 kDa (Fig. 4b). Meanwhile, the blots of native AmPLA2 and native bee venom (positive control) yielded a similar double-band. By contrast, no immunoreactivity was detected in negative control. The results indicate that there was cross-reaction between the expressed fusion protein in Tn cells and anti-AmPLA2 polyclonal serum. One mixed venom sample of Chinese honeybee was reported to have three bands detected for the natural AccPLA2 with molecular weight 15 kDa using the anti-AmPLA2 antibody, which was caused by the characters of BvPLA2 glycosylation (Li et al., 2005). These results also indicate that the expressed AccPLA2 protein can be glycosylated in Tn cells.
3.5. Enzymatic activity assay of the expressed fusion protein
The hydrolyzation analysis of the total infected cellular protein on egg yolk substrate revealed that the PLA2 enzymatic activity is about 3.16 μmol/(min∙mg) (Table 1). The result demonstrates that the crude fusion protein extracts of AccPLA2 expressed in Tn cells could directly yield enzymatic activity of hydrolyzation on egg yolk substrate. The crude enzymatic activity of the pure fusion protein of AccPLA2 was estimated at 63.3 μmol/(min∙mg), which is about 20 times that of the total protein of the cells. However, the enzymatic activity of the purified AccPLA2 from Tn cells cannot be detected because of the low expression of AccPLA2.
Table 1.
Enzymatic activity of the expressed AccPLA2
| Samples | Enzymatic activity (μmol/(min·mg)) |
| Native AmPLA2 | 193.75±8.84 |
| Native bee venom | 15.84±1.18 |
| Extracts from Tn cells infected with rBacmid-AccPLA2 | 3.16±1.13 |
| Extracts from normal Tn cells | Not detected |
4. Discussion
Previous studies have concluded that the amino acid sequences deduced from the cDNAs of AccPLA2 and AmPLA2 are composed of 134 amino acids with 5 disulfide bridges, 2 catalytic domains, and 3 calcium binding sites. The sequences of AccPLA2 and AmPLA2 share 95% homologies (Shen et al., 2002). This study confirmed that the activity and immunological properties of AccPLA2 fusion protein expressed in Tn cells are in agreement with those of the native AmPLA2 isolated from the worker bee venom glands of the European honeybee. Based on the molecular and biochemical homologies of AccPLA2 and AmPLA2, it could be speculated that the recombinant protein of AccPLA2 might have a native-like conformation in Tn cells.
Baculovirus-mediated expression in insect cells is well established for the production of recombinant glycoproteins. With regard to protein folding and post-translational processing, insect cells are second only to mammalian cell lines. Evidence indicated that many processing events known in mammalian systems also occurred in insects (Luckow et al., 1993; Altmann et al., 1999). Numerous experiments were conducted with insect cells. For instance, human β-interferon and O-glycan core 2β-1,6-N-acetylglucosaminyltransferase (C2GnT) with high levels of activity have been successfully expressed in Sf-9 cells (Smith et al., 1983; Toki et al., 1997), and Tn cells showed high glycosylation potential for the recombinant insect glycoprotein, BvHya from the European honeybee (Soldatova et al., 1998). However, its capability to produce human-like complex type N-glycans has been a matter of controversy for many years. The lack of complex glycosylation has limited the use of the insect cell baculovirus expression vector system. Tn cells have been found to produce a small amount of galactose-terminated N-glycans, which prevent glycoproteins from being used as therapeutics proteins (Altmann et al., 1999). Therefore, two novel cell lines, A7S from Pseudaletia unipuncta and DpN1 from Danaus plexippus that possess the capability of complex glycosylation, can overcome such a problem (Palomares et al., 2003). However, the similar problems should not be taken account of for the naturally occurring glycoproteins such as BvPLA2 and BvHya from insects to be expressed in Tn cell system. The Bac-to-Bac system and Tn cells may serve as a general probe for elucidation of some of the regulatory factors influencing glycosylation of insect protein.
BvPLA2 is the most important bee venom glycoprotein with a single oligosaccharide attached to asparagin-13, which is responsible for its enzymatic activity and allergenicity (Scott et al., 1990a; 1990b; Kubelka et al., 1993). Prior to this study, BvPLA2 has not been expressed in insect cells. Here, the AccPLA2 gene was expressed in Tn cells. The expressed fusion protein was active, indicating that Tn cells could express properly modified AccPLA2. The appearance of recombinant protein in SDS-PAGE and Western blot is a double band and the glycosylation capacity of Bac-to-Bac system and Tn cells for AccPLA2 was verified, which provides further insights into the advantages of baculovirus and its active foreign eukaryotic insect protein. Our previous work on the difference of BvPLA2 glycosylation among individuals of Chinese honeybee showed that AccPLA2 was glycosylated during 8–12 d old at adult stage, and the AccPLA2 in venom was a mixture of three different glycosides. The sequencing for all three bands of proteins excised from the NC membrane of Western blot showed that they had the same N-terminal sequence, IIYPGTLW, which is the N-terminal sequence of the mature AccPLA2. The difference in the molecular weights of the three bands was caused by their different glycosylations (Li et al., 2005). Altmann et al. (1991) found that AmPLA2 from the European honeybee consisted of three isoforms with approximate molecular masses of 16, 18, and 20 kDa, respectively. The sequencing data on N-terminal amino acid sequence showed that the PLA2-18 and PLA2-20 carried oligosaccharide residues, and PLA2-16 escaped glycosylation during biosynthesis. The electrophoretic separation of the three isoforms was based on structural features of glycosylation of AmPLA2. Soldatova et al. (1998) also found that recombinant BvHya expressed in Tn cells appeared as a double band of 43 to 44 kDa with different glycosylations. Our results are consistent with these previous reports (Li et al., 2005; Altmann et al., 1991; Soldatova et al., 1998). Since the glycosylation of recombinant protein is an important factor that affects protein function, the present study combined with our previous findings may lay a new scientific basis for the molecular biological utilization of AccPLA2 in the future.
The present study showed a low level of expression of AccPLA2 protein in Tn cells, which might be caused by the allergen and toxic characters of BvPLA2 and may have harmed insect cells. It also showed that the splicing time of the insect cells infected by the bacmid containing AccPLA2 protein is much less than that of the cells infected by the bacmids containing other foreign genes.
In conclusion, we constructed Tn cells by transfecting the recombinant bacmid DNA that was inserted in a new BvPLA2 (AccPLA2) gene from the Chinese honeybee (Apis cerana cerana) venom glands to express the recombinant AccPLA2 protein, a native BvPLA2-like structure with corresponding biological activities that can be glycosylated in Tn cells. Our work may provide fundamental knowledge for potential genetic engineering to produce AccPLA2 in the pharmaceutical industry.
Acknowledgments
We are grateful to Prof. Chuan-xi ZHANG and Prof. Jia-an CHENG in the Institute of Insect Science, Zhejiang University for their kind instruction in experiment. We are also grateful to Dr. Jian-xiang WU in Zhejiang University for his assistance in preparing the antibody against AmPLA2. We would like to acknowledge Dr. Jia-cai WU in Zhejiang University for his technical assistance, and Dr. Zhen-hua LIU in Tufts University and Dr. Song-bai LIU in Harvard University for editing the manuscript.
Footnotes
Project (No. 2007AA10Z324) supported by the National High-Tech Research and Development Program (863) of China
References
- 1.Altmann F, Kubelka V, Staudacher E, Uhl K, Marz L. Characterization of the isoforms of phospholipase A2 from honeybee venom. Insect Biochem. 1991;21(5):467–472. doi: 10.1016/0020-1790(91)90099-Z. [DOI] [Google Scholar]
- 2.Altmann F, Staudacher E, Wilson IBH, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J. 1999;16(2):109–123. doi: 10.1023/A:1026488408951. [DOI] [PubMed] [Google Scholar]
- 3.Annand RR, Kontoyianni M, Penzotti JE, Dudler T, Lybrand TP, Gelb MH. Active site of bee venom phospholipase A2: the role of histidine-34, As-partate-64 and Tyrosine-87. Biochemistry. 1996;35(14):4591–4601. doi: 10.1021/bi9528412. [DOI] [PubMed] [Google Scholar]
- 4.Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531(1):2–6. doi: 10.1016/S0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- 5.Dennis EA. Diversity of group types, regulation and function of phospholipase A2 . J Biochem. 1994;269(18):13057–13060. [PubMed] [Google Scholar]
- 6.Dennis EA. A growing phospholipase A2 superfamily of signal transduction enzymes. Trends Biochem Sci. 1997;22(1):1–2. doi: 10.1016/S0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 7.Dudler T, Chen WQ, Wang SS, Schneider T, Annand RR, Dempcy RO, Crameri R, Gmachl M, Suter M, Gelb MH. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2 . Biochim Biophys Acta. 1992;1165(2):201–210. doi: 10.1016/0005-2760(92)90188-2. [DOI] [PubMed] [Google Scholar]
- 8.Fenard D, Lambeau G, Valentin E, Lefebvre JC, Lazdunski M, Doglio A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J Clin Invest. 1999;104(5):611–618. doi: 10.1172/JCI6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenard D, Lambeau G, Maurin T, Lefebvre JC, Doglio A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol Pharmacol. 2001;60(2):341–347. doi: 10.1124/mol.60.2.341. [DOI] [PubMed] [Google Scholar]
- 10.Habermann E. Bee and wasp venoms. Science. 1972;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 11.King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Imm. 2000;123(2):99–106. doi: 10.1159/000024440. [DOI] [PubMed] [Google Scholar]
- 12.Kubelka V, Almann F, Staudacher E, Tretter V, Marz L, Hard K, Kamerling JP, Vliegenthart JF. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2 . Eur J Biochem. 1993;213(3):1193–1204. doi: 10.1111/j.1432-1033.1993.tb17870.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuchler K, Gmachl M, Sippl MJ, Krell G. Analysis of the cDNA for phospholipase A2 from honeybee venom glands-the deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur J Biochem. 1989;184(1):249–254. doi: 10.1111/j.1432-1033.1989.tb15014.x. [DOI] [PubMed] [Google Scholar]
- 14.Li JH, Zhang CX, Shen LR, Tang ZH, Cheng JA. Expression and regulation of phospholipase A2 in venom gland of the Chinese honeybee, Apis cerana cerana . Arch Insect Biochem Physiol. 2005;60(1):1–12. doi: 10.1002/arch.20075. [DOI] [PubMed] [Google Scholar]
- 15.Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli . J Virol. 1993;67(8):4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingarro I, Prez-Paya E, Pinilla C, Appel JR, Houghten RA, Blondelle SE. Activation of bee venom phospholipase A2 through a peptide-enzyme complex. FEBS Lett. 1995;372(1):131–134. doi: 10.1016/0014-5793(95)00964-B. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee AB, Miele L, Pattabiraman N. Phospholipases A2 enzymes: regulation and hysiological role. Biochem Pharmacol. 1994;48(1):1–10. doi: 10.1016/0006-2952(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Kudo I. Phospholipase A2 . J Biochem. 2002;131(2):285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima S, Kitamoto K, Arioka M. The catalytic activity, but not receptor binding, of PLA2s plays a critical role for neurite outgrowth induction in PC12 cells. Brain Res. 2004;1015(1/2):207–211. doi: 10.1016/j.brainres.2004.04.069. [DOI] [PubMed] [Google Scholar]
- 20.Oldroyd BP, Wongsiri S. Asian Honeybees. Biology, Conservation, and Human Interactions. Cambridge, Massachusetts, USA: Harvard University Press; 2006. pp. 36–64. [Google Scholar]
- 21.Owen MD, Pfaff LA, Reisman RE, Wypych J. Phospholipase A2 in venom extracts from honey bees (Apis mellifera L.) of different ages. Toxicon. 1990;28(7):813–820. doi: 10.1016/S0041-0101(09)80004-4. [DOI] [PubMed] [Google Scholar]
- 22.Palomares LA, Joosten CE, Hughes PR, Granados RR, Shuler ML. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol Prog. 2003;19(1):185–192. doi: 10.1021/bp025598o. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez de Turco EB, Jackson FR, DeCoster MA, Kolko M, Bazan NG. Glutamate signalling and secretory phospholipase A2 modulate the release of arachidonic acid from neuronal membranes. J Neurosci Res. 2002;68(5):558–567. doi: 10.1002/jnr.10239. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. New York, USA: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- 25.Schmidt JO. Toxinology of venoms from the honeybee genus Apis . Toxicon. 1995;33(7):917–927. doi: 10.1016/0041-0101(95)00011-A. [DOI] [PubMed] [Google Scholar]
- 26.Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB. Interfacial catalysis: the mechanism of phospholipase. Science. 1990;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott DL, Otwinowski Z, Gelb MH, Sigler PB. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990;250(4987):1563–1566. doi: 10.1126/science.2274788. [DOI] [PubMed] [Google Scholar]
- 28.Shang JY, Shao YM, Lang GJ, Yuan G, Tang ZH, Zhang CX. Expression of two types of acetylcholinesterase gene from the silkworm, Bombyx mori, in insect cells. Insect Sci. 2007;14(6):443–449. [Google Scholar]
- 29.Shen LS, Zhang CZ, Cheng JA. Cloning and sequencing of genes encoding phospholipase A2 from the venom of Apis cerana cerana and A. mellifera . J Agric Biotechnol. 2002;10(1):29–32. (in Chinese) [Google Scholar]
- 30.Shipolini RA, Callewaert GL, Cottrell RC, Vernon CA. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur J Biochem. 1974;48(2):465–476. doi: 10.1111/j.1432-1033.1974.tb03787.x. [DOI] [PubMed] [Google Scholar]
- 31.Shipolini RA, Doonan S, Vernon CA. The disulphide bridges of phospholipase A2 from bee venom. Eur J Biochem. 1974;48(2):477–483. doi: 10.1111/j.1432-1033.1974.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith GE, Summers MD, Fraser M. Production of human belta interferon in insect cell with baculovirus expression vectors. J Mol Cell Biol. 1983;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soldatova LN, Crameri R, Gmachl M, Kemeny DM, Schmidt M, Weber M, Mueller UR. Superior biologic activity of the recombinant bee venom allergen hyaluronidase expressed in baculovirus infected insect cells as compared with Escherichia coli . J Allergy Clin Immunol. 1998;101(5):691–698. doi: 10.1016/S0091-6749(98)70179-4. [DOI] [PubMed] [Google Scholar]
- 34.Toki D, Sarkar M, Yip B, Reck F, Joziasse D, Fukuda M, Schachter H, Brockhausen I. Expression of stable human O-glycan core 2β-1,6-N-acetylgluco-saminyltransferase in Sf9 insect cells. Biochem J. 1997;325(1):63–69. doi: 10.1042/bj3250063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield CW, Behura SK, Berlocher SH, Clark AGJ, Johnston S, Sheppard WS, Smith DR, Suarez AV, Weaver D, Tsutsui ND. Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera . Science. 2006;314(5799):642–645. doi: 10.1126/science.1132772. [DOI] [PubMed] [Google Scholar]
- 36.Xu P, Shi M, Chen XX. Antimicrobial peptide evolution in the Asiatic honey bee Apis cerana . PLoS ONE. 2009;4(1):e4239. doi: 10.1371/journal.pone.0004239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang CX, Tang XD, Cheng JA. The utilization and industrialization of insect resources in China. Entomol Res. 2008;38(S1):S38–S47. doi: 10.1111/j.1748-5967.2008.00173.x. [DOI] [Google Scholar]
- 38.Zhao M, Brunk UT, Eaton JW. Delayed oxidant-induced cell death involves activation of phospholipase A2 . FEBS Lett. 2001;509(3):399–404. doi: 10.1016/S0014-5793(01)03184-2. [DOI] [PubMed] [Google Scholar]