Abstract
Tobacco mesophyll protoplasts were previously shown to respond to naphthaleneacetic acid by modifying their transmembrane potential difference. In the present work, evacuolated protoplasts were used to show that this response resides only at the plasmalemma. This electrical response was investigated by using polyclonal antibodies directed against plasma membrane antigens presumably involved in the reception and transduction of the auxin signal. An IgG fraction from an antiserum directed against the membrane auxin-binding protein from maize coleoptile completely inhibited the naphthaleneacetic acid-induced response of tobacco protoplasts. The suppression of the auxin-induced variation in the transmembrane potential difference by an IgG preparation directed against the plasmalemma ATPase from yeast demonstrated the involvement of the ATPase in the electrical response. Variation induced by fusicoccin in the transmembrane potential difference of tobacco protoplasts was unaffected by the anti-auxin-binding protein IgG fraction but was completely suppressed by the anti-ATPase IgG preparation. These results demonstrate the presence of a membrane receptor for auxin at the plasmalemma, the binding of the hormone to this receptor leading to the activation of the proton-pumping ATPase. They also show that at least the primary steps of activation by naphthaleneacetic acid are distinct from those of the fusicoccin-induced response.
Keywords: electrical potential difference, evacuolated protoplasts, ATPase
Full text
PDF

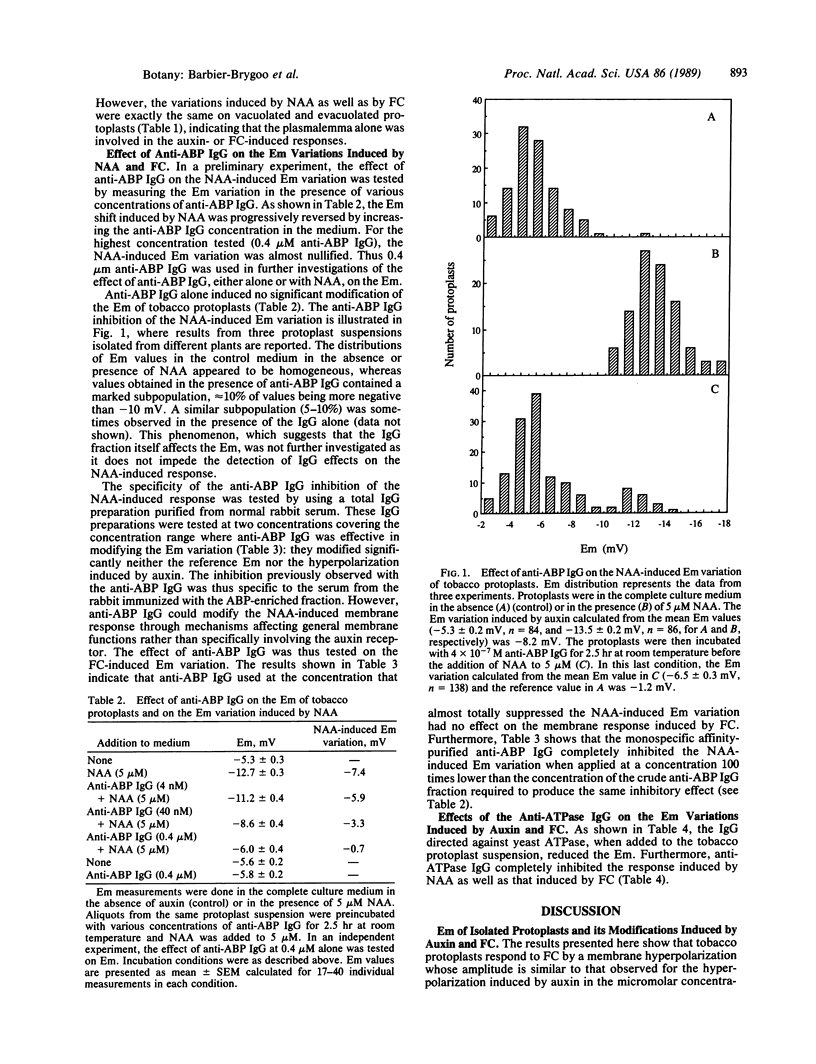
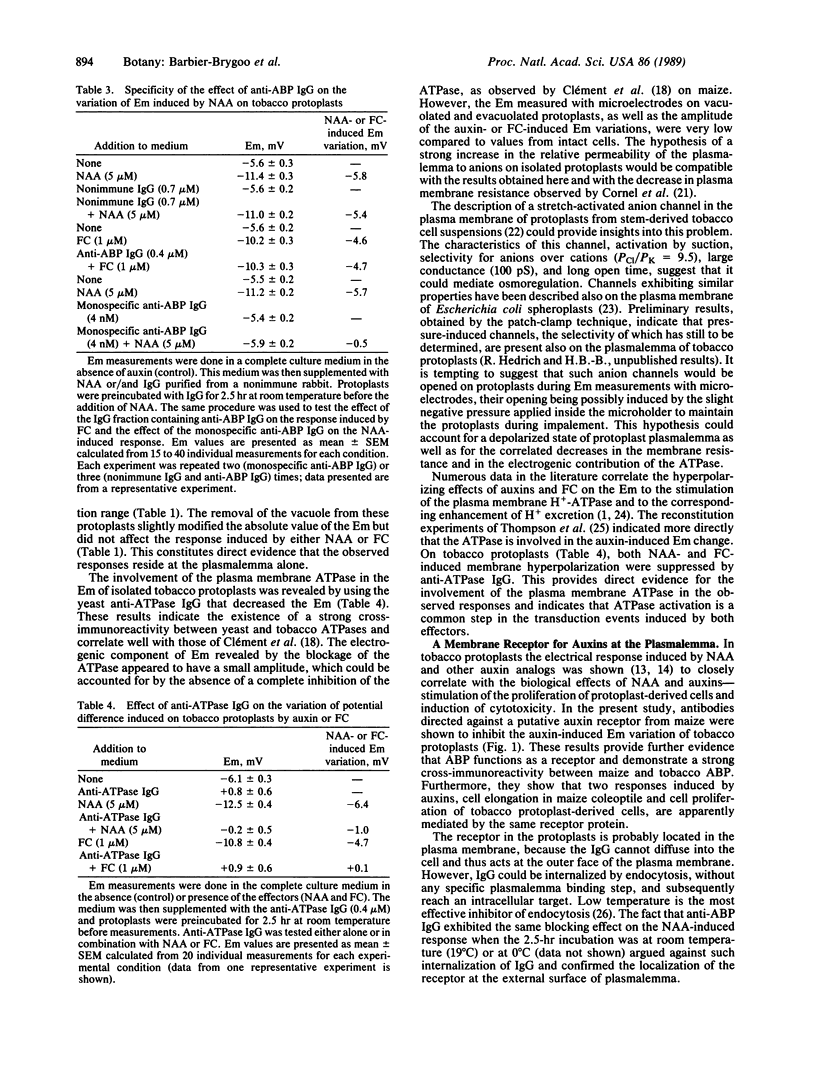

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caboche M., Muller J. F., Chanut F., Aranda G., Cirakoglu S. Comparison of the growth promoting activities and toxicities of various auxin analogs on cells derived from wild type and a nonrooting mutant of tobacco. Plant Physiol. 1987 Apr;83(4):795–800. doi: 10.1104/pp.83.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephritikhine G., Barbier-Brygoo H., Muller J. F., Guern J. Auxin effect on the transmembrane potential difference of wild-type and mutant tobacco protoplasts exhibiting a differential sensitiity to auxin. Plant Physiol. 1987 Apr;83(4):801–804. doi: 10.1104/pp.83.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Brummer B., Bertl A., Parish R. W. Indole-3-acetic acid and fusicoccin cause cytosolic acidification of corn coleoptile cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8992–8995. doi: 10.1073/pnas.83.23.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). I. Purification by immunological methods and characterization. J Biol Chem. 1985 Aug 15;260(17):9848–9853. [PubMed] [Google Scholar]
- Löbler M., Klämbt D. Auxin-binding protein from coleoptile membranes of corn (Zea mays L.). II. Localization of a putative auxin receptor. J Biol Chem. 1985 Aug 15;260(17):9854–9859. [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman J. B., Cinti D. L. Preparation of microsomes with calcium. Methods Enzymol. 1978;52:83–89. doi: 10.1016/s0076-6879(78)52008-9. [DOI] [PubMed] [Google Scholar]
- Shen W. H., Petit A., Guern J., Tempé J. Hairy roots are more sensitive to auxin than normal roots. Proc Natl Acad Sci U S A. 1988 May;85(10):3417–3421. doi: 10.1073/pnas.85.10.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura S., Sotobayashi T., Futai M., Fukui T. Purification and properties of an auxin-binding protein from maize shoot membranes. J Biochem. 1986 May;99(5):1513–1524. doi: 10.1093/oxfordjournals.jbchem.a135621. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M., Krull U. J., Venis M. A. A chemoreceptive bilayer lipid membrane based on an auxin-receptor ATPase electrogenic pump. Biochem Biophys Res Commun. 1983 Jan 14;110(1):300–304. doi: 10.1016/0006-291x(83)91295-0. [DOI] [PubMed] [Google Scholar]



