Abstract
Chromophobe renal cell carcinoma (ChRCC) metastatic to the testis has not, to the best of our knowledge, been reported in the literature. Nor have there been reports of delayed bilateral adrenal metastasis of ChRCC. Here we report a case of metachronous contralateral testicular and bilateral adrenal metastasis of ChRCC in a 70-year-old man who underwent right radical nephrectomy for RCC six years ago. He was admitted to the hospital because of left intrascrotal enlargement of two-month duration. Ultrasonography revealed a mass in the upper pole of the left testis. Computed tomography (CT) showed irregular masses in the bilateral adrenal area. Left radical orchiectomy and laparoscopic bilateral adrenalectomy were performed. The pathologic examination showed metastatic ChRCC in the left testis and bilateral adrenal gland. Postoperative follow-up showed that the patient had survived for at least 56 months without recurrence. The case highlights the unique behavior of RCC with an unusual site of metastasis and favorable survival after multiple metastasectomy.
Keywords: Renal cell carcinoma (RCC), Testicular metastasis, Adrenal metastasis, Metastasectomy
1. Introduction
It has been reported that testicular metastasis from renal cell carcinoma (RCC) is predominantly ipsilateral and invariably on the left side. It usually presents simultaneously with the renal primary tumors or precedes the diagnosis of renal tumors (Nabi et al., 2001). The histological subtype of RCC metastatic to the testis almost always shows clear cell carcinoma (Datta et al., 2001; Steiner et al., 1999). Only one case of metachronous contralateral testicular metastasis from RCC has been reported (Nabi et al., 2001), and no case of chromophobe RCC (ChRCC) metastatic to the testis was reported, to the best of our knowledge. Delayed bilateral adrenal metastasis of RCC is also very rare (Antonelli et al., 2006; Bonnet et al., 2008). To date, there have been no reports on delayed bilateral adrenal metastasis of ChRCC. Here we report a case of metachronous contralateral testicular and bilateral adrenal metastasis of ChRCC and review the literature.
2. Case report
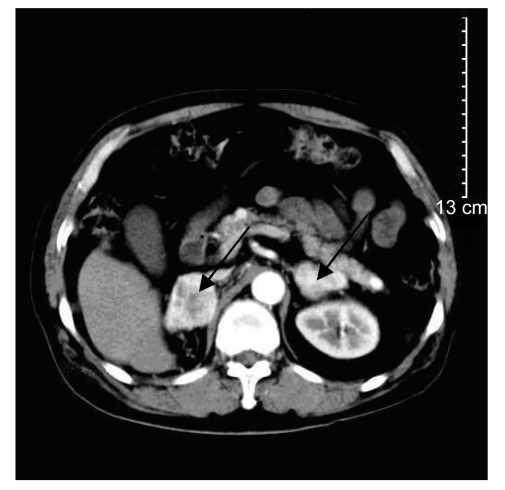
A 70-year-old male patient was admitted to the hospital because of left intrascrotal enlargement of two-month duration. He underwent right radical nephrectomy for right RCC in a local hospital six years ago. Physical examination revealed a palpable mass of the left testis with 4 cm×3 cm×2 cm in size. Superficial lymph nodes were not palpable. Serum alpha fetoprotein (AFP) and β-human chorionic gonadotropin (β-HCG) were within normal limits. B-ultrasound showed a 3.9 cm×2.8 cm mass in the left testis. A positive computed tomography (CT) (Fig. 1) scan of the abdomen revealed that total right nephrectomy had been done, and irregular masses were seen in bilateral adrenal areas. The right adrenal mass was 7.7 cm×5.5 cm in size and the left one 5.6 cm×6.0 cm. Single photon emission computed tomography (SPECT) of bone showed no bony metastasis.
Fig. 1.
CT showed irregular masses of bilateral adrenal areas (black arrows)
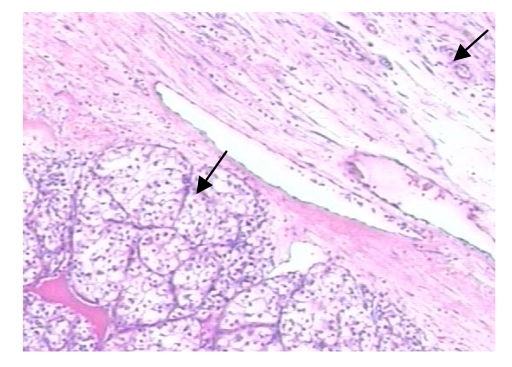
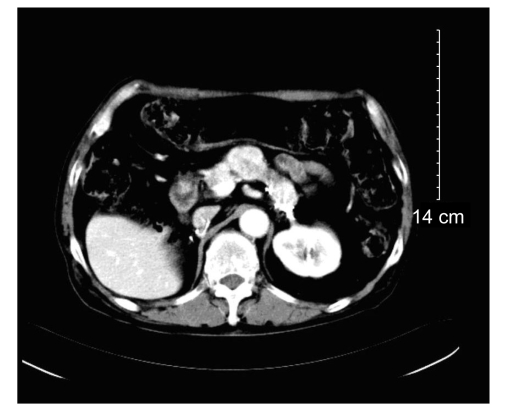
The patient underwent left radical orchiectomy. The pathologic examination showed ChRCC of the left testis (Fig. 2). Immunohistochemistry of the left testicular tumor was positive for intra-cellular cytokeratin and vimentin. The patient accepted laparoscopic bilateral adrenalectomy seven days after left radical orchiectomy. The pathological findings indicated metastatic ChRCC of the bilateral adrenal glands. His postoperative course was uneventful. Postoperative follow-up showed that the patient had survived for at least 56 months without recurrence (Fig. 3).
Fig. 2.
Hematoxylin and eosin-stained sections of left testicular tumor
Left down arrow showed renal chromophobe cell carcinoma. Right upper arrow showed testicular convoluted seminiferous tubule (×100)
Fig. 3.
CT showed no recurrent mass of bilateral adrenal area at 56 months after operation
3. Discussion
The incidence rate of secondary testicular tumors ranges from 0.3% to 3.6% (Dieckmann et al., 1988). The most frequent origin of them is the prostate (Dutt et al., 2000; Llarena Ibarguren et al., 2008). Very rarely, intrascrotal metastasis from RCC has been reported (Schmorl et al., 2008). The pathologic diagnosis of RCC metastatic to the testis almost always reveals a clear cell tumor (Datta et al., 2001; Steiner et al., 1999).
After radical nephrectomy, approximately 25% of RCC patients developed metachronous metastasis (Bonnet et al., 2008), among whom less than 2% presented with metastasis confined to the adrenal glands (Antonelli et al., 2006; Siemer et al., 2004). In a recent study, a series of 1179 patients were treated for RCC, and it was found that the global incidence of adrenal metastasis among those patients was 3.7%, of which 1.9% were ipsilateral, 1.5% contralateral, 0.3% bilateral, 2.7% synchronous with the renal tumor, and only 1% metachronous (Antonelli et al., 2006). The incidence rate of metachronous bilateral adrenal metastasis from RCC is even more rare. There have only been three cases reported (Antonelli et al., 2006; Bonnet et al., 2008). And there are no reports concerning delayed bilateral adrenal metastasis of ChRCC.
ChRCC is biologically a tumor of low malignant potential with a 5-year survival rate of 78% to 100% and a 10-year survival rate of 80% to 90% (Amin et al., 2008). Metastasis of ChRCC constitutes less than 5% of all ChRCC cases and less than 1% of all metastatic RCC (Choueiri et al., 2008). Patients with ChRCC are more likely to experience liver metastasis (Hoffmann et al., 2008). Treatment modalities for metastatic RCC are limited. Chemotherapy and radiotherapy have proven to have low response rates. Immunotherapeutic combination regimens are currently being examined in clinical trials, including cytokine therapy combined with interleukin-2 (IL-2) and interferon-α (IFN-α), or targeted agents such as multitargeted tyrosine kinase inhibitors (TKIs), mammalian target of rapamycin (mTOR) inhibitors, and the monoclonal antiangiogenic antibodies. Most of the studies were focused on clear cell RCC and only a few on the ChRCC (Choueiri et al., 2008; Guevremont et al., 2009; Motzer et al., 2008). Although these agents show a consistent efficacy in clinical trials, surgery remains the only known curative therapy for RCC. Several studies described surgical intervention for metastatic RCC and showed that metastasectomy promoted long-term survival in the patients with a solitary metastasis (Bonnet et al., 2008; Daliani et al., 2009; Eggener et al., 2008; Swanson, 2004). Median survival for metastatic RCC varied from 23 months (Kierney et al., 1994) to 41 months in patients with resected solitary metastatic lesions (Wroński et al., 1996). It remains a topic of debate whether metastasectomy is feasible for the patients who have nonsolitary lesions of RCC metastasis. A study showed that, compared to resection of nonsolitary lesions, resection of solitary metastasis did not necessarily lead to longer survival. However, an interval shorter than two years between primary tumor and metastases was correlated with a shorter disease-specific survival (van der Poel et al., 1999).
The laparoscopic approach to adrenal malignancy still remains controversial. More recently, some authors considered laparoscopic adrenalectomy (LA) to be safe for metastatic adrenal lesions, with equivalent long-term oncological outcomes to the open surgery and an additional benefit of being less invasive. LA has been recommended as a feasible initial approach for isolated adrenal metastasis (Bonnet et al., 2008; Porpiglia et al., 2004; Moinzadeh and Gill, 2005). Moinzadeh and Gill (2005) reported that 31 patients underwent a total of 33 LAs for adrenal carcinoma and no operative mortality was reported. One case was electively converted to open surgery. Mean tumor size was 5 cm (ranging from 1.8 to 9 cm). There were metastatic cancers in 26 cases and primary adrenal malignancy in 7 cases according to the pathological findings. Cancer specific survival at a median follow-up of 42 months was 53% and a 5-year actuarial survival was 40%. Seven patients had local recurrence (23%). There was no port site metastasis. We have accomplished LA for five patients with adrenal metastatic tumors. The mean follow-up was 18 months (from 3 to 56 months). Among the five patients, there was no endoperitoneal or trocar port-site seeding found, though one case died of systemic dissemination of the disease 14 months after the operation. We consider LA a feasible option following the principles of oncological surgery. Adrenalectomy for metastasis, with intent of prolonging survival, should be offered to patients with favourable tumor biology. Nevertheless, further investigations are required to evaluate the appropriateness of this operation.
In conclusion, we present a very rare case that highlights the unique behavior of RCC with an unusual metastatic site and favorable survival after multiple metastasectomy. We consider that, when the metastatic RCC is resectable, it should be surgically treated. The patients with chromophobe histological subtype or a long interval between primary tumor and metastases may live longer with no recurrence after metastasectomy.
References
- 1.Amin MB, Paner GP, Alvarado-Cabrero I, Young AN, Stricker HJ, Lyles RH, Moch H. Chromophobe renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 145 cases. Am J Surg Pathol. 2008;32(12):1822–1834. doi: 10.1097/PAS.0b013e3181831e68. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Cozzoli A, Simeone C, Zani D, Zanotelli T, Portesi E, Cosciani Cunico S. Surgical treatment of adrenal metastasis from renal cell carcinoma: a single-centre experience of 45 patients. BJU Int. 2006;97(3):505–508. doi: 10.1111/j.1464-410X.2006.05934.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet S, Gaujoux S, Leconte M, Thillois JM, Tissier F, Dousset B. Laparoscopic adrenalectomy for metachronous metastasis from renal cell carcinoma. World J Surg. 2008;32(8):1809–1814. doi: 10.1007/s00268-008-9539-3. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Plantade A, Elson P, Nergrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26(1):127–131. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 5.Daliani DD, Tannir NM, Papandreou CN, Wang X, Swisher S, Wood CG, Swanson DA, Logothetis CJ, Jonasch E. Prospective assessment of systemic therapy followed by surgical removal of metastases in selected patients with renal cell carcinoma. BJU Int. 2009;104(4):456–460. doi: 10.1111/j.1464-410X.2009.08490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta MW, Ulbright TM, Young RH. Renal cell carcinoma metastatic to the testis and its adnexa: a report of five cases including three that accounted for the initial clinical presentation. Int J Surg Pathol. 2001;9(1):49–56. doi: 10.1177/106689690100900108. [DOI] [PubMed] [Google Scholar]
- 7.Dieckmann KP, Düe W, Loy V. Intrascrotal metastasis of renal cell carcinoma. Case reports and review of the literature. Eur Urol. 1988;15(3-4):297–301. doi: 10.1159/000473457. [DOI] [PubMed] [Google Scholar]
- 8.Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology. 2000;37(4):323–331. doi: 10.1046/j.1365-2559.2000.00983.x. [DOI] [PubMed] [Google Scholar]
- 9.Eggener SE, Yossepowitch O, Kundu S, Motzer RJ, Russo P. Risk score and metastasectomy independently impact prognosis of patients with recurrent renal cell carcinoma. J Urol. 2008;180(3):873–878. doi: 10.1016/j.juro.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guevremont C, Jeldres C, Perrotte P, Karakiewicz PI. Sorafenib in the management of metastatic renal cell carcinoma. Curr Oncol. 2009;16(Suppl. 1):S27–S32. doi: 10.3747/co.v16i0.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann NE, Gillett MD, Cheville JC, Lohse CM, Leibovich BC, Blute ML. Differences in organ system of distant metastasis by renal cell carcinoma subtype. J Urol. 2008;179(2):474–477. doi: 10.1016/j.juro.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 12.Kierney PC, van Heerden JA, Segura JW, Weaver AL. Surgeon’s role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1(4):345–352. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 13.Llarena Ibarguren R, García-Olaverri Rodríguez J, Azurmendi Arin I, Olano Grasa I, Pertusa Peña C. Metachronic testicular metastasis secondary to clear cell renal adenocarcinoma. Arch Esp Urol. 2008;61(4):531–533. doi: 10.4321/S0004-06142008000400012. [DOI] [PubMed] [Google Scholar]
- 14.Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005;173(2):519–525. doi: 10.1097/01.ju.0000149038.89467.30. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 16.Nabi G, Gania MA, Sharma MC. Solitary metachronous contralateral testicular metastasis from renal cell carcinoma. Indian J Pathol Microbiol. 2001;44(4):487–488. [PubMed] [Google Scholar]
- 17.Porpiglia F, Fiori C, Tarabuzzi R, Giraudo G, Garrone C, Morino M, Fontana D, Scarpa RM. Is laparoscopic adrenalectomy feasible for adrenocortical carcinoma or metastasis? BJU Int. 2004;94(7):1026–1029. doi: 10.1111/j.1464-410X.2004.05098.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmorl P, Ostertag H, Conrad S. Intratesticular metastasis of renal cancer. Der Urologe A. 2008;47(8):1001–1003. doi: 10.1007/s00120-008-1738-x. [DOI] [PubMed] [Google Scholar]
- 19.Siemer S, Lehmann J, Kamradt J, Loch T, Remberger K, Humke U, Ziegler M, Stöckle M. Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol. 2004;171(6):2155–2159. doi: 10.1097/01.ju.0000125340.84492.a7. [DOI] [PubMed] [Google Scholar]
- 20.Steiner G, Heimbach D, Pakos E, Müller S. Simultaneous contralateral testicular metastasis from a renal clear cell carcinoma. Scand J Urol Nephrol. 1999;33(2):136–137. doi: 10.1080/003655999750016168. [DOI] [PubMed] [Google Scholar]
- 21.Swanson DA. Surgery for metastases of renal cell carcinoma. Scand J Surg. 2004;93(2):150–155. doi: 10.1177/145749690409300211. [DOI] [PubMed] [Google Scholar]
- 22.van der Poel HG, Roukema JA, Horenblas S, van Geel AN, Debruyne FM. Metastasectomy in renal cell carcinoma: a multicenter retrospective analysis. Eur Urol. 1999;35(3):197–203. doi: 10.1159/000019849. [DOI] [PubMed] [Google Scholar]
- 23.Wroński M, Arbit E, Russo P, Galicich JH. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996;47(2):187–193. doi: 10.1016/S0090-4295(99)80413-0. [DOI] [PubMed] [Google Scholar]