Abstract
Biomolecular nucleophilic substitution reactions, SN2, are fundamental and commonplace in chemistry. It is the well documented experimental finding in the literature that vicinal substitution with bulkier groups near the reaction center significantly slows the reaction due to steric hindrance, but theoretical understanding in the quantitative manner about factors dictating the SN2 reaction barrier height is still controversial. In this work, employing the new quantification approach that we recently proposed for the steric effect from the density functional theory framework, we investigate the relative contribution of three independent effects, steric, electrostatic, and quantum, to the SN2 barrier heights in gas phase for substituted methyl halide systems, R1R2R3CX, reacting with fluorine anion where R1, R2, and R3 denote substituting groups and X=F or Cl. We found that in accordance with the experimental finding, for these systems the steric effect dominates the transition state barrier, contributing positively to barrier heights, but this contribution is largely compensated by the negative, stabilizing contribution from the quantum effect due to the exchange-correlation interactions. Moreover, we find that it is the component from the electrostatic effect that is linearly correlated with the SN2 barrier height for the systems investigated in the present study. In addition, we compared our approach with the conventional method of energy decomposition in density functional theory, as well as examined the steric effect from the wavefunction theory for these systems via the natural bond orbital analysis.
I. Introduction
The biomolecular nucleophilic substitution, SN2, is one of the most common and best understood reactions in chemistry.1 It occurs at an aliphatic sp3 carbon center with an electronegative leaving group directly attached. The nucleophile enters on the opposite side of the carbon to the leaving group, leading to a trigonal bipyramidal transition state. The breaking of the carbon – leaving group bond and the formation of the new carbon – nucleophile bond occur simultaneously, so the reaction is second order overall, involving both leaving and entering groups in the slow and rate-determining step. Many factors have been found to affect the SN2 reaction rate, such as the basicity of the leaving group, the size of the nucleophile, the polarity of the solvent, etc. 2-5
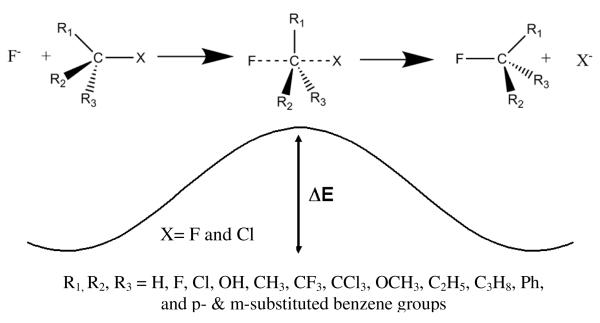
The steric effect from substituents of the carbon at the reaction center has been a well-known factor that considerably influences the SN2 reaction rate in solutions.2-9 Originated from the steric hindrance, bulkier substituents for R1, R2, or R3 groups in Scheme 1 significantly slows the reaction because larger groups effectively block the backside approach of the incoming nucleophile. Experimental data in the gas phase are limited but available data indicate the same ordering as in solution.10-15 Much has been devoted to understand steric hindrance of these reactions earlier,16,17 but it is still of considerable research interest in the recent literature.18,19 One of the reasons for the continuous investigation and controversy about them in our opinion is that no better approach to quantify steric effect has been available until very recently.
Scheme 1.
The steric effect is one of the most widely used concepts in chemistry. It originates from the fact that each atom in a molecule occupies a certain amount of space. When atoms are brought together, hindrance will be induced at the expense of shape, energy, reactivity, etc. Weisskopf20 originally attributed the steric effect to the “kinetic energy pressure”. Since no physically observable variable is associated with the steric effect, quantum mechanically speaking, there is no unique way to define it. Two approaches of quantifying steric effect are available in the literature thus far, one from the wavefunction theory 21 and the other from density functional theory (DFT).22
The wavefunction approach of quantifying the steric effect employs the Pauli Exclusion Principle. When two electrons are parallel in spin, the Principle dictates that they move apart, thus occupying more space and yielding larger steric effect than when the spins are anti-parallel. One problem with this quantification is that no dynamic electron correlation effect is included so this definition cannot be extended to post Hartree-Fock methods such as MP2 and coupled-cluster methods. The dynamic correlation between anti-parallel spin electrons is also repulsive, and thus contributes to the steric effect as well. Another concern is that the kinetic energy contribution from the Pauli Exclusion Principle is not included either.23-27 Also, it is well known that for the kinetic energy, there is not only an exchange component, termed the Pauli energy, but also a dynamic correlation ingredient, Tc.28-34 In addition, there is no distinction between quantum and pure steric effects with the present wavefunction approach of steric effect description.
Recently, we proposed a new approach of quantifying the steric effect from DFT.22 Assuming that the total energy comes from the independent contribution of three effects, steric, quantum and electrostatic, we have established that the energy component from the steric effect is precisely defined by the Weizsäcker kinetic energy.35 Physically, it represents the minimal space of a hypothetical state occupied by the electrons when all of them are placed in the lowest orbital. Several appealing properties have been disclosed such as exclusiveness, repulsiveness and extensiveness. It is consistent with Weisskopf’s attribution that the steric effect is kinetic in nature. It is extendable to any level of theory besides DFT, because only the electron density and its gradient are needed to compute the quantity. More importantly, our approach can be extended to atomic and functional group concepts if Bader’s atoms-in-molecules (AIM) approach 36 is adopted. Then, atoms attain balanced steric repulsion by sharing a zero steric repulsion interface when they form a molecule. We have applied this new quantification to understand molecular conformation changes,37-41 and also compared this new theoretical scale with the experimental scale of steric effect by Taft42 with reasonable agreement between the two observed.43
In this work, we apply our scheme of energy decomposition to SN2 reactions in gas phase in order to unravel different energy contributions, steric, electrostatic, and quantum, to their reaction barrier height. We find that in most cases steric effect is indeed the dominant factor, contributing positively to the energy difference between the transition state and reactants, but its contribution is largely compensated by the negative, stabilizing contribution from the quantum effect, with the correlation coefficient R2 between the two larger than 0.99. It is the contribution from the electrostatic effect that is found to be linearly proportional to the barrier height of SN2 reactions investigated in this study. The correlation coefficient R2 is 0.95. Comparisons of our scheme with other approaches, the steric effect description from the wavefunction theory using the Pauli Exclusion Principle and the conventional method of the total energy partition in DFT, have also been carried out.
II. Methods and Computations
In DFT,44 the conventional energy difference ΔE between the transition state and reactants, is as follows37-41
| (1) |
where TS, Ee and Exc are the non-interacting kinetic, the electrostatic, and the exchange-correlation energy density functionals, respectively, and
| (2) |
with Vne, J, and Vnn defining the nuclear-electron attraction, classical electron-electron Coulomb repulsion, and nuclear-nuclear repulsion energies, respectively.
In the new energy decomposition scheme,22,45 we assume that the energy difference comes solely from three independent effects, steric, electrostatic, and quantum (due to the exchange-correlation interaction),
| (3) |
where the steric contribution is found to be the Weizsäcker kinetic energy35
| (4) |
and the contribution from the quantum effect is the following
| (5) |
The main difference between Eqs. (1) and (3) is the identification of the Pauli component in the kinetic energy,23-27 EPauli ≡ TS - TW, which must be included as part of the contribution to the quantum effect.
The physical meaning of this description of steric effect in the framework of density functional theory is the introduction of a hypothetical reference state, where electrons are assumed to be Bosons. Physically, Eq. (4) represents the minimal space of the hypothetical state occupied by the electrons when all of them are placed in the lowest orbital. This picture is in contrast with the wavefunction quantification of the steric effect where the Pauli Exclusion Principle was employed, which applies only to the same-spin electrons to account for their static (exchange) correlation.
Two points need to be clarified about the quantum effect. First, because of the above reference state using bosons, it is more appropriate to call the quantum effect in Eq. (5) “fermionic effect” or “fermionic quantum effect”.46 Secondly, the contribution from the quantum effect, Eq, is due to the exchange-correlation interactions among electrons. It is fundamentally different from Exc in Eq. (1) because Eq defined in Eq. (5) contains exchange-correlation energy contributions from the kinetic energy whereas Exc is purely a potential energy component.
From the information theory perspective, Eq. (4) is nothing but the Fisher information (without a multiplying factor 1/8).38,40,46 The Fisher information is a measure of the narrowness or sharpness or heterogeneity of the density distribution. Equation (4) is then a measure of the compactness of the system and thus must have to do with the effect from the spacious viewpoint. Notice that the intrinsic relationship between Shannon entropy and Fisher information for atomic and molecular systems has recently been revealed by one of the authors.38
In the present work, we consider the energy difference between the transition state and the reactant complex in the gas phase for 59 self-exchange SN2 reactions of R1R2R3C-F reacting with fluorine anion, where R1, R2, and R3 represent substituting groups including H, F, Cl, OH, CH3, CF3, OCH3, C2H5, C3H8, Ph, and para- and meta-substituted benzene groups (Scheme 1). The SN2 reaction in the gas-phase is known to proceed via a pre-transition state association type of complex. We computed all energy differences in above equations with respect to this complex. To confirm what we have found for the self-exchanging SN2 reaction, we also considered R1R2R3C-Cl systems reacting with fluorine and the same number of central-carbon substitutions (Scheme 1). We represent these systems in terms of three substituted groups in the format of (R1R2R3). The central purpose of this study is to understand the nature of SN2 reaction barriers in terms of the three independent effects, steric, electrostatic and quantum, and with special focus on the impact of the steric effect to dictate the SN2 reaction rate in a quantitative manner.
All calculations were performed at the DFT B3LYP/6-311+G(d) level of theory.47 Calculations of the reactions paths were performed with Gaussian 03 package version E0248 with tight SCF convergence and ultra fine integration grids. For transition-state structure searches, the quadratic synchronous transit approach49 was employed and single-point frequency calculation was followed to ensure that the final structure obtained has only one imaginary frequency and that the vibration mode of the negative frequency corresponds to the bond formation that is anticipated. In addition, an intrinsic reaction coordinate50 run was ensued to verify the relevance of transition-state structures. The new energy decomposition analysis has been implemented in the NWChem suite 51 of software at the same level of theory. NWChem Version 5.0 52 as developed and distributed by Pacific Northwest National Laboratory, P. O. Box 999, Richland, Washington 99352 USA, and funded by the U. S. Department of Energy, was used. The single point calculation was also done at the DFT B3LYP/6-311+G(d) level of theory. We have shown earlier 37 that the new energy decomposition scheme does not qualitatively depend on the choice of either basis set or density functionals. As a comparison, we also calculated the steric energy from the wavefunction theory using natural bond orbitals for all the systems. The NBOFILE keyword in NWChem was used to create an input file to be used as the input for the stand-alone natural bond orbital analysis code, NBO version 5.0. 53 We term the result from this calculation as the NBO steric energy thereafter with the difference denoted by ΔNBOSteric.
III. Results and Discussion
Table 1 shows the results from the two energy decomposition analyses, Eqs. (1) and (3), together with the steric energy difference result from the wavefunction theory for the 59 self-exchange SN2 reactions of substituted methyl halide systems, R1R2R3CX, with F− and R1, R2, and R3 = H, F, Cl, OH, CH3, CF3, OCH3, C2H5, C3H8, Ph, and para- and meta-substituted benzene groups. Gas-phase SN2 barrier height data from both computational and experimental studies have been reported elsewhere. 14,16,54-56 For example, for CH3F (i.e., the HHH species in Table 1), the experimental value of the barrier height in enthalpy difference is about 13.3 kcal/mol, 14 our results are 9.22 kcal/mol in energy difference and 13.8 kcal/mol in enthalpy difference, indicating that our results are in general in reasonable agreement with the experimental data. Nevertheless, we notice that earlier studies have shown that to accurately reproduce the experimental data, high-level computational approaches at the G2, G3, or even higher level of theory are required.52-54 Since the main purpose of this work is to qualitatively understand the nature of the barrier height in terms of energy decomposition schemes, the high accuracy and agreement with experimental data are not our major concern in this work.
Table 1.
SN2 energy barrier heights for 59 systems investigated in this study together with their energy decomposition analyses based on Eqs. (1) and (3). For the comparison purpose, the steric energy difference from the NBO analysis is also tabulated for the systems. Units are in kcal/mol.
| Models (R1R2R3) | ΔE | ΔEs | ΔEq | ΔEe | ΔTs | ΔExc | Δ(NBOSteric) |
|---|---|---|---|---|---|---|---|
| HHH | 9.22 | 89.40 | −93.73 | 13.56 | −22.84 | 18.50 | −30.17 |
| CH3HH | 14.67 | 122.83 | −130.24 | 22.08 | −27.92 | 20.51 | −15.58 |
| CH3CH3H | 19.66 | 145.01 | −155.68 | 30.33 | −35.80 | 25.12 | −17.15 |
| CH3CH3CH3 | 27.13 | 148.69 | −161.12 | 39.55 | −40.53 | 28.10 | −7.15 |
| PhCH3CH3 | 29.42 | 158.89 | −168.47 | 39.00 | −38.99 | 29.41 | −27.96 |
| C2H5C2H5C2H5 | 24.90 | 126.99 | −136.16 | 34.06 | −37.23 | 28.07 | −0.92 |
| C2H5C2H5CH3 | 25.52 | 134.71 | −144.12 | 34.92 | −37.98 | 28.57 | −11.23 |
| C2H5CH3CH3 | 26.21 | 139.65 | −150.60 | 37.15 | −39.22 | 28.27 | −9.40 |
| PhPhCH3 | 30.75 | 154.87 | −166.14 | 42.03 | −42.89 | 31.62 | −23.87 |
| PhPhPh | 24.48 | 150.45 | −160.59 | 34.62 | −43.22 | 33.08 | −52.53 |
| PhCH3H | 21.66 | 170.70 | −178.00 | 28.96 | −34.16 | 26.87 | −27.65 |
| PhHH | 16.36 | 161.63 | −170.21 | 24.93 | −32.82 | 24.25 | −10.39 |
| C2H5HH | 13.79 | 118.08 | −125.34 | 21.05 | −27.67 | 20.41 | 2.60 |
| C3H8C2H5C2H5 | 24.84 | 126.33 | −135.31 | 33.82 | −37.06 | 28.08 | −1.67 |
| C3H8C3H8C2H5 | 26.80 | 108.45 | −119.76 | 38.11 | −39.46 | 28.15 | −4.42 |
| C3H8C3H8C3H8 | 26.69 | 106.40 | −118.04 | 38.33 | −39.70 | 28.06 | −6.39 |
| CCl3HH | 18.98 | 9.08 | −8.21 | 18.11 | −18.11 | 18.99 | 5.13 |
| CF3CH3H | 20.93 | 90.78 | −99.76 | 29.91 | −32.06 | 23.08 | −42.65 |
| CF3HH | 17.28 | 89.53 | −98.54 | 26.29 | −27.97 | 18.96 | −28.41 |
| ClClCl | 13.05 | −5.54 | 0.12 | 18.47 | −31.60 | 26.18 | −93.70 |
| ClClH | 32.43 | 32.34 | −55.72 | 55.81 | −36.52 | 13.14 | −44.86 |
| ClHH | 20.40 | 85.25 | −97.26 | 32.41 | −29.52 | 17.51 | −32.41 |
| FClCl | 13.31 | −20.49 | 14.76 | 19.04 | −29.09 | 23.36 | −98.20 |
| FFCl | 14.19 | −41.23 | 32.61 | 22.81 | −28.13 | 19.50 | −97.11 |
| FFF | 15.94 | −61.09 | 49.61 | 27.43 | −27.12 | 15.64 | −64.78 |
| FFH | 28.80 | 14.14 | −34.51 | 49.18 | −37.73 | 17.35 | −19.86 |
| FHH | 17.15 | 70.32 | −80.97 | 27.80 | −29.37 | 18.72 | −13.49 |
| OCH3FH | 25.35 | 25.67 | −42.07 | 41.76 | −35.27 | 18.87 | −7.90 |
| OCH3HH | 19.01 | 116.61 | −127.03 | 29.43 | −33.06 | 22.64 | 7.07 |
| OHFF | 74.78 | 49.10 | −81.88 | 107.56 | −62.89 | 30.12 | −24.67 |
| OHHH | 38.98 | 231.56 | −241.01 | 48.43 | −34.06 | 24.60 | 39.86 |
| OHOHH | 34.38 | 127.41 | −138.82 | 45.79 | −25.27 | 13.85 | 22.60 |
| p-Ph(CCH)CH3H | 20.87 | 168.74 | −175.35 | 27.48 | −33.14 | 26.52 | −25.42 |
| p-Ph(CCH)HH | 8.32 | 98.10 | −99.58 | 9.79 | −18.41 | 16.94 | −6.23 |
| p-Ph(CH3)CH3H | 21.73 | 171.60 | −179.70 | 29.82 | −35.09 | 26.99 | −25.89 |
| p-Ph(CH3)HH | 16.47 | 162.07 | −170.01 | 24.41 | −32.12 | 24.17 | −10.33 |
| p-Ph(CN)CH3H | 20.33 | 166.21 | −172.50 | 26.62 | −31.90 | 25.61 | −24.90 |
| p-Ph(CN)HH | 8.87 | 114.41 | −114.39 | 8.85 | −16.81 | 16.83 | −0.85 |
| p-Ph(Cl)CH3H | 21.82 | 169.25 | −177.44 | 30.00 | −34.16 | 25.97 | −25.35 |
| p-Ph(Cl)HH | 16.61 | 162.14 | −171.20 | 25.67 | −32.47 | 23.40 | −9.33 |
| p-Ph(F)CH3H | 22.06 | 171.41 | −179.11 | 29.77 | −34.20 | 26.50 | −26.78 |
| p-Ph(F)HH | 16.81 | 160.44 | −169.92 | 26.29 | −33.24 | 23.76 | −8.50 |
| p-Ph(NH2)CH3H | 22.51 | 179.26 | −186.69 | 29.94 | −34.92 | 27.49 | −22.16 |
| p-Ph(NH2)HH | 17.18 | 161.93 | −170.73 | 25.98 | −32.96 | 24.16 | −1.00 |
| p-Ph(NO2)CH3H | 19.31 | 161.15 | −168.39 | 26.55 | −32.01 | 24.77 | −32.81 |
| p-Ph(NO2)HH | 1.53 | 39.39 | −33.55 | −4.30 | 5.38 | 0.45 | 26.40 |
| p-Ph(OCH3)CH3H | 23.83 | 213.73 | −218.29 | 28.39 | −34.90 | 30.34 | −28.18 |
| p-Ph(OCH3)HH | 18.58 | 198.18 | −204.76 | 25.16 | −33.66 | 27.09 | −3.51 |
| p-Ph(OH)CH3H | 22.96 | 177.50 | −185.49 | 30.95 | −35.41 | 27.42 | −20.94 |
| p-Ph(OH)HH | 17.69 | 160.60 | −170.22 | 27.31 | −33.71 | 24.09 | −8.68 |
| m-Ph(CCH)HH | 16.69 | 162.38 | −171.31 | 25.63 | −33.24 | 24.30 | −5.73 |
| m-Ph(CH3)HH | 16.72 | 162.60 | −171.73 | 25.85 | −33.43 | 24.30 | −7.66 |
| m-Ph(CN)HH | 16.57 | 163.93 | −172.32 | 24.96 | −32.48 | 24.09 | −12.21 |
| m-Ph(Cl)HH | 16.36 | 161.20 | −170.71 | 25.86 | −33.57 | 24.07 | −11.04 |
| m-Ph(F)HH | 16.21 | 162.07 | −170.57 | 24.70 | −32.44 | 23.94 | −12.59 |
| m-Ph(NH2)HH | 29.37 | 247.74 | −256.66 | 38.29 | −35.94 | 27.02 | 10.45 |
| m-Ph(NO2)HH | 16.69 | 162.80 | −172.22 | 26.11 | −33.79 | 24.37 | −9.59 |
| m-Ph(OCH3)HH | 20.07 | 176.82 | −187.73 | 30.99 | −36.02 | 25.11 | −0.32 |
| m-Ph(OH)HH | 16.52 | 162.71 | −171.26 | 25.07 | −32.69 | 24.14 | −12.11 |
From the Table, it is seen that (i) the SN2 reaction barrier height, defined as the energy difference between the reactant complex and the transition state (Scheme 1), is always positive, ranging from 1.53 kcal/mol for p-Ph(NO2)HH to 74.78 kcal/mol for OHFF; and (ii) the steric energy difference ΔEs is positive in almost all cases (except ClClCl, FClCl and ClFF systems) indicating that steric effect generally contributes positively to the barrier height. That is, the larger the steric effect, the higher the SN2 reaction barrier, and thus the slower the chemical reaction, consistent with the experimental finding. Moreover, looking at the general trend of the energy difference from three effects, steric ΔEs, electrostatic ΔEe and quantum ΔEq, we find that ΔEq is generally negative, contributing in stabilizing the transition state of SN2 reactions. The quantity of ΔEe is positive but much less than ΔEs in magnitude, suggesting that in most cases the steric effect ΔEs is the dominant factor controlling the barrier height.
As examples, let us look at the first five systems in Table 1. When R1 changes from the hydrogen atom to the spaciously larger, methyl group with R2 and R3 fixed to H, the steric energy contribution increases from 89.40 to 122.83 kcal/mol. When R2 and R3 are subsequently altered also to the methyl group, ΔEs keeps increasing to 145.01 and then to 148.69 kcal/mol, respectively. Notice that the amount of each steric hindrance increase from one methyl group to two to three methyl groups, 33.4, 22.2, 3.7 kcal/mol, respectively, is quickly decreased. This is because the vicinal substituted methyl groups rapidly occupy all available space near the reaction center, leading to that the steric hindrance becomes saturated. Furthermore, when one of the three methyl groups is replaced by a bulkier benzene group, the steric energy contribution gets even larger, becoming 158.89 kcal/mol. These results exhibit that not only the steric effect is the dominant factor of the SN2 barrier, but it is also proportional the barrier height in some cases. For these five systems, the correlation coefficient R2 between ΔEs and ΔE is 0.88.
This relationship between ΔEs and ΔE however is not universal, as is witnessed by many examples in the Table. For instance, from CH3CH3CH3 to C2H5C2H5C2H5, both ΔEs and ΔE are decreased, so are the differences from PhCH3CH3 to PhPhPh. Again, the same as for the above methyl substitution case, this is because the bulkier groups quickly occupied all available spaces around the reaction center and thus the repulsion due to the steric hindrance promptly becomes saturated. Even though in almost all cases ΔEs is positive and dominantly contributes to ΔE, the overall correlation between ΔEs and ΔE for all data points in Table 1 is statistically insignificant.
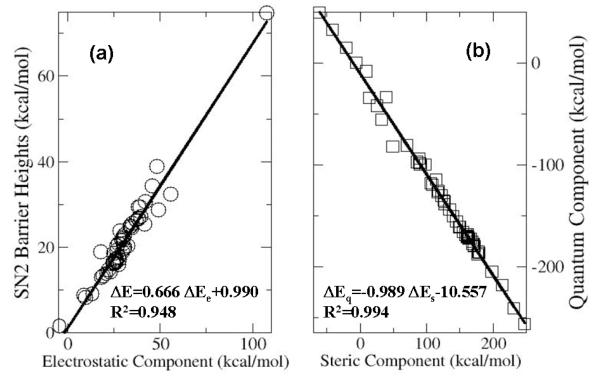
In contrast, we find that there exists a strong linear relationship between the barrier height ΔE and the electrostatic energy difference ΔEe, as shown in Fig. 1a, with the correlation coefficient R2 equal to 0.95. Removing the point with the largest barrier height (OHFF) does not change the fitting statistics. Also shown in Fig. 1 is the even stronger correlation between ΔEs and ΔEq, Fig. 1b, with R2 = 0.99 for the 59 data points in Table 1. Notice that though much smaller in magnitude than ΔEs, ΔEe is positive as well in most cases in the Table, except p-Ph(NO2)HH, and thus also contributes positively to the barrier height in SN2 reactions. Taken together, these results suggest that (i) the steric effect dominates the SN2 reaction barrier height; (ii) steric and quantum effects are mutually compensated; and (iii) it is the electrostatic effect that is linearly correlated with the barrier height.
Figure 1.
Strong linear correlations (a) between the transition state barrier height ΔE and the total electrostatic energy difference ΔEe and (b) between the steric energy difference ΔEs and the quantum energy difference ΔEq for the SN2 reactions of CR1R2R3F with fluorine anion and 59 substituted groups investigated in this study. See discussion in detail in the text.
The above strong correlation between the two components, ΔEs and ΔEq, in Eq. (3) is seemingly in contradiction with the assumption that they represent the contribution from independent physiochemical, steric and quantum, effects. This is not the case. First, the relationship exists only at the solution point, not universal in general. If the two quantities, defined separately in Eqs. (4) and (5), were intrinsically related, the much harder quantum contribution Eq in Eq. (5) involving two most difficult quantities in DFT, non-interacting kinetic energy density functional TS and exchange-correlation energy density functional Exc, would then have a much simpler explicit solution from Eq. (4). Secondly, similar correlations such as viral theorems also exist in the solution point for other quantities. Thirdly, although this relationship existed for some of the systems in our early work on conformational changes, 39 it was not observed in others cases. More importantly, we found that the correlation between Es and Eq before the difference between the reactant and SN2 transition state is taken is much less statistically significant. For both reactants and transition state of the R1R2R3C-F system, the correlation coefficient R2 between Es and Eq is only 0.71, whereas R2 of the difference correlation, ΔEs vs. ΔEq, between the two systems is 0.99. Based on these arguments, we conclude that the strong correlation between ΔEs and ΔEq is not universal.
The quantity ΔEe is also included in Eq. (1), the conventional energy decomposition scheme in DFT. 44,57,58 Does there exist any correlation between ΔTS and ΔExc as well? The answer is no, based on the data in Table 1. Also, the last column of the Table tabulates the steric energy Δ(NBOSteric) computed from the wavefunction approach where the Pauli Exclusion Principle is employed as a measure of the steric repulsion. From the Table, it is seen that in almost all cases (except C2H5HH, CCl3HH, OCH3HH, OHHH, OHOHH and m-Ph(NH2)HH systems), its contribution to the SN2 barrier is negative, meaning that the steric description from the wavefunction theory gives that the larger the vicinal substituted group, the smaller the barrier height, which is in apparent contradiction with the well-documented experimental findings. In addition, no statistically significant correlation between Δ(NBOSteric) and other quantities in Table 1 has been observed.
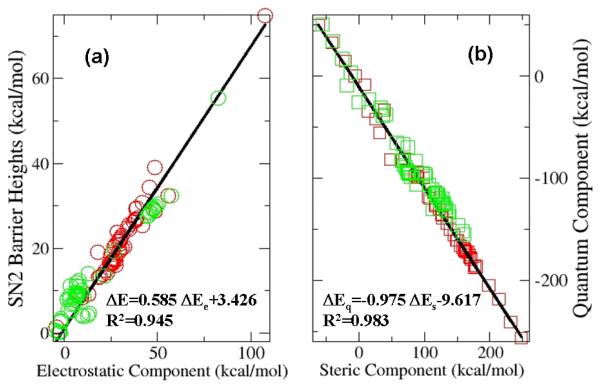
To confirm our findings from the self-exchange SN2 reaction for R1R2R3C-F and exclude the possibility that these results are not generally applicable, we then considered the SN2 reaction of R1R2R3C-Cl with fluorine anion and the same number of substitutions. Part of the results (in green color) for these systems together with those (in red color) from R1R2R3C-F are shown in Fig. 2, where it is seen that these two kinds of systems give similar results and the least-square-fitting statistics of the two systems is similar in accuracy as well. Again, the contribution from the steric effect is dominant but largely compensated by the stabilizing contribution from the quantum effect. A strong correlation between the electrostatic effect and the barrier height is observed. No correlation between ΔTS and ΔExc or between Δ(NBOSteric) and any other quantity is distinguished. One noticeable difference from the R1R2R3C-F system is that there are more (62%) species within the R1R2R3C-Cl system whose Δ(NBOSteric) values turned positive, indicating that for this system, the wavefunction quantification approach of the steric effect also predicts its positive contribution to the barrier height for a majority of the species considered in this study. In addition, the experimental barrier height in enthalpy of CH3Cl is 2.9 kcal/mol 14, much smaller than that of CH3F, and our computational barrier height result in enthalpy for the CH3Cl SN2 reaction is 1.8 kcal/mol, in reasonable agreement with the experimental data.
Figure 2.
Strong linear correlations (a) between the transition state barrier height ΔE and the total electrostatic energy difference ΔEe and (b) between the steric energy difference ΔEs and the quantum energy difference ΔEq for SN2 reactions of 59 substituted CR1R2R3F (red color) and CR1R2R3Cl (green color) systems reacting with fluorine anion investigated in this study. See discussion in detail in the text.
Finally, we mention in passing that we have also considered possible correlations of the SN2 barrier heights for these systems with structural and electronic properties on the reaction center as well as other theoretical quantities from DFT or conceptual DFT frameworks such as changes in bond lengths, NBO charges, molecular electrostatic potential,59,60 natural atomic bond energies, chemical potential, hardness, electrophilicity, etc.44,45 No statistically significant correlation of the SN2 barrier height with any of these examined quantities has been identified for the entire data set of these species. In addition, to determine the main atomic or functional group interactions that contribute to the quantities shown in Figs. 1 and 2, that is, to find out which atom or group contributes most to the total and component energy differences and whether or not it is the interaction between the nucleophile and the carbon atom on which the leaving group is attached, we will make use of the steric energy partition scheme that we recently reported.43 This and other works along the line will be investigated and presented elsewhere.
IV. Conclusions
The biomolecular nucleophilic substitution, SN2, is one of the most common and best understood reactions in chemistry. Though the reaction is fundamentally important in chemistry, factors dictating its barrier height are still not quantitatively well understood. In this contribution, we employ the energy decomposition scheme that we recently proposed to investigate the relative contributions from three independent effects – steric, electrostatic and quantum – for 118 SN2 reactions in gas phase. The present study becomes possible, because new insight into and quantification of the steric effect have recently been accomplished within the framework of density functional theory. We found that while the steric effect dominates the barrier height, in accordance with the well documented experimental findings in the literature, it is largely compensated by the opposing, stabilizing contribution from the quantum effect. Moreover, we found that it is the positively contribution from the electrostatic effect that is best correlated with the SN2 barrier height for the two types of systems that we investigated in this study, R1R2R3C-F and R1R2R3C-Cl reacting with fluorine anion with R1, R2, and R3 as substituting groups. Comparisons with the conventional approach of energy decomposition in density functional theory as well as with the quantitative description of the steric effect from the wavefunction theory have been carried out and discussed. This work offers new thoughts and insights to a fundamental class of chemical reactions and can similarly be applied to other categories of chemical reactions as well.
Acknowledgments
Helpful discussion with Daniel Ess, Robert G. Parr, and Cynthia K. Schauer of University of North Carolina at Chapel Hill, Robert C. Morrison of East Carolina University, Paul W. Ayer of McMaster University, Canada, and Stephen L. Craig of Duke University is gratefully acknowledged. This work was supported in part by UNC EFRC: Solar Fuels and Next Generation Photovoltaics, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001011, the National Institute of Health (HL-06350), NSF (FRG DMR-0804549), and the Intramural Research Program of NIH, NIEHS. We acknowledge the use of the computational resources provided by the Research Computing Center at University of North Carolina at Chapel Hill. Part of the work was presented in the 50th Sanibel Symposium at St. Thomas Island, GA.
References
- (1).Wade LG. Organic Chemistry. 5th ed Prentice Hall; New Jersey: 2003. [Google Scholar]; Shaik SS, Schlegel HB, Wolfe S. Theoretical Aspects of Physical Organic Chemistry: The SN2 Mechanism. John Wiley & Sons; New York: 1992. [Google Scholar]
- (2).Newman MS, editor. Steric Effects in Organic Chemistry. John Wiley and Sons; New York: 1956. [Google Scholar]
- (3).Hughes ED, Ingold CK. J. Chem. Soc. 1935:244. [Google Scholar]
- (4).Dostrovsky I, Hughes ED, Ingold CK. J. Chem. Soc. 1946:173. [Google Scholar]
- (5).Reichardt C. Solvents and Solvent Effects in Organic Chemistry. VCH; New York: 1988. [Google Scholar]
- (6).Ingold CK. Structure and Mechanism in Organic Chemistry. 2nd ed Cornell University Press; Ithaca: 1969. [Google Scholar]
- (7).Lowry TH, Richardson KS. Mechanism and Theory in Organic Chemistry. Harper Collins; New York: 1987. [Google Scholar]
- (8).DeTar DF, McMullen DF, Luthra NP. J. Am. Chem. Soc. 1978;100:2484. [Google Scholar]
- (9).Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, CA: 2006. [Google Scholar]
- (10).Caldwell G, Magnera TF, Kebarle P. J. Am. Chem. Soc. 1984;106:959. [Google Scholar]
- (11).Olmstead WN, Brauman JI. J. Am. Chem. Soc. 1977;99:4219. [Google Scholar]
- (12).Craig SL, John I, Brauman JI. Science. 1998;276:1536. [Google Scholar]
- (13).Chabinyc ML, Craig SL, Regan CK, Brauman JI. Science. 1998;279:1882. doi: 10.1126/science.279.5358.1882. [DOI] [PubMed] [Google Scholar]
- (14).Laerdahl JK, Uggerud E. Inter. J. Mass. Spec. 2002;214:277. [Google Scholar]
- (15).Regan CK, Craig SL, Brauman JI. Science. 2002;295:2245. doi: 10.1126/science.1068849. [DOI] [PubMed] [Google Scholar]
- (16).Jensen F. Chem. Phys. Lett. 1992;196:368. [Google Scholar]
- (17).Vayner G, Houk KN, Jorgensen WL, Brauman JI. J. Am. Chem. Soc. 2004;126:9054. doi: 10.1021/ja049070m. [DOI] [PubMed] [Google Scholar]
- (18).Kim Y, Cramer CJ, Truhlar DG. J.Phys. Chem.A. 2009;113:9109. doi: 10.1021/jp905429p. [DOI] [PubMed] [Google Scholar]
- (19).Chen X, Regan CK, Craig SL, Krenske EH, Houk KN, Jorgensen WL, Brauman JI. J. Am. Chem. Soc. 2009;131:16162. doi: 10.1021/ja9053459. [DOI] [PubMed] [Google Scholar]
- (20).Weisskopf VF. Science. 1975;187:605. doi: 10.1126/science.187.4177.605. [DOI] [PubMed] [Google Scholar]
- (21).Badenhoop JK, Weinhold F. J. Chem. Phys. 1997;107:5406. [Google Scholar]; Int. J. Quantum Chem. 1999;72:269. [Google Scholar]
- (22).Liu SB. J. Chem. Phys. 2007;126:244103. doi: 10.1063/1.2747247. [DOI] [PubMed] [Google Scholar]
- (23).March NH. Phys. Lett. A. 1986;113:476. [Google Scholar]
- (24).Levy M, Ou-Yang H. Phys. ReV. A. 1988;38:625. [Google Scholar]
- (25).Herring C, Chopra M. Phys. ReV. A. 1988;37:31. doi: 10.1103/physreva.37.31. [DOI] [PubMed] [Google Scholar]
- (26).Savin A, Becke AD, Flad J, Nesper R, Preuss H, von Schnering HG. Angew. Chem., Int. Ed. 1991;30:409. [Google Scholar]
- (27).Holas A, March NH. Phys. ReV. A. 1991;44:5521. doi: 10.1103/physreva.44.5521. [DOI] [PubMed] [Google Scholar]
- (28).Levy M, Perdew JP. Phys. Rev. A. 1985;32:2010. doi: 10.1103/physreva.32.2010. [DOI] [PubMed] [Google Scholar]
- (29).Liu SB, Parr RG. Phys. Rev. A. 1996;53:2211. doi: 10.1103/physreva.53.2211. [DOI] [PubMed] [Google Scholar]
- (30).Liu SB, Karasiev V, Lopez-Boada R, De Proft F. Int. J. Quantum Chem. 1998;69:513. [Google Scholar]
- (31).Liu SB, Nagy A, Parr RG. Phys. Rev. A. 1999;59:1131. [Google Scholar]
- (32).Nagy A, Liu SB, Parr RG. Phys. Rev. A. 1999;59:3349. [Google Scholar]
- (33).Liu SB, Morrison RC, Parr RG. J. Chem. Phys. 2006;125:174109. doi: 10.1063/1.2378769. [DOI] [PubMed] [Google Scholar]
- (34).Liu SB, Parr RG. J. Phys. Chem. A. 2007;111:10422. doi: 10.1021/jp073322z. [DOI] [PubMed] [Google Scholar]
- (35).von Weizsäcker CF. Z. Phys. 1935;96:431. [Google Scholar]
- (36).Bader RFW. Atoms in Molecules - A Quantum Theory. Oxford University Press; Oxford: 1990. [Google Scholar]
- (37).Liu SB, Govind N. J. Phys. Chem. A. 2008;112:6690. doi: 10.1021/jp800376a. [DOI] [PubMed] [Google Scholar]
- (38).Liu SB. J. Chem. Phys. 2007;126:191107. doi: 10.1063/1.2741244. [DOI] [PubMed] [Google Scholar]
- (39).Liu SB, Govind N, Pedersen LG. J. Chem. Phys. 2008;129:094104. doi: 10.1063/1.2976767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Nagy A, Liu SB. Phys. Lett. A. 2008;372:1654. [Google Scholar]
- (41).Uğur I, Vleeschouwer FD, Tüzün N, Aviyente V, Geerlings P, Liu SB, Ayers PW, De Proft F. J. Phys. Chem. A. 2009;113:8704. doi: 10.1021/jp903371b. [DOI] [PubMed] [Google Scholar]
- (42).Taft RW., Jr J. Am. Chem. Soc. 1952;74:2729. [Google Scholar]
- (43).Torrent-Sucarrat M, Liu SB, De Proft F. J. Phys. Chem. A. 2009;113:3698. doi: 10.1021/jp8096583. [DOI] [PubMed] [Google Scholar]
- (44).Parr RG, Yang W. Density Functional Theory of Atoms and Molecules. Oxford University Press; Oxford: 1989. [Google Scholar]
- (45).Liu SB. Acta Phys. -Chim. Sin. 2009;25:590. [Google Scholar]
- (46).Nagy A. Chem. Phys. Lett. 2007;449:212. [Google Scholar]
- (47).Becke AD. Phys. Rev. A. 1988;38:3098. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]; J. Chem. Phys. 1993;98:5648. [Google Scholar]; Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- (48)(a).Frisch MJ, et al. Gaussian, Inc.; Wallingford CT: 2004. [Google Scholar]; (b) Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision E.02. Gaussian, Inc.; Wallingford, CT: 2004. [Google Scholar]
- (49).Peng C, Ayala PY, Schlegel HB, Frisch MJ. J. Comp. Chem. 1996;17:49. [Google Scholar]
- (50).Hratchian HP, Schlegel HB. J. Chem. Phys. 2004;120:9918. doi: 10.1063/1.1724823. [DOI] [PubMed] [Google Scholar]
- (51).Straatsma TP, Aprà E, Windus TL, Bylaska EJ, de Jong W, Hirata S, Valiev M, Hackler M, Pollack L, Harrison R, Dupuis M, Smith DMA, Nieplocha J, Tipparaju V, Krishnan M, Auer AA, Brown E, Cisneros G, Fann G, Früchtl H, Garza J, Hirao K, Kendall R, Nichols J, Tsemekhman K, Wolinski K, Anchell J, Bernholdt D, Borowski P, Clark T, Clerc D, Dachsel H, Deegan M, Dyall K, Elwood D, Glendening E, Gutowski M, Hess A, Jaffe J, Johnson B, Ju J, Kobayashi R, Kutteh R, Lin Z, Littlefield R, Long X, Meng B, Nakajima T, Niu S, Rosing M, Sandrone G, Stave M, Taylor H, Thomas G, van Lenthe J, Wong A, Zhang Z. NWChem, A Computational Chemistry Package for Parallel Computers, Version 4.6. Pacific Northwest National Laboratory; Richland, Washington 99352-0999, USA: 2004. [Google Scholar]
- (52).Kendall RA, Aprà E, Bernholdt DE, Bylaska EJ, Dupuis M, Fann GI, Harrison RJ, Ju J, Nichols JA, Nieplocha J, Straatsma TP, Windus TL, Wong AT. High Performance Computational Chemistry: an Overview of NWChem a Distributed Parallel Application. Computer Phys. Comm. 2000;128:260. [Google Scholar]
- (53).Glendening ED, Badenhoop J,K, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin; Madison: 2001. [Google Scholar]
- (54).Glukhovtsev MN, Pross A, Radom L. J. Am. Chem. Soc. 1995;117:2024. [Google Scholar]
- (55).Glad SS, Jensen F. J. Am. Chem. Soc. 1997;119:227. S.S. [Google Scholar]
- (56).Deng L, Branchadell V, Ziegler T. J. Am. Chem. Soc. 1994;116:10645. [Google Scholar]
- (57).Rong C, Lian S, Yin D, Zhong AG, Zhang RQ, Liu SB. Chem. Phys. Lett. 2007;434:149. [Google Scholar]
- (58).Rong C, Lian S, Yin D, Shen B, Zhong AG, Bartolotti L, Liu SB. J. Chem. Phys. 2006;125:174102. doi: 10.1063/1.2378830. [DOI] [PubMed] [Google Scholar]
- (59).Liu SB, Pedersen LG. J. Phys. Chem. A. 2009;113:3648. doi: 10.1021/jp811250r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Liu SB, Schauer CK, Pedersen LG. J. Chem. Phys. 2009;131:164107. doi: 10.1063/1.3251124. [DOI] [PMC free article] [PubMed] [Google Scholar]