Abstract
Atherosclerotic renal artery stenosis reduces blood flow and perfusion pressures to the post-stenotic kidney producing renovascular hypertension and threatening GFR. Little is known regarding regional tissue oxygenation in human renovascular disease that develops slowly. We compared stenotic and contralateral kidneys regarding volume, tissue perfusion, blood flow measured by multidetector CT and blood oxygen level dependent (BOLD) MR values in cortex and medulla in 14 patients with unilateral stenosis (mean =71% by quantitative CT) and in 14 essential hypertensive patients during 150 mEq/d sodium intake and renin-angiotensin blockade. Stenotic kidney volume was reduced compared to contralateral (118.6 ± 9.9 vs 155.4 ± 13.7 mL, p <.01), as was total blood flow (269.7 ± 42.2 vs 383.7 ± 49, p=.02) mainly due to reduced cortical volume. Tissue perfusion was similar but lower than essential hypertension(1.5 vs 1.2 mL/min/cc, p<.05). BOLD MR at 3 Tesla confirmed elevated R2* values (a measure of deoxyhemoglobin) in deep medullary regions in all three sets of kidneys (38.9 ± .7 vs cortex 17.8 ± .36 sec −1, p<.0001). Despite reduced blood flow, R2* values did not differ between atherosclerotic and essential hypertensive kidneys, although furosemide-suppressible fall in medullary R2* was reduced in stenotic kidneys (5.7±1.8 vs 9.4±1.9 sec-1, p<.05). Renal venous oxygen levels from the stenotic kidney were higher than those from essential hypertensives (65.1 ± 2.2 vs 58.1 ± 1.2, p=.006). These data indicate that although stenosis reduced blood flow and volume, cortical and medullary oxygenation was preserved under these conditions.
Keywords: renal artery stenosis, renovascular hypertension, BOLD MR, ischemia, hypertension, oxygen, renin
Introduction
Atherosclerotic renal artery stenosis (ARAS) commonly reduces perfusion pressure to the affected kidney, activates the release of renin and produces renovascular hypertension. ARAS also can lead to rarefaction of renal microvessels in experimental models associated with tubulointerstitial fibrosis that ultimately threatens kidney viability 1. Antihypertensive therapy for renovascular hypertension now often includes agents that block the rennin-angiotensin system, including angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB’s) that reduce systemic arterial pressures but also reduce post-stenotic perfusion pressures to the kidney 2. Experimental studies in 2 kidney-1-clip- renovascular hypertension indicate that post-stenotic cortical blood flow, oxygen levels and renal venous oxygen levels are reduced in rats 3. Much of the hypoxia and increase in oxidative stress in this model is reversed by an ARB (candesartan) or a free radical scavenger (tempol), but is more severe in those treated with an ACE inhibitor (enalapril) 4. The authors interpret these data to suggest that oxygen availability in the post-stenotic kidney is modulated in part by activation of the AT1 receptor that was not observed during ACE inhibition. How these findings apply to human renovascular disease is not known.
Although post-stenotic pressures and blood flow are reduced, some human studies challenge the premise that renovascular disease produces whole kidney “ischemia” 5. This inference derives from the fact that erythropoietin levels are not elevated and venous oxygen saturation in the post-stenotic kidney is not depressed, but actually may be higher, as compared to the levels from the contralateral kidney (CLK) 6. In fact, the kidney receives more blood flow than needed for its basic metabolic function, unlike the heart or brain 7. Severe vascular occlusive disease may reduce blood flow for the whole kidney, but associated reductions in kidney volume may preserve regional perfusion (expressed as blood flow per cc of tissue). Whether reduced blood flow to either cortical or medullary segments of the kidneys in humans leads to similar reduction in tissue oxygenation and/or increased overall oxygen consumption is not well understood.
Until recently, evaluation of tissue oxygenation in the human kidney in-vivo was not technically feasible. Recent studies using blood oxygen level dependent (BOLD) MR at 1.5 Tesla indicate that alterations in cortical and medullary deoxyhemoglobin measured as the relaxation coefficient (R2*) can provide assessment of local oxygenation in-vivo 8. Levels of R2* change with acute reduction in renal perfusion pressure 9, ureteral obstruction 10, acute kidney injury or acute allograft rejection 11. Studies with higher magnet field strength (3 Tesla as compared with 1.5 Tesla) improve differentiation between medullary and cortical oxygen consumption based on inhibiting tubular solute transport 12;13.
The purpose of the current study was to examine renal blood flow, renal venous oxygen tension and regional tissue oxygenation using BOLD MR in post-stenotic (STK) and contralateral kidneys (CLK) from hypertensive human subjects with atherosclerotic renal artery stenosis (ARAS group), as compared with patients with essential hypertension without renal artery stenosis (EH group). These studies were undertaken during therapy with either an ACE inhibitor or an ARB during conditions of known, stable sodium intake. Our results suggest that oxygen consumption in the post-stenotic kidney beyond minimum metabolic requirements is actually reduced under these conditions.
METHODS
Non-diabetic patients with either unilateral ARAS (n=14) or essential hypertension (n=14) participated in this study during a three-day inpatient protocol in the Clinical Research Unit of St. Mary’s Hospital, Rochester, MN. Dietary intake was regulated at 150 mEq sodium with an isocaloric diet prepared on site. Patients with qualifying renal artery stenosis were identified using criteria similar to those stipulated for recruitment in the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial 14, with the exception of limiting studies to unilateral stenosis with serum creatinine levels below 1.7 mg/dL . Informed, written consent was obtained as approved by the Institutional Review Board of the Mayo Clinic. Severity of renal artery stenosis was estimated by Doppler ultrasound measurements above 260 cm/sec in the affected artery and quantitative vascular imaging using CT images as described below.
The first study day included measurement of sodium excretion and of GFR by iothalamate clearance (iothalamate meglumine, Conray, Mallinkrodt, St. Louis) following oral hydration (20 ml/kg) over three 30-minute timed collection periods, as previously described 15 16.
Blood pressure (BP) was measured by automated oscillometric recordings including three values taken three times daily (an automated oscillometric unit, Omron BP, each time BP measured at 5 min, 7 min and 9 min after a 5 minutes rest). Patients continued previous medications and all but one were treated with ACE inhibitors or ARB’s.
On the second day, BOLD MR examination was performed on GE Twin Signa EXCITE 3.0T system (GE Medical Systems, Waukesha,WI) using an 8 channel torso phased array coil 13 . Three-plane single shot fast spin echo localizers were performed during suspended respiration followed by additional scout images (single shot fast spin echo) oriented parallel to the long axis of each kidney. These long axis scout images were then used to prescribe transverse BOLD images in a plane orthogonal to the long axis. BOLD imaging consisted of a 2D fast spoiled gradient echo sequence with multiple echo times (TE’s). Eight echoes were obtained for each slice location, with TE’s ranging from 2.5 ms to 32 ms. Imaging parameters for the BOLD acquisition included: repetition time (TR) 140 ms, flip angle 45 degrees, slice thickness 5 mm, imaging matrix 224×160−192, field of view (FOV) 32–40 cm, with 0.7–1.0 partial phase FOV (PFOV). Imaging protocols to establish TEs in normal subjects were performed in volunteers without furosemide. Image matrix and TR were adjusted in patients with limited breath hold capacity, and the FOV and PFOV adjusted according to patient size. Transverse slice BOLD images were acquired during suspended respiration through the mid pole hilar region of each kidney. Parametric images of R2* were then generated by fitting signal intensity versus TE data to an exponential function on a voxel-by-voxel basis. Following the first BOLD acquisition, furosemide (20 mg) was administered intravenously and flushed with 20 ml of saline. BOLD measurements for each kidney were repeated 15 minutes later.
BOLD images were analyzed on an Advantage Windows workstation version 4.2 (GE Healthcare, London, UK) using CineTool software (GE Healthcare). This program generates a set of parametric images of R2* from the BOLD sequence data by fitting signal intensity data from each echo on a voxel by voxel basis to an exponential function describing the expected signal decay as a function of TE and solving for the unknown value of R2* (the magnetic rate of relaxation of the tissue, or the inverse of the T2* relaxation time).
For data analysis, individual anterior, lateral and posterior regions of interest (ROIs) were traced in the cortex and medulla manually on the 7 msec TE image or any other image yielding optimal contrast between cortex and medulla, and then implemented at the parametric R2* image to determine average values of R2* within the ROI. Special care was taken to ensure that each region of interest fell within identifiable medullary and cortical sections that remained within the segment upon repeat scanning after furosemide. As previously described 13, we considered for comparisons the mean values of R2* of three areas (anterior, lateral, and posterior) for cortex and medulla within the selected slice.
On the third day of the protocol, the common femoral vein was cannulated using a 5F Cobra catheter (Cook, Inc, Bloomington, IN) inserted into the right, left, and infrarenal inferior vena cava to collect selective renal vein samples for venous oxygen and plasma renin activity. Samples for oxygen tension were collected in Portex® 3ml Line Draw Arterial Blood Sample Syringe with Dry Lithium Heparin for gases and electrolytes and transferred immediately for analysis by the clinical laboratory. The catheter was left in place for central venous injection of contrast for transit time studies using a multidetector CT (MDCT), as previously described 17;18. MDCT imaging was obtained using a dual-source 64-slice helical MDCT scanner (SOMATOM Definition, Siemens Medical Solutions, Germany) after a bolus injection of iopamidol-370 (0.5 ml/kg up to a maximum of 40 ml) using a power injector during respiratory suspension. Perfusion scans were performed at 120 kVp and 160 mAs (adjusted per level of SNR of the scan) with 20 × 1.2 collimation and zero table feed. The flow study was composed of 35 scans divided into 3 consecutive scanning sequences (each 20 sec long) followed by 10 additional scans at 8 sec intervals, for a total of 45 scans. The total scanning time lasted about 158 sec. The longest breath-hold was 20 sec. Images representing the four slices (5 mm thickness) localized in the hilum region were acquired and reconstructed using a B40f kernel.
Fifteen minutes after completion of the perfusion study, a kidney volume study (5 mm thick slices) was performed to determine both cortical and medullary regional volumes. Additional images for quantitative vascular stenosis evaluation (quantitative CT angiography) were reconstructed at 0.6 mm slice thickness, with a 0.3 mm overlap, at either b10f or b18f kernel settings.
Image analysis was performed using ANALYZE (Biomedical Imaging Resource Center, Mayo Clinic, Rochester, MN). Analysis of MDCT flow studies were undertaken by selecting ROI in cross-sectional images from the aorta, individual kidney cortex and medulla. The computer then generated curves reflecting the change in tissue density produced by transit of contrast in that region. Regional perfusion, or blood flow normalized per unit tissue (mL blood/minute /cm3 tissue) was conventionally calculated as: Perfusion = 60 * Blood volume/Mean Transit Time/ (1- blood volume), where (1-blood volume) is a correction for dynamic changes in blood volume that occur in vivo 19.
Cortical and medullary volumes were calculated using the stereology module within ANALYZE. ROIs for the cortical and medullary regions were defined on each successive slice, and subsequently multiplied by slice width; these were then summed to obtain cortical, medullary, and total renal volume. Renal blood flow for each kidney was determined as the renal perfusion (mL/min/cc tissue) * kidney volume (cc tissue). Quantitative evaluation of vascular stenosis was undertaken by CT angiography comparing the maximally narrowed cross-sections of the artery to proximal segments and expressed in percentage of vascular occlusion.
Statistical analysis
Results were expressed using mean values and standard error of the mean (SEM). Comparison between groups with essential hypertension or ARAS were performed with t-tests as appropriate. Comparisons between stenotic and contralateral kidneys were performed using paired-t tests.
Results
Clinical characteristics of the patients studied are summarized in TABLE 1. Ages ranged from 50 to 84, with the average age in each group approaching 66 years. Serum creatinine was higher and measured iothalamate GFR was lower in subjects with ARAS (p<.01). All subjects with ARAS and all but one subject with essential hypertension were taking an ACE inhibitor or ARB, and 22 out of 28 were receiving a thiazide-class diuretic. Fifteen out of 28 were taking statin therapy. The severity of arterial stenosis in the stenotic kidneys (FIGURE 1) ranged between 62 and 81 % as estimated by quantitative CT angiography. Antihypertensive therapy was similar in the EH and ARAS groups (average number of antihypertensive drugs was respectively 2.5 and 2.9). Median urinary sodium collected on day 1 was 159.6 mEq/ day.
Table 1.
Clinical and Demographic characteristics of patients with essential hypertension (EH) and atherosclerotic renal artery stenosis (ARAS):
| Characteristic | EH N=14 |
ARAS N=14 |
|---|---|---|
| Age (years) | 67.1 ± 2.8 | 65.5 ± 2.8 |
| Gender | 7F/7M | 6F/8M |
| Duration of the disease (years) | 12.6 ± 1.7 | 12 ± 5 |
| GFR (mL/min/1.73 m2) | 84.1 ± 5.2 | 65.2 ± 5.3* |
| Creatinine (mg/dL) | 0.8 ± 0.0 | 1.2 ± 0.1† |
| Body mass index (kg/m2) | 27.5 ± 1.1 | 27.9 ± 1 |
| Median urinary sodium (mEq)/24H |
165.9 | 159.3 |
| Number of antiHTN drugs | 2.5 ± 0.2 | 2.9 ± 0.3 |
| ACEI/ARB | 8/5 | 5/9 |
| Mean SBP/ | 139.2 ± 5.8 | 131.9 ± 5 |
| / DBP (mmHg) | 74.9 ± 3.6 | 70.4 ± 2.2 |
| Doppler peak systolic velocity (cm/s) |
NA | 371.6 ± 38.8 |
| Degree of stenosis (%)‡ | NA | 71 ± 5.5 |
Mean ± SEM,
p<0.05,
p<0.01
GFR=glomerular filtration rate
AntiHTN=antihypertensive
ACEI=angiotensin converting enzyme inhibitors
ARB=angiotensin receptors blockers
SBP=systolic blood pressure
DBP=diastolic blood pressure
NA=not applicable
by quantitative CT angiography
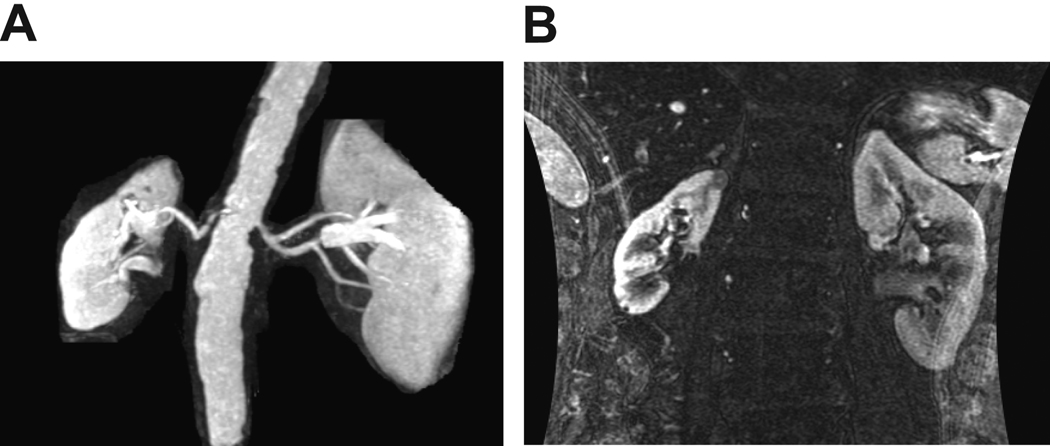
Figure 1.
(A) MR angiogram of a patient with unilateral renal artery stenosis with reduced kidney volume in the post-stenotic kidney. (B) Coronal view of both kidneys enhanced by gadolinium emphasizing the difference in single kidney volume. This was associated with a reduced single-kidney blood flow and glomerular filtration (see TABLE 2). Single-kidney cortical and medullary perfusion (renal blood flow per cc volume as measured by multidetector CT (MDCT)) were reduced in post-stenotic kidneys, with elevation of renal vein renin values (see text).
The total volume of stenotic kidneys was reduced, primarily due to reduction in cortical volume as compared to the contralateral kidney (Table 2, p<.01)). Both medullary and cortical perfusion (expressed as mL/min/cc of tissue volume) tended to be reduced in the stenotic and contralateral kidneys compared to subjects identified with essential hypertension (p=.06), but did not differ from each other. Whole kidney blood flow was reduced in the stenotic as compared to both the contralateral kidney and essential hypertensives, primarily on the basis of reduced cortical and medullary volumes. Despite reduction in blood flow and volume, tissue oxygenation as reflected by cortical and medullary R2* values did not differ in the post-stenotic kidney as compared either to the contralateral or essential hypertensive kidneys (TABLE 2).
Table 2.
Kidney functional parameters in patients with essential hypertension (EH) and atherosclerotic renal artery stenosis (ARAS):
| Single Kidney: | EH N=28 ‖ |
ARAS | |
|---|---|---|---|
| Stenotic N=14 |
Contralateral N=14 |
||
| Total Kidney Volume (CT) (mL) | 144.3 ± 6.6 | 118.6 ± 9.9 * § | 155.4 ± 13.7 |
| - Cortical volume (mL) | 96.1 ± 5.9 | 80.1 ± 7.8 § | 111.8 ± 12.1 |
| - Medullary volume (mL) | 48.2 ± 3.5 | 38.5 ± 4.5 | 43.6 ± 6.1 |
| Renal Tissue Perfusion | |||
| - Cortex (mL/min/cc tissue) | 3.5 ± 0.2 | 2.7 ± 0.3 * § | 2.9 ± 0.3 |
| - Medulla (mL/min/cc tissue) | 1.5 ± 0.1 | 1.2 ± 0.1 † | 1.2 ± 0.1 † |
| Total Renal Blood Flow (mL/min) | 404.6 ± 28.9 | 269.7 ± 42.2 * § | 383.7 ± 49.0 |
| - Cortical flow (mL/min) | 331.6 ± 27.2 | 219.1 ± 35.1 * § | 330.2 ± 44 |
| - Medullary flow (mL/min) | 68.1 ± 5.4 | 49 ± 8.9 ‡ | 55 ± 10.2 |
| MRI studies: BOLD MR | |||
| Basal cortical R2* (s−1) | 18.6 ± 0.6 | 17.0± 0.6 | 16.9 ± 0.5 |
| Basal medullary R2* (s−1) ¶ | 38.2 ± 1.0 | 37.5 ± 1.5 | 41.9 ± 1.4 |
| Change in R2* after Furosemide | 7.0±1.1 | 5.7±1.8 § | 9.4± 1.9 |
| PRA (ng/mL/H) | 8.2 ± 2.1 | 24.3 ± 5.0 * § | 18.3 ± 4.3 |
Mean values ± SEM,
p<.05 vs EH,
p=.06 vs EH,
p=.08 vs EH,
p<.05 vs Contralateral
data from both kidneys were compiled
All values of medullary R2* were higher than cortical values (p<.0001)
PRA=plasma renin activity
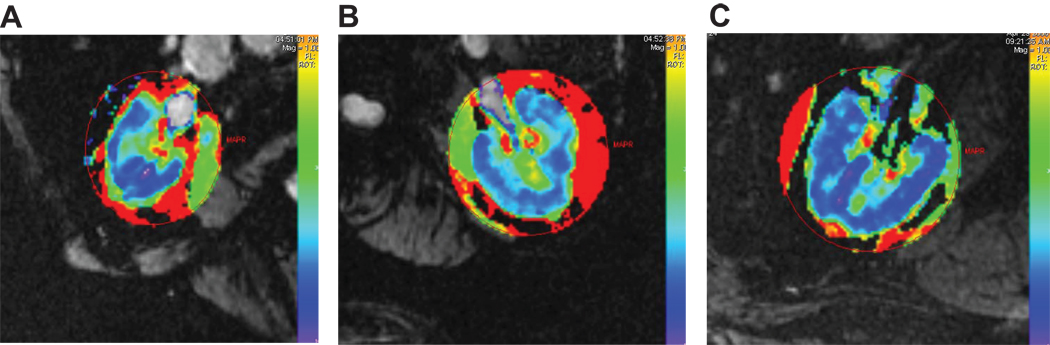
Examples of R2* parametric maps for cortex and medulla in stenotic and contralateral kidneys of this individual are illustrated in FIGURE 2 (A) and (B) and compared to that of a patient with essential hypertension (FIGURE 2C). Cortical levels were lower (16.9 to 18.6 sec−1) as compared to medullary R2* levels (37.5–41.9 sec−1) for all patients (p<.001) and for each kidney group (TABLE 2). Average levels did not differ between stenotic and contralateral kidneys and essential hypertension. These values did not differ from basal levels of cortical and medullary R2* in healthy volunteers (n=6 kidneys: Cortical R2*=16.4±0.3 sec^-1, Medullary R2*=39.±1.9 sec^-1).
Figure 2.
Parametric maps from transverse axial cuts at the hilum depicting R2* levels in cortical and medullary regions in the stenotic (A) and contralateral (B) kidneys depicted in Figure 1, as well as from a patient with essential hypertension (C). These maps underscore the heterogeneities of R2* values and the progressive rise to higher values located in the deeper sections of medulla. Despite reduced blood flow, mean cortical levels of R2* were low (17.0 ±0.6 sec-1) as compared to medulla (37.5± 1.5 sec-1). Mean levels of cortical deoxyhemoglobin did not differ from those in the contralateral kidney (16.9 ± 0.7 sec-1) or essential hypertension (18.6 ± 0.6 sec-1) (TABLE 2).
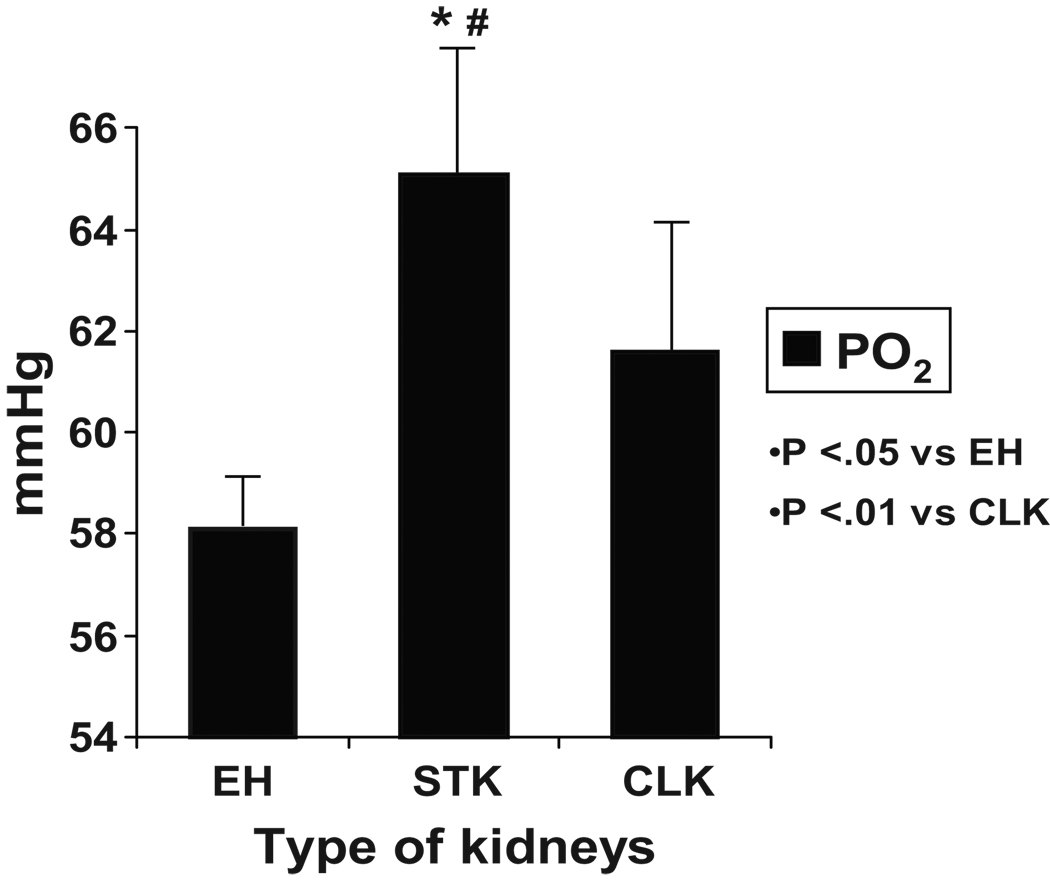
Renal vein levels of plasma renin activity from both the stenotic and contralateral kidneys were elevated as compared to essential hypertensive kidneys (TABLE 2, p<.01). Renal vein oxygen levels (mmHg, FIGURE 3) from the stenotic kidney were higher than those obtained from patients with essential hypertension and as compared to the contralateral kidney. No differences in renal vein oxygen levels were apparent in ARAS patients treated with ACE inhibitors (64 mmHg, n= 5) vs. ARB’s (64.6 mm Hg, n= 9).
Figure 3.
Levels of venous oxygen tension (PO2, mmHg) in renal vein. Levels obtained from the post-stenotic kidney were higher than those from essential hypertension (p<.01).
EH=essential hypertension group
STK=stenotic kidneys
CLK=contralateral kidney
* p<.05 vs EH
# p<.01 vs CLK
Discussion
In this paper we present measurements of single kidney volume, blood flow and tissue oxygenation in hypertensive patients with unilateral atherosclerotic renal artery stenosis as compared to essential hypertension. Despite reduced blood flow to the whole organ, our results demonstrated elevation of venous oxygen levels in post-stenotic kidneys. The fact that deep medullary and cortical oxygenation as measured by regional BOLD MR did not differ despite reduced blood flow suggests that loss of kidney volume was not associated with “ischemia” within either region under these conditions. We interpret the elevation of venous oxygen levels to likely reflect reduced oxygen consumption in the stenotic kidney as compared to both the contralateral kidney and essential hypertension. Reduced furosemide-suppressible changes in medullary oxygen consumption in the stenotic kidneys as compared to the contralateral kidney were consistent with this interpretation and suggest less energy-requiring solute transport in the post-stenotic kidneys.
It should be emphasized that our patients had relatively preserved GFR, although lower than the two-kidney GFR in essential hypertensives. None of the patients with renal artery stenosis had total occlusion and all had measurable single kidney blood flow by MDCT. The actual perfusion (blood flow per volume of tissue) was only modestly reduced in the stenotic kidney (20% as compared to essential hypertension). Small differences in perfusion were apparent between the stenotic and contralateral kidney, consistent with the inference that longstanding small vessel changes within both kidneys may be more important than the large vessel occlusive disease evident in the stenotic kidney. These results extend the observations that histological and functional changes in ARAS are present in both kidneys 20. On the other hand, the average difference in kidney volume between STK and CLK was 23% in our subjects, suggesting that the difference in blood flow between ARAS kidneys was related primarily to changes in volume rather than tissue perfusion. Our results demonstrate that despite changes in volume, both cortical and medullary R2* levels were preserved at levels not different from essential hypertension, consistent with the known excess of blood flow to the kidney as compared to its basal metabolic energy requirements 8.
Levels of plasma renin activity were elevated in our subjects, in part because nearly all were treated with inhibitors of the renin-angiotensin system and diuretics. As expected, the highest levels were obtained from the post-stenotic kidney. The renal vein renin levels from both stenotic and contralateral kidneys were higher than those from essential hypertension. We interpret this to reflect stimulation of renin from combined diuretic therapy before sampling because long-term thiazide effects were magnified by the dose of intravenous furosemide administered the day before as part of BOLD imaging. Previous studies in experimental renovascular hypertension demonstrate that activation of the renin-angiotensin system alters levels of oxidative stress and efficiency of sodium transport within the post-stenotic kidney 3. Administration of an ARB improves venous oxygenation in 2-kidney-1-clip hypertension in rats after 3 weeks, whereas an ACE inhibitor leads to lower venous oxygen levels and elevated oxidative stress that can be reversed with tempol 4. Studies of other experimental models of chronic kidney disease demonstrate that blockade of angiotensin alters the efficiency of sodium transport favorably to reduce oxidative stress, a mechanism that may be renoprotective 21. The precise role of rennin-angiotensin blockade in our studies cannot be determined with the data available, but our data indicate that both ACE inhibitors and ARB’s were associated with preserved oxygenation in humans under these conditions. The range of values for normal subjects not treated either with ACE/ARB therapy or diuretics overlapped those of both essential hypertension and renal artery stenosis.
These human studies differ from changes observed with more acute models of renal artery occlusion. R2* levels rise during acute, progressive renovascular occlusion that reduces blood flow in a swine model 9. Direct measurement of tissue oxygen tension using microelectrodes confirm that this reduction produces reductions of both cortical and medullary oxygen tensions to desaturated levels 22. On the other hand, our results are supported by a previous study showing little difference in basal R2* values in chronically stenotic swine kidneys 23 Previous studies of venous erythropoietin levels and oxygen saturation are consistent with the postulate that whole kidney “ischemia” is not common in human ARAS 5;6. Our interpretation of these differences is that gradual, incremental reduction in perfusion to the kidney triggers compensatory mechanisms that preserve oxygenation in both cortex and medulla. This occurs even in the deepest medullary segments sampled in the present studies. Some compensation may be provided by development of collateral circulation, although this could not be determined in this study. Much of the oxygen consumption in the kidney beyond minimal metabolic requirements is related to energy-dependent solute transport that may decline as a function of reduced filtration 24 Reduced solute filtration and transport may provide one explanation for the difference observed in the medullary R2* response after administration of furosemide in the stenotic kidney as compared to either contralateral or essential hypertensive kidneys. Considerable data support the presence of preglomerular arteriovenous shunting within the kidney that affects local distribution of oxygen 24. We cannot exclude a role of enhanced shunting to medullary sites during long-term adaptation to reduced perfusion that may play a role in our findings. It is possible that such long-term adaptation plays a role in the functional “hibernation” that allows preservation and potential recovery of function after restoration of the renal vascular supply 25.
Taken together, these results support the premise that atherosclerotic renal artery stenosis can reduce kidney volume and blood flow under some conditions without evident tissue ischemia during blockade of the rennin-angiotensin system.
Perspectives
The role of large vessel occlusion in promoting renovascular hypertension and loss of kidney function continues to pose vexing challenges to clinicians caring patients with ARAS. Determining the underlying mechanisms by which vascular occlusion begins to threaten tissue oxygenation and injury is fundamental to identifying individuals likely to benefit from restoration of renal blood flow with endovascular procedures. These studies partly explain the stability of kidney function during trials of medical therapy that include rennin-angiotensin system blockade for renal vascular disease. Up to now, these trials fail to establish major benefits from renal revascularization, perhaps because the oxygenation of both cortex and medulla for many patients can be preserved during antihypertensive therapy similar to that employed in the patients studied here. It is likely that similar strategies with more severe vascular occlusion will define more precisely those post-stenotic kidneys at high risk for injury related to tissue ischemia that may benefit from restoring the circulation. 26 27.
Acknowledgements
We would like to thank all the team which made this study possible and in particular Beverly Tietje, John Crane, John Woollard, Dr Hui Tang and the Clinical Research Unit from the Saint Marys Hospital, Rochester, Minnesota.
Sources of Funding
The project described was supported by Award number P01HL085307 from National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Aterioscler Thromb Vascu Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 2.Hackam DG, Spence JD, Garg AX, Textor SC. The role of rennin-angiotensin system blockade in atherosclerotic renal artery stenosis and renovascular hypertension. Hypertension. 2007;50:998–1003. doi: 10.1161/HYPERTENSIONAHA.107.097345. [DOI] [PubMed] [Google Scholar]
- 3.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K, 1C kidney. Hypertension. 2003;41:692–696. doi: 10.1161/01.HYP.0000052945.84627.8F. [DOI] [PubMed] [Google Scholar]
- 4.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II Type 2 receptors and nitric oxide sustain oxygenation in theclipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51:1–7. doi: 10.1161/HYPERTENSIONAHA.107.097832. [DOI] [PubMed] [Google Scholar]
- 5.Wiecek A, Kokot F, Kuczera M, Grzesszczak W, Kierstztejn M. Plasma erythropoietin concentration in renal venous blood of patients with unilateral renovascular hypertension. Nephrol Dial Transplant. 1992;7:221–224. doi: 10.1093/oxfordjournals.ndt.a092109. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen K, Rehling M, Henriksen JH. Renal vein oxygen saturation in renal artery stenosis. Clin Physiol. 1992;12:179–184. doi: 10.1111/j.1475-097x.1992.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 7.Epstein FH. Oxygen and renal metabolism. Kidney Int. 1997;51:381–385. doi: 10.1038/ki.1997.50. [DOI] [PubMed] [Google Scholar]
- 8.Heyman SN, Khamaisi M, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia response and the progression of chronic kidney disease. Am J Nephrol. 2008;28:998–1006. doi: 10.1159/000146075. [DOI] [PubMed] [Google Scholar]
- 9.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65:944–950. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen M, Dissing TH, Morkenborg J, Stodkilde-Jorgensen H, Hansen LH, Pedersen LB, Grenier N, Frokiaer J. Validation of quantitative BOLD MRI measurements in the kidney: application to unilateral ureteral obstruction. Kidney International. 2005;67:2305–2312. doi: 10.1111/j.1523-1755.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 11.Djamali A, Sadowski EA, Muehrer RJ, Reese S, Smavatkul C, Vidyasagar A, Fain SB, Lipscomb RC, Hullett DH, Samaniego-Picota M, Grist TM, Becker BN. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Renal Physiol. 2007;292:F513–F522. doi: 10.1152/ajprenal.00222.2006. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Vu AT, Li BSY, Dunkle E, Prasad PV. Evaluation of intrarenal oxygenation by BOLD MRI at 3.0 T. J Magn Reson Imaging. 2004;20:901–904. doi: 10.1002/jmri.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC. Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol. 2009;44:566–571. doi: 10.1097/RLI.0b013e3181b4c1e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy TP, Cooper CJ, Dworkin LD, Henrich WL, Rundback JH, Matsumoto AH, Jamerson KA, D'Agostino RB. The Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) Study: Rationale and Methods. J Vasc Interv Radiol. 2005;16:1295–1300. doi: 10.1097/01.RVI.0000176301.69756.28. [DOI] [PubMed] [Google Scholar]
- 15.Textor SC, Turner ST. Renal vascular response to sodium loading in sons of hypertensive parents. Hypertension. 1991;17:982–988. doi: 10.1161/01.hyp.17.6.982. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DM, Bergert JH, Larson TS, Liedtke BS. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis. 1997;30:646–652. doi: 10.1016/s0272-6386(97)90488-1. [DOI] [PubMed] [Google Scholar]
- 17.Lerman LO, Taler SJ, Textor SC, Sheedy PF, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int. 1996;49:846–854. doi: 10.1038/ki.1996.117. [DOI] [PubMed] [Google Scholar]
- 18.Doghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs using 64-slice multidetector CT: comparison with electron beam CT. Radiology. 2007;243(2):405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 19.Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Non--invasive measurement of concurrent, single-kidney perfusion, glomerular filtration and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 20.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA. Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant. 2001;16:765–770. doi: 10.1093/ndt/16.4.765. [DOI] [PubMed] [Google Scholar]
- 21.Deng A, Tang T, Singh P, Wang C, Satriano J, Thomson SC, Blantz RC. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney International. 2009;75:197–204. doi: 10.1038/ki.2008.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, Romero JC. Regional decrease in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R67–R71. doi: 10.1152/ajpregu.90677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez SI, Warner L, Haas JA, Bolterman RJ, Textor SC, Lerman LO, Romero JC. Increased hypoxia and reduced renal tubular response to furosemide detected by BOLD magnetic resonance imaging in swine renovascular hypertension. Am J Physiol Renal Physiol. 2009;297:F981–F986. doi: 10.1152/ajprenal.90757.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans RG, Gardiner BS, Smith DW, O'Conner PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol. 2008;295:F1259–F1270. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]
- 25.Cheung CM, Shurrab AE, Buckley DL, Hegarty J, Middleton RJ, Mamtora H, Kalra PA. MR-derived renal morphology and renal function in patients with atherosclerotic renovascular disease. Kidney Int. 2006;69:715–722. doi: 10.1038/sj.ki.5000118. [DOI] [PubMed] [Google Scholar]
- 26.Bax L, Woittiez AJ, Kouwenberg HJ, Mali PTM, Buskens E, Beek FJA, Braam B, Huysmans FTM, Kool LJS, Rutten MJCM, Doorenbos CJ, Aarts JCNM, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PMT, van de Ven PJG, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ. Stent Placement in Patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Int Med. 2009;150:999. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 27.Textor SC. Atherosclerotic renal artery stenosis: overtreated, but underrated? J Am Soc Nephrol. 2008;19:656–659. doi: 10.1681/ASN.2007111204. [DOI] [PubMed] [Google Scholar]