Abstract
Constitutive B-cell lymphoma 6 (Bcl-6) expression was undetectable in multiple myeloma (MM) cell lines, except U266 cells. However, it was up-regulated by coculture with bone marrow (BM) stromal cell-culture supernatant (SCCS). Bcl-6 expression in patient MM cells in the BM was positive. Anti–interleukin-6 (IL-6)–neutralizing antibody significantly blocked SCCS-induced Bcl-6 in MM cells. Indeed, IL-6 strongly triggered Bcl-6 expression in MM cells, whereas Janus kinase inhibitor and STAT3 siRNA down-regulated Bcl-6. Tumor necrosis factor-α (TNF-α) also triggered Bcl-6, but independently of STAT3, whereas IκB kinaseβ inhibitor down-regulated TNF-α–induced Bcl-6, indicating that the canonical nuclear factor-κB pathway mediates TNF-α–induced Bcl-6 expression. Importantly, down-regulation of Bcl-6 by shRNA significantly inhibited MM cell growth in the presence of SCCS. Our results therefore suggest that Bcl-6 expression in MM cells is modulated, at least in part, via Janus kinase/STAT3 and canonical nuclear factor-κB pathways and that targeting Bcl-6, either directly or via these cascades, inhibits MM cell growth in the BM milieu.
Introduction
B-cell lymphoma 6 (Bcl-6) is a 95-kDa nuclear protein belonging to the Pox virus zinc finger transcription factor family. It is a proto-oncogene encoding a transcriptional repressor, which regulates germinal center B-cell differentiation. BCL6 is constitutively expressed in a significant fraction of B-cell lymphomas. Importantly, Bcl-6 is deregulated either by chromosomal translocations (3q27) or aberrant somatic hypermutation in a subset (35%-40%) of diffuse large B-cell lymphomas (DLBCLs),1,2 and its biologic significance has been extensively studied in this setting.3
Bcl-6 function is regulated by acetylation; specifically, histone deacetylase-2 (HDAC2) binds to Bcl-6 and modulates its function.4 Conversely, inhibition of HDAC2 induces hyperacetylation of Bcl-6, resulting in loss of its function. Therefore, HDAC inhibitors (especially class I inhibitors) have been used for functional inhibition of Bcl-6.5 Most importantly, a small peptide inhibitor of BCL-6 induces cytotoxicity in primary human DLBCL cells both in vitro and in vivo, without affecting normal lymphoid tissue,6,7 suggesting that Bcl-6 is a promising novel therapeutic target in DLBCLs. However, the biologic significance of Bcl-6 in multiple myeloma (MM) has not yet been elucidated.
Methods
Detailed information pertinent to tumor cell lines and primary tumor specimens, growth of long-term bone marrow stromal cells (BMSCs), reagents, immunoblotting, cell growth assays, real-time polymerase chain reaction (RT-PCR), and shRNA infection are included in the supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).8–13 Primary CD138+ tumor cells from MM patients were obtained using negative selection, as previously described8 after Institutional Review Board–approved informed consent (Dana-Farber Cancer Institute) and in accordance with the Declaration of Helsinki protocol.
Results and discussion
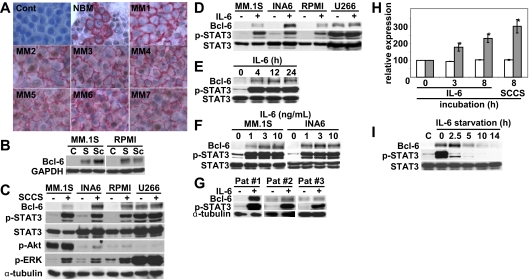
To examine whether patient MM cells in the BM express Bcl-6, we first performed immunohistochemical analysis on BM tissue microarrays from both healthy donors (NBM) and MM patients. Importantly, Bcl-6 was strongly expressed within the nucleus in MM cells of all cases (Figure 1A), suggesting that Bcl-6 might play a role in MM pathogenesis. To examine whether soluble factors modulated Bcl-6 expression in MM cells in the context of the BM microenvironment, MM.1S and RPMI8226 cells were cultured with stromal cell-culture supernatants (SCCSs) or BMSCs for 12 hours. Induction of Bcl-6 was similarly up-regulated by SCCS and BMSC coculture (Figure 2B), suggesting that induction of Bcl-6 by BMSCs was predominantly by soluble factors, and we carried out further experiments using SCCSs. Although MM cell lines express weak or undetectable constitutive Bcl-6 expression, it is markedly up-regulated by SCCSs (Figure 2C). Because the pattern of Bcl-6 induction by SCCSs was similar to phospho-STAT3 and phospho-ERK, we hypothesized that interleukin-6 (IL-6) in SCCSs may be triggering Bcl-6 in MM cells. We therefore cultured MM cell lines with recombinant IL-6 and confirmed that Bcl-6 was markedly up-regulated, as was p-STAT3 (Figure 2D). Interestingly, U266 has high baseline Bcl-6 and p-STAT3 expression, which is associated with constitutive phosphorylation of gp130 (supplemental Figure 1). Dose-dependent (Figure 2E) and time-dependent (Figure 2F) effects of IL-6 on Bcl-6 expression showed maximum induction by 4 hours with 3 and 10 ng/mL IL-6. Importantly, IL-6 also triggered Bcl-6 expression in patient MM cells (Figure 2G). Real-time RT-PCR showed that IL-6, in a time-dependent fashion, significantly increased Bcl-6 mRNA levels in INA6 cells (Figure 2H), indicating that IL-6–induced transcriptional up-regulation of Bcl-6. We also examined the kinetics of Bcl-6 down-regulation after IL-6 withdrawal. As shown in Figure 2I, Bcl-6 expression rapidly decreased to baseline levels at 5 to 10 hours after IL-6 withdrawal.
Figure 1.
IL-6 in SCCSs induces Bcl-6. (A) Immunohistochemical analysis for Bcl-6 expression was performed on bone marrow (BM) tissue microarrays from healthy donors (NBM) and multiple myeloma (MM) patients. Representative results are shown. CD138 is stained in red; Bcl-6 is stained in brown. Control sample was stained without anti–Bcl-6 antibodies or anti-CD138. See supplemental Methods for image-acquisition information. (B) MM.1S and RPMI8226 cells were cultured with stromal cell–culture supernatants (SCCSs; S) or bone marrow stromal cells (BMSCs; Sc) for 12 hours. (C) MM.1S, interleukin-6 (IL-6)–starved INA6, RPMI8226, and U266 cells were cultured with SCCSs for 12 hours. (D) MM.1S, IL-6–starved INA6, RPMI8226, and U266 cells were cultured with IL-6 (5 ng/mL) for 12 hours. (E) MM.1S cells were cultured with IL-6 (5 ng/mL) for the indicated time periods. (F) MM.1S and IL-6–starved INA6 cells were cultured with IL-6 (1, 3, or 10 ng/mL) for 12 hours. (G) Patient MM cells were cultured with IL-6 (5 ng/mL) for 12 hours. (H) INA6 cells were cultured with IL-6 (5 ng/mL for 3 hours and 8 hours) or SCCSs for 8 hours. Total RNA was extracted, and Bcl-6 gene expression was examined by real-time RT-PCR ( ) and normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (□), which served as an internal control. *P < .01. (I) INA6 cells were cultured with or without IL-6 (5 ng/mL) for 12 hours. Cells were then washed and cultured for the indicated time periods. Whole-cell lysates were subjected to immunoblotting with indicated antibodies. Phospho-STAT3 served as positive control for IL-6–induced signal transduction.
) and normalized to expression of glyceraldehyde-3-phosphate dehydrogenase (□), which served as an internal control. *P < .01. (I) INA6 cells were cultured with or without IL-6 (5 ng/mL) for 12 hours. Cells were then washed and cultured for the indicated time periods. Whole-cell lysates were subjected to immunoblotting with indicated antibodies. Phospho-STAT3 served as positive control for IL-6–induced signal transduction.
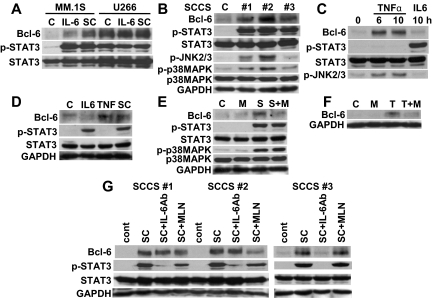
Figure 2.
TNF-α up-regulates Bcl-6 expression. (A) MM.1S and U266 cells were cultured with IL-6 (5 ng/mL) or SCCSs (SC) for 12 hours. (B) RPMI8226 cells were cultured with 3 different SCCS (#1, #2, #3) for 12 hours. (C) RPMI8226 cells were cultured with tumor necrosis factor-α (TNF-α; 2.5 ng/mL) or IL-6 (5 ng/mL) for the indicated time periods. (D) Patient MM cells were cultured with IL-6 (5 ng/mL), TNF-α (2.5 ng/mL), or SCCSs (SC) for 12 hours. (E) RPMI8226 cells were cultured with or without SCCSs (S), in the presence or absence of MLN120B (M, 10μM) for 12 hours. (F) Patient MM cells were cultured with TNF-α (T) for 12 hours in the presence or absence of MLN120B (M, 10μM). (G) RPMI8226 cells were cultured with 3 different SCCSs (sc#1, sc#2, and sc#3), in the presence or absence of neutralizing IL-6 antibodies (5 μg/mL) or MLN120B (M, 10μM) for 12 hours. Whole cell lysates were subjected to immunoblotting with indicated antibodies.
To define the extent to which IL-6 accounts for SCCS-induced Bcl-6 expression, we next cultured MM cells with SCCSs, in the presence or absence of neutralizing anti–IL-6 antibodies. Neutralizing anti–IL-6 antibodies only partially inhibited SCCS-induced Bcl-6 and p-STAT3 expression (Figure 2A). Because other gp130 family cytokines also trigger phosphorylation of STAT3 and ERK, and BMSCs also secrete oncostatin M (OSM; supplemental Figure 2), we examined whether exogenous OSM also triggered Bcl-6 in MM cell lines. As expected, OSM markedly up-regulated Bcl-6 expression in MM.1S and INA6 cell lines, associated with induction of p-STAT3 (supplemental Figure 3). In contrast, other cytokines, including insulin-like growth factor-1, vascular endothelial growth factor, stromal cell–derived factor-1α, or IL-3, did not induce Bcl-6 expression (data not shown). These results suggest that gp130 family cytokines trigger Bcl-6 expression in MM cells.
Our studies indicated that phosphorylation of both ERK and STAT3 is correlated with Bcl-6 expression (Figure 1C). MEK inhibitor U0126 completely blocked both constitutive and IL-6–induced phospho-ERK; however, it did not inhibit phospho-STAT3 or Bcl-6 expression (supplemental Figure 4A). In contrast, pan-Janus kinase (JAK) inhibitor AG490 markedly down-regulated phospho-STAT3 and Bcl-6 expression (supplemental Figure 4B). We further confirmed the significance of STAT3 down-regulation using STAT3 siRNA: STAT3 siRNA transfectants have lower levels of Bcl-6 than scrambled siRNA transfectants (supplemental Figure 4C). Taken together, these results suggest that IL-6–induced Bcl-6 expression is modulated via JAK/STAT3 pathway.
We next examined the impact of IL-6 on Bcl-6 expression in MM cells in the context of the BM microenvironment by culturing 2 IL-6 responsive of MM cell lines (MM.1S, U266) with SCCSs or IL-6. IL-6 was a more potent inducer of p-STAT3 than SCCSs; however, SCCSs more potently induced Bcl-6 than IL-6 in MM.1S (Figure 2A). We further examined 3 distinct SCCSs and observed that induction of Bcl-6 by SCCSs was correlated with phosphorylation of JNK and p38MAPK (Figure 2B). Because we have previously shown that transcription and secretion of IL-6 by BMSCs are regulated by nuclear factor-κB (NF-κB)14 and tumor necrosis factor-α (TNF-α) is a potent activator of NF-κB, JNK, and p38MAPK, we hypothesized that TNF-α in SCCSs may also trigger Bcl-6 expression. As expected, TNF-α markedly up-regulated Bcl-6 in RPMI8226 cells (Figure 2C). This up-regulation was completely independent of p-STAT3, indicating that IL-6 or other gp130 family cytokines were not induced by the TNF-α–NF-κB axis in this setting. Importantly, both SCCSs and TNF-α also triggered Bcl-6 in patient MM cells even when IL-6 did not (Figure 2D). We have previously shown that IκB kinaseβ inhibitor MLN120B blocks TNF-α–induced NF-κB activation.15 We next showed that MLN120B completely blocked TNF-α–induced Bcl-6 expression in patient MM cells (Figure 2E) and RPMI8226 cells (Figure 2F), suggesting that TNF-α–induced Bcl-6 expression is mediated via the canonical NF-κB pathway. Because secretion of cytokines from primary BMSCs varies, we further compared the induction of Bcl-6 in RPMI8226 cells by SCCSs from 3 different persons, in the presence or absence of neutralizing anti–IL-6 antibodies or MLN120B (Figure 2F). All SCCSs markedly induced Bcl-6 expression; however, the inhibitory effect of neutralizing anti–IL-6 antibodies or MLN120B on Bcl-6 induction varied (Figure 2G). These results suggest that SCCSs may have different cytokines and that the signaling cascades triggering Bcl-6 may therefore also differ. These results indicate that up-regulation of Bcl-6 by SCCS is not induced by a single cytokine but the total effect of cytokines in the BM milieu.
To determine the biologic significance of Bcl-6, we next knocked down Bcl-6 expression by lentiviral Bcl-6 shRNA. MM.1S cells were cultured with SCCS to induce Bcl-6. Both sh #1 and sh #2 constructs sufficiently down-regulated Bcl-6 expression (supplemental Figure 5A). Importantly, Bcl-6 knockdown infectants had decreased numbers of viable cells compared with noninfected or Sc shRNA infectants (supplemental Figure 5B). Analogous experiments were carried out in U266 cells with high constitutive Bcl-6 expression without SCCSs, and we observed that Bcl-6 shRNA showed cell growth inhibition associated with down-regulation of Bcl-6 (supplemental Figure 6). In this study, our results therefore suggest that Bcl-6 expression is mediated via both JAK/STAT3 and NF-κB pathways in MM cells and that targeting these cascades could both inhibit Bcl-6 expression and inhibit growth of MM cells in the BM microenvironment.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (SPORE IP50 grants CA10070, PO-1 78378, and RO-1 CA 50947), the Multiple Myeloma Research Foundation (T.H., D.C., N.R., C.M.), and the LeBow Family Fund to Cure Myeloma (K.C.A.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.H. performed experiments and prepared the manuscript; C.M., D.C., and K.C.A. designed experiments and analyzed the data; H.I., G.G., and H.H. performed experiments; N.R. designed experiments; N.C.M. and P.G.R. analyzed the data; and D.R.C. designed and performed the experiments.
Conflict-of-interest disclosure: C.M. has received consultant honoraria from Millennium, Novartis, Bristol-Myers Squibb, Merck, Kosan, and Pharmion, as well as research funding from Amgen, AVEO Pharma, EMD Serono, and Sunesis. K.C.A., N.C.M., and N.R. are consultants and on the advisory boards for Millennium, Celgene, and Novartis. P.G.R. is a consultant and on the advisory boards for Millennium and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.
References
- 1.Kerckaert JP, Deweindt C, Tilly H, Quief S, Lecocq G, Bastard C. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nat Genet. 1993;5(1):66–70. doi: 10.1038/ng0993-66. [DOI] [PubMed] [Google Scholar]
- 2.Ye BH, Lista F, Lo Coco F, et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262(5134):747–750. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 3.Ci W, Polo JM, Melnick A. B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Curr Opin Hematol. 2008;15(4):381–390. doi: 10.1097/MOH.0b013e328302c7df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bereshchenko OR, Gu W, Dalla-Favera R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32(4):606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 5.Lemercier C, Brocard MP, Puvion-Dutilleul F, Kao HY, Albagli O, Khochbin S. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J Biol Chem. 2002;277(24):22045–22052. doi: 10.1074/jbc.M201736200. [DOI] [PubMed] [Google Scholar]
- 6.Polo JM, Dell'Oso T, Ranuncolo SM, et al. Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med. 2004;10(12):1329–1335. doi: 10.1038/nm1134. [DOI] [PubMed] [Google Scholar]
- 7.Cerchietti LC, Yang SN, Shaknovich R, et al. A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 2009;113(15):3397–3405. doi: 10.1182/blood-2008-07-168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hideshima T, Chauhan D, Kiziltepe T, et al. Biologic sequelae of IκB kinase (IKK) inhibition in multiple myeloma: therapeutic implications. Blood. 2009;113(21):5228–5236. doi: 10.1182/blood-2008-06-161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen D, Nong Y, Morgan JG, et al. A selective small molecule IκB kinase β inhibitor blocks nuclear factor κB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317(3):989–1001. doi: 10.1124/jpet.105.097584. [DOI] [PubMed] [Google Scholar]
- 10.Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107(10):4053–4062. doi: 10.1182/blood-2005-08-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hideshima T, Chauhan D, Teoh G, et al. Characterization of signaling cascades triggered by human interleukin-6 versus Kaposi's sarcoma-associated herpes virus-encoded viral interleukin 6. Clin Cancer Res. 2000;6(3):1180–1189. [PubMed] [Google Scholar]
- 12.Carrasco DR, Sukhdeo K, Protopopov M, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11(4):349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hideshima T, Catley L, Raje N, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138(6):783–791. doi: 10.1111/j.1365-2141.2007.06714.x. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan D, Uchiyama H, Akbarali Y, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-κB. Blood. 1996;87(3):1104–1112. [PubMed] [Google Scholar]
- 15.Hideshima T, Ikeda H, Chauhan D, et al. Bor-tezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood. 2009;114(5):1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.