Abstract
After detection of a high prevalence of scrapie in a large dairy goat herd, 72 infected animals were examined by immunohistochemistry with prion protein (PrP) antibody Bar224 to study the pathogenesis of the infection. Tissues examined included the brain and thoracic spinal cord (TSC), a wide selection of lymphoreticular system (LRS) tissues, the distal ileum and its enteric nervous system (ENS), and other organs, including the mammary gland. The whole open reading frame of the PRNP gene was sequenced and antibodies to caprine arthritis-encephalitis virus (CAEV) infection were determined. Unexpectedly, accumulation of disease-associated PrP (PrPd) in the brain was more frequent in methionine carriers at codon 142 (24/32, 75.0%) than amongst isoleucine homozygotes (14/40, 35.0%). The latter, however, showed significantly greater amounts of brain PrPd than the former (average scores of 9.3 and 3.0, respectively). A significant proportion of the 38 goats that were positive in brain were negative in the ENS (44.7%) or in the TSC (39.5%). These results, together with the early and consistent involvement of the circumventricular organs and the hypothalamus, point towards a significant contribution of the haematogenous route in the process of neuroinvasion. Chronic enteritis was observed in 98 of the 200 goats examined, with no association with either scrapie infection or presence of PrPd in the gut. Lymphoproliferative interstitial mastitis was observed in 13/31 CAEV-positive and scrapie-infected goats; PrPd in the mammary gland was detected in five of those 13 goats, suggesting a possible contribution of CAEV infection in scrapie transmission via milk.
Keywords: scrapie, goat, prion neuroinvasion, transmissible spongiform encephalopathy, CAEV
1. INTRODUCTION
Scrapie, the prototype of the transmissible spongiform encephalopathies (TSE), was originally described in sheep more than two centuries ago (reviewed by Schneider et al. [20]), but was subsequently found to occur naturally also in goats [3, 4, 10, 31]. It is well documented that the susceptibility of sheep to natural scrapie is related to polymorphisms at certain codons of the prion protein gene (PRNP; [6, 12]), and more recent reports also describe genetic differences in the susceptibility of goats to scrapie. Thus, in goat scrapie, incomplete protective effects on natural infection have been associated with the presence of methionine (M) at codon 142 [8], and of serine (S), histidine (H), glutamine (Q) and lysine (K) at codons 146, 154, 211 and 222, respectively (reviewed by [25]). In sheep scrapie, PRNP polymorphisms also appear to modulate the pathogenesis of the infection, at least in respect of the relative accumulation of disease-associated prion protein (PrPd) in the brain and lymphoreticular system (LRS) tissues. For example, sheep of the ARR/VRQ genotype (V, valine; A, alanine; R, arginine) at codons 136, 154 and 171, despite being susceptible to infection and development of clinical disease, have been reported to accumulate PrPd in the LRS less often than sheep of other susceptible genotypes [7, 11], rarely [5], or not at all [1, 13, 26]. Similarly in goats, accumulation of PrPd in the brain without LRS involvement appears to be a rare event, linked to the M polymorphism at codon 142 [8].
It is generally believed that sheep acquire natural scrapie by the oral route, and that the infectious agent gains access to the central nervous system (CNS) through sympathetic and parasympathetic neural pathways, after initial replication in the enteric nervous system (ENS). This prevailing view of neural neuroinvasion is mostly based on observations of natural sheep scrapie [27, 28] and of experimental infections in sheep [29] and hamster models [2, 18], which show that accumulation of PrPd in the ENS precedes that in the CNS, and that the earliest CNS sites targeted are the dorsal motor nucleus of the vagus nerve (DMNV) and the intermedio-lateral column (ILC) of the thoracic spinal cord. It has also been suggested that prior amplification of PrPd in the lymphoid follicles of the intestinal Peyer’s patches favours, at least in some cases, infection of the ENS. More recently, an alternative or complementary route of neuroinvasion has been suggested to involve haematogenous spread of TSE agents and their entry in the brain through the circumventricular organs (CVO [22, 23]); these specialized structures of the brain have fenestrated capillaries and therefore lack a blood-brain barrier.
Sheep and goats reared in normal livestock management systems are naturally exposed to diverse infectious and non-infectious conditions. The effect of the co-existence of scrapie and other diseases on the pathogenesis and dissemination of the prion infection has received some attention in recent years. Thus, it has been shown that co-occurrence of maedi-visna (MV) virus-associated indurative mastitis and scrapie in sheep can lead to accumulation of PrPd in the mammary gland [17], and to release of infectivity in milk [15]. It has also been shown that, in a murine experimental model, Salmonella typhimurium-induced colitis increases susceptibility to subsequent oral scrapie challenge [21]. Similar studies have not been conducted for goat scrapie.
Following the cull, due to scrapie, of a large dairy goat herd, detailed studies showed a high prevalence of sub-clinical infection on a selection of 200 animals. The clinico-epidemiological aspects of the infection, including the effect of PRNP genotype and age, as well as those of tissue distribution of PrPd and its diagnostic repercussions have been described in a previous publication [8]. The present report concentrates on the analysis of results related to the pathogenesis of the infection, in particular, its modulation by the PRNP genotype, the assessment of the possible routes of neuroinvasion, and the significance of co-existing conditions.
2. MATERIALS AND METHODS
Details of the origin, evolution and composition of the herd, as well as of its scrapie history and aspects of the prevalence, diagnosis and epidemiology of the infection have been described previously [8]. This report concentrates on aspects of the pathogenesis of scrapie in the 72 goats that were found to be scrapie-infected when a selection of 200 goats was examined after the whole herd was culled.
On post-mortem of the 72 goats, the following tissues were collected and fixed in 10% formaldehyde: (i) central nervous system: sagittally-sectioned half brain and thoracic spinal cord (TSC; segments 10–12), (ii) LRS tissues: palatine tonsil, spleen, nictitating membrane, medial retropharyngeal, pre-scapular, distal jejunal, mammary and pre-femoral lymph nodes, (iii) alimentary system tissues: distal ileum and rectum, for examination of both gut-associated lymphoid tissue and ENS, (iv) other tissues: eye – for examination of optic nerve, retina and ocular muscles –, adrenal gland and mammary gland. These samples were processed for immunohistochemical (IHC) detection of PrPd, as already described [8].
The magnitude of PrPd accumulation in all those tissue samples was subjectively scored from 0–3 (where 0 = absent, 1 = mild, 2 = moderate and 3 = severe). In the brain, 10 different areas (frontal cerebral cortex, corpus striatum, septal area, hypothalamus, thalamus, midbrain tegmentum, substantia nigra, cerebellum, and medulla oblongata at the levels of the middle cerebellar peduncles and the obex) were subjected to such a scoring system, which has been detailed and illustrated elsewhere [22], so that the maximum score for an individual goat was 30. Particular care was taken to obtain representation of the CVO, specifically of the area postrema (AP), the median eminence (ME), the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT), where accumulation of PrPd was also scored in the same way as for the brain parenchyma. As for the collected segments of the TSC, three different sections 1 cm apart were examined. Also, two different pieces of distal ileum, 2–3 cm apart, were processed and examined.
Genotyping of the whole open reading frame of the PRNP gene was performed as previously described [8], and antibodies to caprine arthritis encephalitis virus (CAEV) were detected by an agar-gel immuno-diffusion test (Maeditec, VLA-Weybridge, UK), following the original protocol described by Winward et al. [30].
3. RESULTS
Detailed individual information on ages, genotypes and IHC results of the 72 scrapie-infected goats are available on line at www.vetres.org as “Supplementary data Table I”.
3.1. Modulation of the pathogenesis of scrapie by polymorphisms at codon 142 of the PRNP gene
In a previous publication [8] we described that, of the 200 goats originally examined, the probability of infection (defined as an IHC positive result in any of the tissues examined) was significantly higher for isoleucine homozygotes at codon 142 (II142, 40/80 = 50.0%), than for those goats carrying methionine at the same codon (IM142 or MM142, 32/120 = 26.7%). We also reported that all goats that displayed definite clinical signs of scrapie (n = 7) were amongst the 72 infected goats and all were II142, and that of the clinical scrapie cases that occurred before the cull, 55/62 were also II142 and seven were IM142 heterozygotes. Therefore, allowing for untraceable differences in exposure – in the unlikely event that these existed –, II142 animals showed a higher susceptibility to infection and were more prone to develop clinical disease than methionine carriers (M142).
Of the 72 scrapie-infected goats, some were PrPd positive only in brain (n = 4), some only in LRS tissues (n = 34) and some in both (n = 34). It was therefore tempting to think that clinical disease amongst II142 animals would be in connection with goats of this genotype accumulating PrPd in the brain more frequently than M142 goats, while these latter animals would be mostly amongst those with PrPd only in LRS tissues. Surprisingly, the analysis of data provided just the opposite results, so that scrapie-infected M142 carriers showed a significantly higher propensity (p < 0.001; Fisher’s exact test) to accumulate PrPd in the brain (24/32 = 75.0%), than did infected II142 homozygotes (14/40 = 35.0%). However, when a quantitative analysis of brain PrPd was performed, it was found that brain-positive II142 goats accumulated significantly higher (p < 0.05; unpaired t-test with Welch correction) amounts of PrPd (mean 9.3, 95% CI 3.9–14.6) than brain-positive M142 carriers (mean 3.0, 95% CI 2.0–4.0).
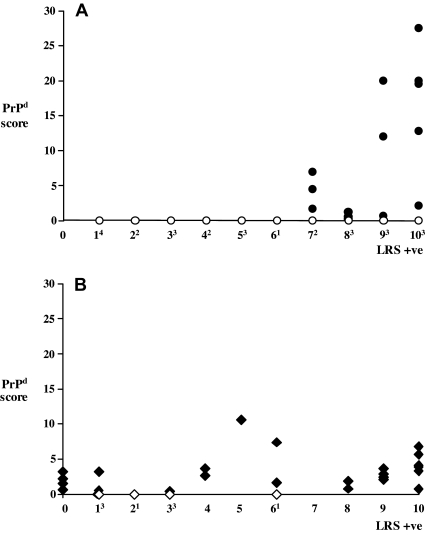
A further analysis revealed clear differences in the relationship between LRS and CNS involvement between the two genotype groups, II142 goats and M142 carriers. Thus, none of the 15 II142 goats with six or less LRS tissues affected were PrPd positive in brain, and only 14 of the 25 with seven or more LRS tissues affected were so (Fig. 1A). In contrast, 12 of the 20 M142 carriers that accumulated PrPd in six or less lymphoid tissues (including those four that were fully negative in the LRS) were PrPd positive in brain, as were all the remaining 12 that had seven or more LRS tissues affected (Fig. 1B). Moreover, while the amount of PrPd in the brain of II142 animals showed a tendency to increase in parallel with the number of lymphoid tissues affected, no such correlation was found for M142 carriers (Fig. 1).
Figure 1.
Magnitude of PrPd accumulation in the brain (y-axis; cumulative score of 10 areas examined) in relation to the number of LRS tissues affected (x-axis; superscripts refer to the number of brain-negative animals for each level of LRS positivity). (A) II142 goats; (B) IM/MM142 goats.
An additional analysis was performed to determine if the differences in CNS/LRS involvement between the two genotype groups were age-dependent. Unpaired t-tests showed that, while brain-positive II142 goats (87 ± 14 months) were significantly older (p < 0.05) than brain-negative goats of the same genotype (68 ± 10 months), differences were not significant amongst M142 carriers (62 ± 11 and 49 ± 14 months for brain positive and negative, respectively; p = 0.1). In other words, scrapie-infected M142 carriers were younger (p < 0.05) than II142 goats regardless of their accumulation or not of PrPd in the brain.
3.2. Routes of neuroinvasion
In order to assess whether the differences in scrapie pathogenesis described above were the result of distinctly different mechanisms of neuroinvasion, the data presented in this section were analyzed separately for the two genotype groups. No differences were found between II142 goats and M142 carriers, suggesting that the PRNP genotype did not have a major impact on the mechanisms of neuroinvasion. Therefore, the following data are presented for the whole of the 72 scrapie-infected goats.
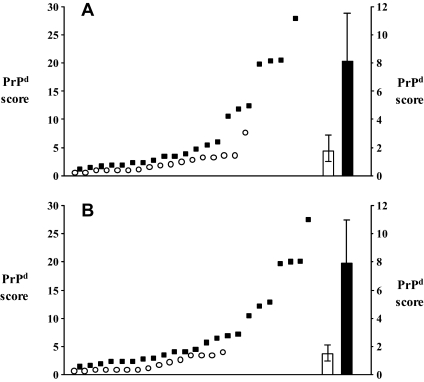
Accumulation of PrPd in the ENS of the distal ileum occurred in only three goats of those 34 that were positive in the LRS alone, and in only 21 of the 38 that accumulated PrPd in the brain (Tab. I, Figs. 2a and 2b). Amongst the latter, the brain PrPd scores of those that were positive in the ENS were significantly higher (mean 8.1, 95% CI 1.7–4.6) than the scores of those that were ENS negative (mean 1.9, 95% CI 1.0–2.8; p < 0.001; Mann–Whitney test; Fig. 3A). The probability of the ENS being PrPd positive was significantly higher (p = 0.001; Fisher’s exact test) if the Peyer’s patches were also positive (19/37, 51.4%), than if these were negative (5/35, 14.3%). It has to be noted, however, that out of the 24 goats with positive ENS, 20 showed PrPd accumulation in seven or more, and none in less than four, of the LRS tissues examined.
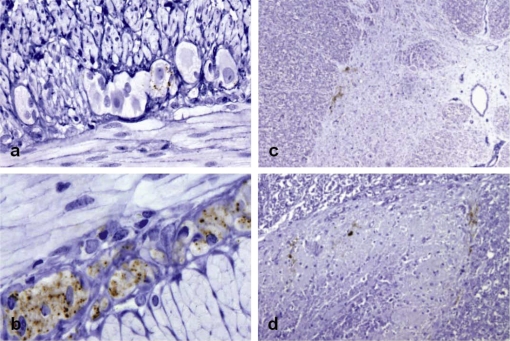
Figure 2.
(a) Trace PrPd deposits in a single ganglion cell of the myenteric plexus of a goat that was positive in 7 of 10 LRS tissues and also in brain (score 1.7). (b) Widespread PrPd accumulation in ganglion and satellite cells of the ENS of a clinically-affected goat (9/10 LRS positive tissues and 20.0 score in brain). Mild PrPd deposition in the intermedio-lateral column (c) and substantia gelationosa (d) of the TSC of a goat that was positive in 7 of 10 LRS tissues and in brain (score 7.0). IHC with Barr224 PrP antibody and haematoxylin counterstaining (magnifications: a, d ×20; b ×40; c ×10). (For a color version of this figure, please consult www.vetres.org.)
Figure 3.
Individual brain PrPd scores (against left y-axis) and average brain PrPd values (against right y-axis; error bars, 95% CI) of goats that were negative (circles and open bar) or positive (squares and solid bar) in the ENS (A) and in the TSC (B).
Table I.
PrPd in the ENS and TSC of brain negative and positive scrapie-infected goats.
| Brain negative |
Brain positive |
|||
|---|---|---|---|---|
| II142 | M142 | II142 | M142 | |
| ENS−ve/TSC−ve | 23 | 8 | 2 | 10 |
| ENS−ve/TSC+ve | 0 | 0 | 2 | 3 |
| ENS+ve/TSC−ve | 3 | 0 | 1 | 2 |
| ENS+ve/TSC+ve | 0 | 0 | 9 | 9 |
| Total | 26 | 8 | 14 | 24 |
II: isoleucine homozygotes; M: methionine carriers (homo- or heterozygotes).
None of the goats that were PrPd negative in brain were positive in the TSC, and only a fraction of those that were positive in brain accumulated PrPd in the TSC (23/38, Tab. I, Figs. 2c and 2d). Once again, the brain PrPd scores of the goats that were positive in the TSC were significantly higher (mean 7.8, 95% CI 4.6–11.0) than those of the goats that were TSC negative (mean 1.4, 95% CI 0.8–2.1; p < 0.001; Mann–Whitney test; Fig. 3B).
Another approach to try to evaluate the possible pathways of access of infection to the brain was to assess the consistency of PrPd deposition in the different areas of the neuroparenchyma examined and the involvement of the CVO (Tab. II). The only area that was positive in all 38 brain-positive goats was the obex, specifically the DMNV. In descending order, the other areas were the hypothalamus (32), the ventral medulla (26), the tegmentum, septal area and substantia nigra (17, 16 and 14, respectively), the cerebellum and thalamus (12 and 10, respectively) and, finally, the corpus striatum and the cerebral cortex (8 and 5, respectively). The data on frequency of PrPd accumulation in those different areas correlated to some extent with the magnitudes of deposition. For instance, for the 32 goats that were positive in obex and hypothalamus, 15 showed higher scores in the DMNV, 8 in the hypothalamus and 9 had the same scores; for those 26 that were positive in hypothalamus and ventral medulla, 16 displayed higher amounts of PrPd in the hypothalamus, 3 in the ventral medulla and 7 had the same scores. A similar analysis indicated that accumulation of PrPd in the CVO either preceded or was in parallel with that in the related areas of the neuroparenchyma (Tab. II and Fig. 4). Thus, the AP was PrPd positive in 35 of 36 goats (97%) in which this CVO could be examined, a proportion that is almost identical to the corresponding DMNV value. The hypothalamus showed a lower frequency of PrPd accumulation (84%) than the three CVO to which it is related, that is, the ME (94%), the SFO (88%) and the OVLT (86%), although these differences were not statistically significant in the Fisher’s exact test. The SFO and the OVLT are also in close contact with the septal area; these two CVO were more frequently affected (p < 0.01 in the Fisher’s exact test) than that area of the neuroparenchyma (42% of cases).
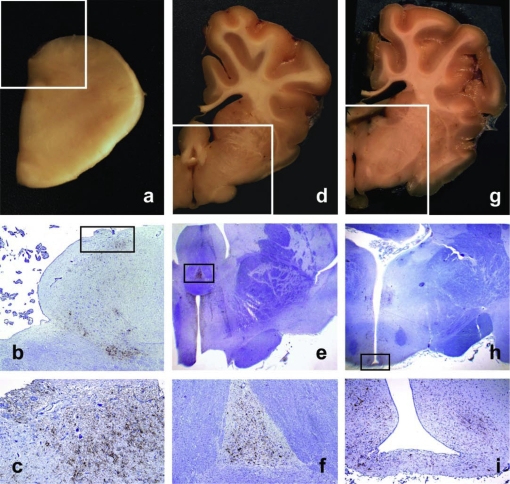
Figure 4.
Accumulation of PrPd in CVO and related brain structures. a, d, g: Gross sections showing location of the AP at the level of the obex (a), of the SFO at the level of the anterior hypothalamus (d), and of the ME at the level of the infundibular hypothalamus (g). b, e, h: Low power micrographs corresponding to the framed areas of a, d and g, respectively. PrPd is seen to accumulate in the framed areas that correspond to the AP (b), SFO (e) and ME (h); IHC with Bar224 PrP antibody and haematoxylin counterstaining (b, ×2; e, ×1; h, ×1). c, f, i: Detail of PrPd accumulating in the same CVO as in b, e and h, respectively; IHC with Bar224 PrP antibody and haematoxylin counterstaining (c, ×10; f, ×4; i, ×4). (For a color version of this figure, please consult www.vetres.org.)
Table II.
Frequency and magnitude of PrPd in different brain areas and in the CVO.
| Positive/exam. (%) | Av. mag.* |
|||
|---|---|---|---|---|
| All examined | Positives | |||
| DMNV | 38/38 | (100) | 1.23 | 1.23 |
| HYTH | 32/38 | (84) | 1.01 | 1.20 |
| VMED | 26/38 | (68) | 0.61 | 0.89 |
| TEGM | 17/38 | (45) | 0.44 | 0.98 |
| SEPT | 16/38 | (42) | 0.44 | 1.06 |
| SUBN | 14/38 | (37) | 0.46 | 1.24 |
| CBEL | 12/38 | (32) | 0.36 | 1.14 |
| THAL | 10/38 | (26) | 0.38 | 1.44 |
| STRI | 8/38 | (21) | 0.19 | 0.89 |
| CCTX | 5/38 | (13) | 0.24 | 1.84 |
| AP | 35/36 | (97) | 1.18 | 1.21 |
| ME | 31/33 | (94) | 1.19 | 1.27 |
| SFO | 22/25 | (88) | 1.11 | 1.26 |
| OVLT | 18/21 | (86) | 0.83 | 0.97 |
DMNV: dorsal motor nucleus of the vagus nerve; HYTH: hypothalamus; VMED: ventral medulla; TEGM: tegmentum; SEPT: septal area; SUBN: substantia nigra; CBEL: cerebellum; THAL: thalamus; STRI: corpus striatum/rhinencephalon; CCTX: cerebral cortex; AP: area postrema; ME: median eminence; SFO: sub-fornical organ; OVLT: organum vasculosum of the lamina terminalis.
Positive/exam.: positive/examined.
* In the left column (“all examined”), average magnitudes (Av. mag.) are calculated including zero values of PrPd negative brain areas; in the right column (“positives”), only the values of PrPd positive brain areas are considered.
3.3. Accumulation of PrPd in LRS tissues
We previously reported that, amongst the 72 infected goats, the medial retropharyngeal lymph node and the palatine tonsil showed a significantly higher frequency of PrPd accumulation (87.3% and 84.5%, respectively) than any of the other eight LRS tissues examined. Extended qualitative and quantitative examinations showed that those differences were most apparent in goats that were negative for PrPd in the brain (Tab. III). Thus, for the medial retropharyngeal lymph node and the palatine tonsil, both the proportion of positive samples and the magnitude of immunolabelling showed only a slight increase when comparing goats that did not and did accumulate PrPd in the brain; this was also the case of the pre-scapular and pre-femoral lymph nodes. Other LRS tissues, such as the mammary lymph node, the Peyer’s patches or the spleen showed a significant (p < 0.05) increase in the proportion of positive samples between brain PrPd-negative and -positive goats, but little variation in the intensity of labelling. Finally, both the proportion of positive samples and the magnitude of PrPd labelling were noticeably higher in the third eyelid, the distal jejunal lymph node and the RAMALT of goats that accumulated PrPd in the brain compared to those that did not.
Table III.
Frequency and magnitude of PrPd in different LRS tissues of goats with and without PrPd accumulation in the brain.
| Brain PrPd negative |
Brain PrPd positive |
|||||
|---|---|---|---|---|---|---|
| Positive/ex.* | (%) | Av. mag.** | Positive/ex. | (%) | Av. mag. | |
| RphLN | 29/34 | (85) | 1.8 | 33/37 | (89) | 1.9 |
| PTons | 27/33 | (82) | 1.6 | 33/38 | (87) | 2.0 |
| MamLN | 18/34 | (53) | 1.1 | 30/38 | (79) | 1.3 |
| PscLN | 17/34 | (50) | 1.5 | 22/38 | (58) | 1.7 |
| 3thEy | 16/34 | (47) | 1.5 | 27/38 | (71) | 2.1 |
| PrfLN | 14/34 | (41) | 1.3 | 19/38 | (50) | 1.4 |
| DjeLN | 13/34 | (38) | 1.2 | 31/38 | (82) | 1.9 |
| PPs | 11/34 | (32) | 1.4 | 26/37 | (70) | 1.7 |
| RAMALT | 9/34 | (26) | 1.2 | 21/38 | (55) | 1.6 |
| Spleen | 6/34 | (18) | 1.5 | 21/38 | (55) | 1.1 |
Results expressed as PrPd-positive samples/examined*, average magnitude of PrPd immunolabelling** in positive LRS tissues of goats that did not or did accumulate PrPd in the brain.
RphLN: medial retropharyngeal lymph node (LN); PTons: palatine tonsil; MamLN: mammary LN; PscLN: pre-scapular LN; 3thEy: third eyelid; PrfLN: pre-femoral LN; DjeLN: distal jejunal LN; PPs: distal ileum Peyer’s patches; RAMALT: recto-anal mucosa-associated lymphoid tissue.
3.4. Accumulation of PrPd in other tissues
Twelve goats (nine II142 homozygotes and three IM142 heterozygotes) accumulated PrPd in tissues other than the CNS, ENS and LRS (Tab. IV). Of the five goats with detectable PrPd in the mammary gland, three were negative and one had a low PrPd score in brain; all the five had seven or more LRS tissues involved. The four goats that were positive in the retina and the optic nerve, two of which also accumulated PrPd in ocular muscles, were those showing the highest PrPd scores in brain, although their LRS tissues were also heavily involved. All the eight goats with PrPd in the medulla of the adrenal gland showed abundant PrPd deposition in LRS tissues and five of them also had high brain scores.
Table IV.
Detection of PrPd in tissues other than CNS, ENS and LRS with regard to CNS and LRS involvement.
| Goat no. | Brain PrPd* | LRS PrPd** | Ret. | Op. N. | Oc. Ms. | Adr. | Mm. Gl. |
|---|---|---|---|---|---|---|---|
| 1143 | 9 | P | |||||
| 1396 | 9 | P | |||||
| 1427 | 10 | P | |||||
| 1439 | 1.7 | 7 | P | ||||
| 1349IM | 4.0 | 10 | P | ||||
| 1386 | 12.8 | 10 | P | ||||
| 1494IM | 6.5 | 10 | P | ||||
| 1451IM | 4.0 | 10 | P | ||||
| 1407 | 19.5 | 10 | P | P | P | ||
| 1475 | 27.5 | 10 | P | P | P | ||
| 1469 | 20.0 | 10 | P | P | P | P | |
| 1447 | 19.5 | 10 | P | P | P | P | P |
Ret.: retina; Op. N.: optic nerve; Oc. Ms.: ocular muscles; Adr.: adrenal gland; Mm. Gl.: mammary gland.
P: positive for PrPd; blank spaces: no PrPd detected.
* Cumulative score in 10 areas examined.
** Number of LRS tissues positive out of 10 examined.
3.5. Effect of co-existing conditions on susceptibility to and transmission of scrapie
Histopathological examination of samples of distal ileum revealed the presence of lesions consistent with non-specific, chronic enteritis in 34 of the 72 scrapie-infected goats. Lesions of variable severity were mainly characterized by atrophy and fusion of intestinal villi and by the presence of mononuclear cell infiltrates in the lamina propria. In 3 of the 34 goats enteritis was of a granulomatous nature; the aetiology was not established although, on the basis of negative Ziehl-Neelsen stain results, they were not mycobacterial. Lesions of enteritis did not appear to be related to the presence of PrPd in the Peyer’s patches, so that these were positive in 20 of 34 goats with enteritis and in another 20 of 38 without such lesions. Examination of the distal ileum for lesions of enteritis was extended to the 128 scrapie-negative goats included in the whole cull, and these presented with a similar frequency of enteric lesions (50%) as the scrapie-infected animals. Detailed data and illustrations are given on line at www.vetres.org as Supplementary files Table SII and Figure S1, respectively.
Antibodies to CAEV were detected in 32 of the 72 scrapie-infected goats (44.4%) and in a similar proportion (50/128 = 39.1%) of the scrapie-negative animals; those proportions were not significantly different in the Fisher’s exact test. Histopathological examinations of mammary gland samples were restricted to the 72 scrapie-infected goats; 13 of them showed lesions of lymphoproliferative, interstitial mononuclear mastitis, and all of them were amongst the CAEV antibody positive goats. None of the 40 scrapie-infected goats that were negative for antibodies to the lentiviral infection showed such pathological changes. Only five goats showed PrPd deposits in the mammary gland; all were amongst the 13 with lesions compatible with CAEV infection. In those animals, PrPd accumulated in the proliferating secondary lymphoid follicles located next to lactiferous ducts; in one case, macrophage-resembling cells with intracytoplasmic PrPd deposits appeared to be crossing the epithelium of the ducts. Detailed data and illustrations are given on line at www.vetres.org as Supplementary files Table SIII and Figure S2, respectively.
4. DISCUSSION
The pathogenesis of scrapie in this heavily infected goat herd appeared to follow two different patterns depending on the polymorphisms at codon 142 of the PRNP gene. In goats of the wild-type genotype (isoleucine homozygotes), neuroinvasion did not occur until a high proportion of LRS tissues were positive, but once it happened, PrPd reached high levels in the brain and was often accompanied by the development of clinical disease. In other words, infection in goats of this genotype appeared to follow a conventional and progressive pattern. In contrast, methionine carriers showed an unpredictable pattern, so that accumulation of PrPd in the brain occurred regardless of the degree of lymphoid involvement, without reaching high values, and these goats rarely progressed to clinical disease. The age at which individual goats were exposed to infection cannot be known. However, even if the age were to be considered as an indicator of the duration of the infection this would not explain the discrepancies. Methionine carriers with PrPd in the brain were clearly younger than brain-positive isoleucione homozygotes but, while isoleucine homozygous goats without brain PrPd were younger than those positive in brain, the ages of methionine carriers with or without brain involvement was not significantly different. One possible explanation of the differences between the two groups would be a certain degree of interference of the methionine polymorphism on the ability of PrPd to accumulate in lymphoid tissues. Thus, it has been shown that sheep homozygous for the VRQ allele have a more extensive and earlier LRS involvement than ARR/VRQ sheep [1, 5, 7, 9, 11, 13, 26], and to a lesser extent than those of the ARQ/VRQ genotype [9, 11, 15, 26]1.
The distinct pathogenesis of disease in codon 142 methionine carriers compared to isoleucine homozygotes did not appear to result from differences in the mechanisms of neuroinvasion. In this respect, several findings were common to most infected goats:
-
(1)
A late and inconsistent involvement, as judged by PrPd accumulation, of the ENS and the TSC, which were negative even in a high proportion (44.7% and 39.5%, respectively) of the 38 goats that showed PrPd in the brain. This is in contrast with the findings reported from oral TSE experiments in sheep [27–29] and hamsters [2, 18]. A problem of low sensitivity of our approach could be argued, as only samples of distal ileum and thoracic segments of the spinal cord were examined. However, those areas of the gut and spinal cord have been repeatedly shown to be the more consistently positive than other gut or spinal segments in early stages of infection in sheep [28, 29].
-
(2)
A highly consistent (68/72) and widespread involvement of LRS tissues, particularly amongst isoleucine homozygotes, which strongly suggests a widespread and persistent haematogenous dissemination of the scrapie agent.
-
(3)
An early involvement of the hypothalamus, which in some cases was the only structure of the neuroparenchyma affected in addition to the DMNV, in agreement with the reports for chronic wasting disease-infected deer [24].
-
(4)
A consistent and early accumulation of PrPd in CVO, which in many cases preceded the involvement of neighbouring areas of the neuroparenchyma, and which is in agreement with recent findings in natural and experimental sheep TSE [22, 23].
Taken together, all these findings strongly suggest an important contribution of the blood in the process of neuroinvasion, which contrasts with the prevailing hypothesis of a neural neuroinvasion pathway, mainly derived from the oral experiments in sheep and rodents mentioned above. Whether this is particular to high prevalence natural infection, or to exposure to the agent by other routes than the oral, remains to be determined.
The early and consistent involvement of the medial retropharyngeal lymph node and the palatine tonsil has been widely reported [1, 7, 16, 19, 26, 27], and it is believed to result from the early exposure of those tissues to infectivity following ingestion of the scrapie agent [1, 27, 29]. If that were the case, it would be difficult to explain the late and inconsistent involvement of the distal ileum Peyer’s patches observed in our study. We actually believe that those two LRS tissues are early and consistently involved due to a combination of pathogenetic mechanisms that include (i) exposure to the infectious agent after ingestion and possibly rumination, (ii) exposure to blood-borne infectious agent, and, distinctly, (iii) exposure to infectious agent which, coming from the blood and through the choroid plexus, would be present in the interstitial fluid of the brain and in the cerebrospinal fluid (CSF), even in the absence of detectable PrPd in the neuroparenchyma; the CSF in the subarachnoidal space would drain through the cribriform plate into an extracranial lymphatic network in the nasal submucosa and nasopharynx to reach the retropharyngeal lymph node [32]. Some of the infectivity contained in the CSF would be excreted in the nasal cavity itself [32], and would represent another source of exposure for the pharyngeal (not examined in our study) and the palatine tonsils, which in turn also drain into the retropharyngeal lymph node.
Although a link between enteritis and susceptibility to scrapie has been reported in an experimental murine model [21], such a relationship has not been found in our study, not even between inflammatory lesions and presence of PrPd in the gut. The most likely explanation would be that, in our study, scrapie infection and the development of enteric lesions were temporally unrelated, with scrapie infection occurring early in life and non-specific enteritis being a late and progressive event.
Perhaps surprisingly, infections by CAE lentivirus and the scrapie agent, which in the 200 goats studied both showed a similar prevalence, also appeared to be unrelated. Transmission of CAEV through milk was demonstrated a long time ago and, more recently, transmission of sheep scrapie via milk has also been shown [14], and it is likely that it also occurs in goats. Moreover, detection of PrPd in the mammary gland of scrapie-infected goats was only recorded in a proportion of those with CAEV-induced lymphoproliferative mastitis, in complete agreement with the findings reported by Lacroux et al. [15] in sheep. These authors, however, also found that milk from ewes both with and without PrPd in the mammary glands was infectious in rodent bioassay. This suggests that accumulation of PrPd in the mammary gland is unnecessary for excretion of the scrapie agent in the milk or for transmission of infection by this route. Assuming this to be true, the absence of a relationship between the two infections in the present study would be explained: goats could transmit via the milk either of the two infections, independently of their co-occurrence or otherwise, and regardless of any associated mastitis.
ONLINE MATERIAL
Supplementary PDF file supplied by authors.
Acknowledgments
These studies were carried out with financial support from Defra’s project SE1956 and FSA’s project MO3065. The authors would like to acknowledge the field staff of the Animal Health (AH) agency for supervising the cull and selecting the goats for transport to the VLA, and the Scrapie Flocks team in AH Central Operations for their co-ordinating activities. We are grateful to VLA staff of the Animal Services Unit, Pathology, Molecular Pathogenesis and Genetics and Laboratory Services for their help and support with animal up-keeping, necropsies, PRNP genotyping and examinations for CAEV antibodies. Lynn Fairlie, Ann Dunachie, Maria Oliva and Caroline Goodsir are acknowledged for their excellent work in processing formalin-fixed tissues and performing immunohistochemistry, and Jim Hope and Mike Dawson for their critical appraisal of the manuscript.
Footnotes
González et al., unpublished data.
References
- 1.Andreoletti O., Berthon P., Marc D., Sarradin P., Grosclaude J., Van Keulen L., et al., Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie, J. Gen. Virol. (2000) 81:3115–3126 [DOI] [PubMed] [Google Scholar]
- 2.Beekes M., McBride P.A., Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie, Neurosci. Lett. (2000) 278:181–184 [DOI] [PubMed] [Google Scholar]
- 3.Brotherston J.C., Renwick C.C., Stamp J.T., Zlotnik I., Pattison I.H., Spread of scrapie by contact to goats and sheep, J. Comp. Pathol. (1968) 78:9–17 [DOI] [PubMed] [Google Scholar]
- 4.Chelle P.-L., Un cas de tremblante chez la chèvre, Bull. Acad. Vet. Fr. (1942) 15:294–295 [Google Scholar]
- 5.Ersdal C., Ulvund M.J., Benestad S.L., Tranulis M.A., Accumulation of pathogenic prion protein (PrPSc) in nervous and lymphoid tissues of sheep with subclinical scrapie, Vet. Pathol. (2003) 40:164–174 [DOI] [PubMed] [Google Scholar]
- 6.Goldmann W., Hunter N., Smith G., Foster J., Hope J., PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie, J. Gen. Virol. (1994) 75:989–995 [DOI] [PubMed] [Google Scholar]
- 7.González L., Dagleish M.P., Bellworthy S.J., Sisó S., Stack M.J., Chaplin M.J., et al., Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa, Vet. Rec. (2006) 158:325–331 [DOI] [PubMed] [Google Scholar]
- 8.González L., Martin S., Sisó S., Konold T., Ortiz-Peláez A., Phelan L., et al., High prevalence of scrapie in a dairy goat herd: tissue distribution of disease-associated PrP and effect of PRNP genotype and age, Vet. Res. (2009) 40:65. [DOI] [PubMed] [Google Scholar]
- 9.Hamir A.N., Richt J.A., Kunkle R.A., Greenlee J.J., Bulgin M.S., Gregori L., Rohwer R.G., Characterization of a US scrapie isolate with short incubation time, Vet. Pathol. (2009) 46:1205–1212 [DOI] [PubMed] [Google Scholar]
- 10.Harcourt R.A., Anderson M.A., Naturally-occurring scrapie in goats, Vet. Rec. (1974) 94:504. [DOI] [PubMed] [Google Scholar]
- 11.Houston E.F., Halliday S.I., Jeffrey M., Goldmann W., Hunter N., New Zealand sheep with scrapie-susceptible PrP genotypes succumb to experimental challenge with a sheep-passaged scrapie isolate (SSBP/1), J. Gen. Virol. (2002) 83:1247–1250 [DOI] [PubMed] [Google Scholar]
- 12.Hunter N., PrP genetics in sheep and the implications for scrapie and BSE, Trends Microbiol. (1997) 5:331–334 [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey M., Begara-Mcgorum I., Clark S., Martin S., Clark J., Chaplin M., González L., Occurrence and distribution of infection-specific PrP in tissues of clinical scrapie cases and cull sheep from scrapie-affected farms in Shetland, J. Comp. Pathol. (2002) 127:264–273 [DOI] [PubMed] [Google Scholar]
- 14.Konold T., Moore S.J., Bellworthy S.J., Simmons H.A., Evidence of scrapie transmission via milk, BMC Vet. Res. (2008) 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacroux C., Simon S., Benestad S.L., Maillet S., Mathey J., Lugan S., et al. , Prions in milk from ewes incubating natural scrapie, PLoS Pathog. (2008) 4:e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langeveld J.P.M., Jacobs J.G., Erkens J.H.F., Bossers A., van Zijderveld F.G., van Keulen L.J.M., Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep, BMC Vet. Res. (2006) 2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ligios C., Sigurdson C.J., Santucciu C., Carcassola G., Manco G., Basagni M., et al. , PrPsc in mammary glands of sheep affected by scrapie and mastitis, Nat. Med. (2005) 11:1137–1138 [DOI] [PubMed] [Google Scholar]
- 18.McBride P.A., Schulz-Schaeffer W.J., Donaldson M., Bruce M., Diringer H., Kretzschmar H.A., Beekes M., Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves, J. Virol. (2001) 75:9320–9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reckzeh C., Hoffmann C., Buschmann A., Buda S., Budras K.-D., Reckling K.-F., et al., Rapid testing leads to the underestimation of the scrapie prevalence in an affected sheep and goat flock, Vet. Microbiol. (2007) 123:320–327 [DOI] [PubMed] [Google Scholar]
- 20.Schneider K., Fangerau H., Michaelsen B., Raab W.H.-M., The early history of the transmissible spongiform encephalopathies exemplified by scrapie, Brain Res. Bull. (2008) 77:343–355 [DOI] [PubMed] [Google Scholar]
- 21.Sigurdson C.J., Heikenwalder M., Manco G., Barthel M., Schwarz P., Stecher B., et al. , Bacterial colitis increases susceptibility to oral prion disease, J. Infect. Dis. (2009) 199:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sisó S., Jeffrey M., González L., Neuroinvasion in sheep transmissible spongiform encephalopathies: the role of the haematogenous route, Neuropathol. Appl. Neurobiol. (2009) 35:232–246 [DOI] [PubMed] [Google Scholar]
- 23.Sisó S., Jeffrey M., Martin S., Houston F., Hunter N., González L., Pathogenetical significance of porencephalic lesions associated with intracerebral inoculation of sheep with the BSE agent, Neuropathol. Appl. Neurobiol. (2009) 35:247–258 [DOI] [PubMed] [Google Scholar]
- 24.Spraker T.R., Zink R.R., Cummings B.A., Sigurdson C.J., Miller M.W., O’Rourke K.I., Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease, Vet. Pathol. (2002) 39:546–556 [DOI] [PubMed] [Google Scholar]
- 25.Vaccari G., Panagiotidis C., Acín C., Peletto S., Barillet F., Acutis P., et al., State-of-the-art review of goat TSE in the European Union, with special emphasis on PRNP genetics and epidemiology, Vet. Res. (2009) 40:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Keulen L.J.M., Schreuder B.E.C., Meloen R.H., Mooij-Harkes G., Vromans M.E.W., Langeveld J.P.M., Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie, J. Clin. Microbiol. (1996) 34:1228–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Keulen L.J.M., Schreuder B.E.C., Vromans M.E.W., Langeveld J.P.M., Smits M.A., Pathogenesis of natural scrapie in sheep, Arch. Virol. Suppl. (2000) 16:57–71 [DOI] [PubMed] [Google Scholar]
- 28.Van Keulen L.J.M., Vromans M.E.W., van Zijderveld F.G., Early and late pathogenesis of natural scrapie infection in sheep, APMIS (2002) 110:23–32 [DOI] [PubMed] [Google Scholar]
- 29.Van Keulen L.J., Vromans M.E., Dolstra C.H., Bossers A., van Zijderveld F.G., Pathogenesis of bovine spongiform encephalopathy in sheep, Arch. Virol. (2008) 153:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winward L.D., Leendersten L., Shen D.T., Microimmunodiffussion test for ovine progressive pneumonia, Am. J. Vet. Res. (1979) 40:564–566 [PubMed] [Google Scholar]
- 31.Wood J.N.L., Done S.H., Pritchard G.C., Wooldridge M.J.A., Natural scrapie in goats: case histories and clinical signs, Vet. Rec. (1992) 131:66–68 [DOI] [PubMed] [Google Scholar]
- 32.Zakharov A., Papaiconomou C., Djenic J., Midha R., Johnston M., Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil, Neuropathol. Appl. Neurobiol. (2003) 29:563–573 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.